b School of Materials Science & Engineering, Beijing Institute of Technology, Beijing 100081, China;
c Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China;
d School of Materials Science & Engineering, Tsinghua University, Beijing 100084, China;
e Department of Materials Science and Engineering, Johns Hopkins University, Baltimore, MD 21218-2608, USA
High-energy-density battery systems have been extensively pursued to catch up with the requirements of portable electronics and electric vehicles [1-6]. Lithium-sulfur (Li-S) battery is endowed by the conversion mechanism with high energy density of 2600 Wh/kg, which is 3-5 times that of conventional Li-ion batteries [7]. Additionally, sulfur is a promising cathode material also due to its natural abundance and environmental benignity. However, the conversion reaction between sulfur and lithium also brings high complexity of the electrochemical system. There are three main technical challenges hindering the practical use of Li-S batteries: the insulating nature of sulfur and lithium sulfides that limits the utilization of active materials, the notorious "shuttle effect" of the polysulfide intermediate product, and the safety concerns raised by potential risk in lithium dendrite penetration [1, 8]. Various carbon hosts have been developed as conductive hosts to accommodate sulfur, which is effective in solving the insulating problem and improve the utilization of sulfur [9-12].
The shuttle effect of polysulfide intermediates refers to the dissolution of polysulfides (Li2Sx, 4 < x < 8) into the electrolyte and diffusion across the separator to the anode side, which induces parasitic reactions between polysulfides and lithium metal. The shuttle effect in Li-S batteries results in the loss of active sulfur materials, lowers the coulombic efficiency, and causes the corrosion of lithium metal anode. The strategies in cathode design cannot fully address the shuttle effect yet [13-16].
The modification of a separator is a facile but very effective route in order to suppress the shuttle effect [17-19]. The modification of separator with ion-selective functional layers of Nafion [20], graphene oxides [21], metal-organic frameworks [22] can prevent the diffusion of polysulfide towards anode side. On the other hand, the incorporation of functional layers that can adsorb polysulfides and promote their redox conversion is also effective in mitigating the shuttle of polysulfides [23-26].
Meanwhile, the uneven deposition of lithium at anode side tends to generate lithium dendrites, which is of potential risk on internal short circuit and safety problems [27]. To protect lithium metal anode, several strategies have been proposed focusing on the construction of protective interlayer on lithium surface [28], formation of stable solid electrolyte interface [29-31], the introduction of three-dimensional (3D) conductive hosts [32, 33], and the modification of separators for lithium metal anodes [34]. In fact, the separators for lithium sulfur batteries need not only to alleviate the shuttle effect caused by polysulfide diffusion, but also to reduce the safety risks of the lithium metal anode.
In this contribution, we proposed a lamellar separator reassembled with exfoliation vermiculites, a type of clay, for Li-S batteries. The vermiculite separator is effective in Li-S batteries in both cathode and anode side. Firstly, the 2D vermiculite flakes are readily assembled to yield a separator structure with the interstitial spaces allowing ion selective transportation [35]. The 2D exfoliated vermiculite sheets are negatively charged to repel the polysulfide anions by electrostatic interaction (Fig. 1a). Meanwhile, the positive equilibrium charges are also presented between the sheets, which enriched positively charged lithium ions after ion exchanging in the interspace of the vermiculite flakes (Fig. 1b). The interlayer space therefore acts as permselective Li-ion transfer channels to avoid the shuttle effect in working Li-S batteries.

|
Download:
|
| Fig. 1. (a) Schematic of exfoliated vermiculite separator in lithium-sulfur battery, which can effectively repel the polysulfide anions and mitigate the "shuttle effect". (b) The structure of vermiculite sheets with enriched interlay. | |
The fabrication procedure of vermiculite separator is as follows. The primary mineral of vermiculite was heated at 500 ℃ for 3 min to make it fully expanded. The thermally-expanded vermiculite was then mechanically sheared to obtain vermiculite powder. Asprepared vermiculite powder was then subjected to a two-step ion-exchange method to replace the interlayer cations with Li ions. 200 mg of vermiculite powder were added into 80 mL saturated sodium chloride (NaCl) solution and heated under 60 ℃ for 24 h, followed by vacuum filtration and repeated washing with deionized water. The resulting product was re-soaked in 80 mL 2 mol/L lithium chloride (LiCl) solution and heated under 60 ℃ for additional 24 h, followed by vacuum filtration and repeated washing with deionized water. The Li+-exchanged vermiculite was further chemically exfoliated in 30 wt% hydrogen peroxide (H2O2) solution for 96 h. The homogeneous dispersion of vermiculite nanosheets was obtained through centrifugation at 5000 revolutions per minute for 10 min. 25 mL of as-obtained vermiculite nano-dispersion was then filtered on a 4 cm-diameter polypropylene (PP) substrate (Celgard 3401). Freestanding vermiculite separator was able to be integrally peeled from the PP matrix while soaking in acetone.
Standard CR2025 coin-type cells were employed for the evaluation of vermiculite separator for Li-S cells. The assembly process was carried out in an Ar-filled glove box with O2 and H2O content blow 1 ppm. The ether-based electrolyte of 1 mol/L LiTFSI-1:1 DOL/DME was utilized in this work. To standardize, equal electrolyte of 25 μL/mgS was added in a cell. The S powder (54 wt%), carbon nanotubes (36 wt%) and poly(vinylidene fluoride) binder (10 wt%) are dispersed in N-methylpyrrolidone solvent. The cathodes were prepared by a doctor blade method with an areal weight loading of 1.1 mg/cm2 to 1.3 mg/cm2. All the Li-S batteries were cycled in the voltage range of 1.7 V to 2.8 V.
Vermiculites are typical clay minerals widely applied in cushion materials, nanocomposite reinforcement, and for the generation of paper-like materials. The vermiculite particles are of twodimensional structures (Fig. 2a), which are composed of silicate layers of vermiculite and inter-lamellar water molecules associated with metallic cations. Due to the presence of interlayer water between the silicate lamellae, the vermiculite is intended to expand vigorously when rapidly heated at a high temperature, and can be partially peeled off by mechanical exfoliation into micronsized sheets easily. Furthermore, hydrogen peroxide expansion method was employed to ensure the high dispersibility of vermiculite silicate sheets, which can be indicated by the significant Tindal effect of the vermiculite sheet dispersion with deionized water as dispersant during the laser irradiation experiment (Fig. 2b). In this work, the vermiculite nanosheets are subjected to an ion-exchange process in LiCl solution to enrich lithium ions in the inter-lamellar structures for rapid lithium ion transportation. The dispersed vermiculite nanosheets were then reassembled into a freestanding membrane via a vacuum filtration technique. The thin vermiculite separator is transparent and flexible (Fig. 2c).

|
Download:
|
| Fig. 2. (a) Optical morphology of vermiculite minerals; (b) laser irradiation experiments with solution containing exfoliated vermiculite sheets (left) and deionized water (right); (c) optical image of assembled freestanding vermiculite separator through facile vacuum filtration. | |
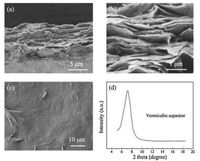
After vacuum filtration, the vermiculite nanosheets dispersed in solution were well reassembled into a lamellar structure. Based on the scanning electron microscopy (SEM) observation at the cross section of the as-obtained vermiculite separator, layer-bylayer vermiculite silicate sheets with densely stacked morphology are observed with a thickness of about 10 μm (Fig. 3a). The thickness of vermiculite separator is highly tuneable by regulating the amount of the vermiculite dispersion to be filtered. The vermiculite silicate nanosheets, which are commonly several tens of nanometers thick, are observed under SEM as well (Fig. 3b). The vermiculite nanosheets with average sizes of about several tens of microns are assembled into a uniform and dense separator (Fig. 3c). X-ray diffraction (XRD) pattern further indicated that the vermiculite sheets stacked are of uniform size with the interlayer cations fully replaced by Li ions as there was only a single broad peak can be observed. Such a compactly stacking structure provides narrow and tortuous channels for ion transport, in which long-chain polysulfides tend to be blocked by steric hindrance and Li+ with much smaller dynamic radius can cross through the separator [36]. The negatively charged vermiculite sheets serving as the well of ion channels also exclude the polysulfide anions but allow rapid transport of lithium cations through the channels. Moreover, the dense surface structure consisted of flat by rigid vermiculite sheets also well suppresses the growth of lithium dendrite for high-safety Li-S batteries.
|
Download:
|
| Fig. 3. Morphology and structural characterizations of vermiculite separator. (a) and (b) cross-sectional SEM image of vermiculite separator; (c) top-view SEM image of vermiculite separator under different resolutions; (d) XRD pattern of the vermiculite separator. | |
To evaluate the electrochemical performance of the vermiculite separator, the separator was assembled into coin cell coupled with sulfur cathode and lithium metal anode for tests. Typical etherbased electrolyte of 1.0 mol/L lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in 1, 3-dioxolane/dimethyl ether (DOL/DME) (1:1) without LiNO3 additive was utilized to demonstrate the effect in the alleviation of shuttle effects. As can be seen in Fig. 4a, when a charge/discharge current density of 0.1C was applied, the cell with a vermiculite coated separator delivered a high initial specific capacity of ca. 1000 mAh/g with an average CE of 90.3% for 50 cycles. In contrast, the cell without any modification usually displays a much lower initial capacity of less than 900 mAh/g with a fluctuant CE below 70%. The incorporation of the vermiculite separator significantly improved the Coulombic efficiency of the cell due to the suppression of shuttle effect by electrostatic repulsion and steric hindrance. The utilization of vermiculite separator also significantly mitigated the parasitic reactions between polysulfides intermediates and Li metal, thereby considerably increasing the discharge capacity. However, with continuous increasing the degree of discharge, a thick Li2S layer is formed, which easily breaks and disperses into the electrolyte and insulating separator. The thick Li2S layer is difficult to be completely charged back to elemental sulfur [37, 38], resulting in the constant loss of capacity despite the introduction of vermiculite separator. Rate performance from 0.1C to 2C was also evaluated to examine the ion transfer resistance when applying vermiculite separator in Li-S battery. It is noteworthy that a high discharge capacities of 700 mAh/g and 600 mAh/g were obtained even at practical rates of 1C and 2C (Fig. 4b), respectively. Compared with a Li-S battery with a routine PP separator, the cells with vermiculite separator presented similar capacity even at high current densities of 2C, which indicated that a fast Li-ion transfer manner was achieved in spite of the dense stacking of vermiculite sheets. This was ascribed to the abundant Li-ion hopping sites present between the vermiculite sheets through the enriched Li ions via ion exchange.

|
Download:
|
| Fig. 4. Electrochemical test of Li-S batteries with vermiculite-based separators or conventional PP separators. (a) long-term cycling at 0.1C and (b) rate performance between 0.1C to 2C. | |
Vermiculite separators, compared with a routine separator based on polymers have significant advantages for Li-S batteries. (1) The 2D vermiculite exhibits a negative surface that can attract abundant cations on the surface. Therefore, the 2D packing of vermiculates layers contributes to physically block the diffusion of long-chain polysulfides while the transport of Li+ with smaller ion radius can be hardly hindered. Therefore, vermiculite separators are capable to regulate the polysulfide ion transportation by electrostatic repulsion and steric hindrance. (2) Although the vermiculite separators induce extra barrier for lithium ions, the fast Li+ transport property among the vermiculite sheets renders good rate performance compared with other composite polymer separator [39-44]. (3) The vermiculite as typical inorganic clay minerals, the flakes of which are highly rigid. The microscale Young's modulus of vermiculite flakes reaches as high as 175 GPa as reported before, which is sufficient to suppress the growth of Li dendrite by mechanical resistance [45]. Consequently, vermiculite separators can suppress the lithium dendrite penetration with its high Young's modulus for rechargeable batteries with high-safety. (3) The as-obtained vermiculite separator allows for stable electrochemical cycles with Coulombic efficiency above 90% and a low capacity decay rate in the electrochemical test of Li-S battery without LiNO3 additive. (5) Vermiculites are of extraordinary chemical and thermal stability, which is helpful for building durable separators for rechargeable batteries. The application of vermiculite-based separator systems can be extended to other electrochemical battery systems with the concerns on safety and ion transportation regulation.
In summary, we demonstrate a 2D vermiculite separator to simultaneously address the challenges in shuttle effect and safety problems. The vermiculite separator assembled with 2D nanosheets can suppress the diffusion of polysulfides through electrostatic interaction and steric hindrance while allowing for fast lithium ion transportation through the interlayer channels. Meanwhile, the inorganic sheets with high strength and Young's modulus prevent the penetration of lithium metal dendrite and potentially improve the safety of the system. The Li-S batteries incorporated with vermiculite separators delivered a superior initial specific capacity of ca. 1000 mAh/g with an average CE of 90.3% for 50 cycles. The high capacity retention obtained in the rate performance evaluation also demonstrates the fast lithium ion transportation behavior in the composite separator. This work elucidated a promising strategy to regulate the diffusion of polysulfides in working Li-S batteries, which can be extended to other electrochemical systems based on multi-electron conversion chemistry.
AcknowledgmentsThis work was supported by National Key Research and Development Program (Nos. 2016YFA0202500 and 2016YFA0200102), the National Natural Scientific Foundation of China (No. 21776019), and Young Elite Scientists Sponsorship Program by CAST (No. YESS20150133). We thank Hong-Jie Peng, Ze-Wen Zhang, Ge Zhang, and Xin-Bing Cheng for helpful discussion.
| [1] |
H.J. Peng, J.Q. Huang, X.B. Cheng, et al., Adv. Energy Mater. 7(2017) 1700260. DOI:10.1002/aenm.v7.24 |
| [2] |
J. Liang, Z.H. Sun, F. Li, et al., Energy Storage Mater. 2(2016) 76-106. DOI:10.1016/j.ensm.2015.09.007 |
| [3] |
A. Manthiram, S.H. Chung, C.X. Zu, Adv. Mater. 27(2015) 1980-2006. DOI:10.1002/adma.v27.12 |
| [4] |
J. Peng, Y.T. Zuo, G. Li, et al., Chin. Chem. Lett. 27(2016) 1559-1562. DOI:10.1016/j.cclet.2016.02.028 |
| [5] |
Z.L. Ma, S. Dou, A.L. Shen, et al., Angew. Chem. Int. Ed. 54(2015) 1888-1892. DOI:10.1002/anie.201410258 |
| [6] |
Y.C. Tu, D.H. Deng, X.H. Bao, J. Energy Chem. 25(2016) 957-966. DOI:10.1016/j.jechem.2016.10.012 |
| [7] |
H.J. Peng, J.Q. Huang, Q. Zhang, Chem. Soc. Rev. 46(2017) 5237-5288. DOI:10.1039/C7CS00139H |
| [8] |
X.Q. Zhang, X.B. Cheng, Q. Zhang, J. Energy Chem. 25(2016) 967-984. DOI:10.1016/j.jechem.2016.11.003 |
| [9] |
Y.P. Xie, H.W. Cheng, W. Chai, et al., Chin. Chem. Lett. 28(2017) 738-742. DOI:10.1016/j.cclet.2016.07.030 |
| [10] |
M.Q. Guo, J.Q. Huang, X.Y. Kong, et al., New Carbon Mater. 31(2016) 352-362. DOI:10.1016/S1872-5805(16)60019-7 |
| [11] |
T.Z. Hou, X. Chen, H.J. Peng, et al., Small 12(2016) 3283-3291. DOI:10.1002/smll.v12.24 |
| [12] |
L. Wei, H.E. Karahan, S.L. Zhai, et al., J. Energy Chem. 25(2016) 191-198. DOI:10.1016/j.jechem.2015.12.001 |
| [13] |
G. Zhou, Y. Zhao, A. Manthiram, Adv. Energy Mater. 5(2015) 1402263. DOI:10.1002/aenm.201402263 |
| [14] |
Y. Ye, F. Wu, Y. Liu, et al., Adv. Mater.(2017), 1700598. |
| [15] |
X.Y. Zhou, F. Chen, J. Yang, J. Energy Chem. 24(2015) 448-455. DOI:10.1016/j.jechem.2015.06.011 |
| [16] |
S.K. Liu, X.B. Hong, Y.J. Li, et al., Chin. Chem. Lett. 28(2017) 412-416. DOI:10.1016/j.cclet.2016.10.038 |
| [17] |
J.Q. Huang, Q. Zhang, F. Wei, Energy Storage Mater. 1(2015) 127-145. DOI:10.1016/j.ensm.2015.09.008 |
| [18] |
Y.Y. Xiang, J.S. Li, J.H. Lei, et al., ChemSusChem 9(2016) 3023-3039. DOI:10.1002/cssc.v9.21 |
| [19] |
N.P. Deng, W.M. Kang, Y.B. Liu, et al., J. Power Sources 331(2016) 132-155. DOI:10.1016/j.jpowsour.2016.09.044 |
| [20] |
T.Z. Zhuang, J.Q. Huang, H.J. Peng, et al., Small 12(2016) 381-389. DOI:10.1002/smll.201503133 |
| [21] |
J.Q. Huang, T.Z. Zhuang, Q. Zhang, et al., ACS Nano 9(2015) 3002-3011. DOI:10.1021/nn507178a |
| [22] |
S. Bai, X. Liu, K. Zhu, et al., Nat. Energy 1(2016) 16094. DOI:10.1038/nenergy.2016.94 |
| [23] |
P.Y. Zhai, H.J. Peng, X.B. Cheng, et al., Energy Storage Mater. 7(2017) 56-63. DOI:10.1016/j.ensm.2016.12.004 |
| [24] |
C.H. Chang, S.H. Chung, A. Manthiram, Small 12(2016) 174-179. DOI:10.1002/smll.v12.2 |
| [25] |
S.H. Chung, C.H. Chang, A. Manthiram, Small 12(2016) 939-950. DOI:10.1002/smll.201503167 |
| [26] |
Z.L. Ma, Z.L. Li, K. Hu, et al., J. Power Sources 325(2016) 71-78. DOI:10.1016/j.jpowsour.2016.04.139 |
| [27] |
X.B. Cheng, R. Zhang, C.Z. Zhao, et al., Chem. Rev. 117(2017) 10403-10473. DOI:10.1021/acs.chemrev.7b00115 |
| [28] |
D. Lin, Y. Liu, Z. Liang, et al., Nat. Nanotechnol. 11(2016) 626-632. DOI:10.1038/nnano.2016.32 |
| [29] |
C. Yan, X.B. Cheng, C.Z. Zhao, et al., J. Power Sources 327(2016) 212-220. DOI:10.1016/j.jpowsour.2016.07.056 |
| [30] |
C.Z. Zhao, X.B. Cheng, R. Zhang, et al., Energy Storage Mater. 3(2016) 77-84. DOI:10.1016/j.ensm.2016.01.007 |
| [31] |
R. Zhang, N.W. Li, X.B. Cheng, et al., Adv. Sci. 4(2017) 1600445. DOI:10.1002/advs.201600445 |
| [32] |
Y. Sun, G. Zheng, Z.W. Seh, et al., Chem 1(2016) 287-297. DOI:10.1016/j.chempr.2016.07.009 |
| [33] |
X. Han, Y. Gong, K. Fu, et al., Nat. Mater. 16(2017) 572-579. |
| [34] |
J.J. Shao, K. Raidongia, A.R. Koltonow, et al., Nat. Commun. 6(2015) 7602. DOI:10.1038/ncomms8602 |
| [35] |
X.B. Cheng, C. Yan, X. Chen, et al., Chem 2(2017) 258-270. DOI:10.1016/j.chempr.2017.01.003 |
| [36] |
H.J. Peng, D.W. Wang, J.Q. Huang, et al., Adv. Sci. 3(2016) 1500268. DOI:10.1002/advs.201500268 |
| [37] |
H.J. Peng, J.Q. Huang, X.Y. Liu, et al., J. Am. Chem. Soc. 139(2017) 8458-8466. DOI:10.1021/jacs.6b12358 |
| [38] |
J.J. Chen, R.M. Feng, J.M. Feng, et al., Chem. Mater. 27(2015) 2048-2055. DOI:10.1021/cm5044667 |
| [39] |
J.Q. Huang, Q. Zhang, H.J. Peng, et al., Energy Environ. Sci. 7(2014) 347-353. DOI:10.1039/C3EE42223B |
| [40] |
T. Yim, S.H. Han, N.H. Park, et al., Adv. Funct. Mater. 26(2016) 7817-7823. DOI:10.1002/adfm.v26.43 |
| [41] |
J. Sun, Y.M. Sun, M. Pasta, et al., Adv. Mater. 28(2016) 9797-9803. DOI:10.1002/adma.201602172 |
| [42] |
M.S. Kim, L. Ma, S. Choudhury, et al., Adv. Mater. Interfaces 3(2016) 1600450. DOI:10.1002/admi.201600450 |
| [43] |
J.D. Zhu, C. Chen, Y. Lu, et al., Carbon 101(2016) 272-280. DOI:10.1016/j.carbon.2016.02.007 |
| [44] |
P. Zhu, J.D. Zhu, J. Zang, et al., J. Mater. Chem. A 5(2017) 15096-15104. DOI:10.1039/C7TA03301J |
| [45] |
J.W. Suk, R.D. Piner, J. An, et al., Thin Solid Films 527(2013) 205-209. DOI:10.1016/j.tsf.2012.12.024 |
 2017, Vol. 28
2017, Vol. 28 


