Exploring low-cost, environmentally friendly, and high-efficient energy storage system has attracted an ever increasing attention due to the increasingly prominent ecological problems and social development needs [1, 2]. Supercapacitor has been considered as one of the most promising candidates because of its superior rate performance, high energy density and long cycle life [3, 4]. According to the charge-storage mechanism, supercapacitors are classified into electrical double-layer capacitors (EDLC) and pseudocapacitors [5-7]. Carbon materials, possessing good electrical conductivity and low cost, have been widely used as the electrode materials of EDLCs [5, 8, 9]. However, the specific capacitance needs to be enhanced to satisfy the current demands. In present, trigging pseudocapacitor is a significant strategy to boost the performance of EDLC [10]. The most effective method is to incorporate heteroatom (such as N [11], P [12], S [13], B [14]) in carbon materials. Furthermore, it has been reported that nitrogen doped carbon materials can effectively tailor the electron donating property and surface polarity to reduce the charge transfer resistance and improve wettability [15, 16]. Recently, considerable efforts have been devoted to synthesizing nitrogen doped carbon for supercapacitors. Nitrogen-containing materials (such as polyaniline [17], pyrrole [18], or polyacrylonitrile [19]) were often utilized as carbon and nitrogen precursors to synthesize nitrogen doped carbon, which is not sustainable and environmentally friendly, and some of the synthesis procedures need to be further simplified for large scale application. Thus, preparation of carbon materials with suitable nitrogen content based on natural, costeffective, and environmentally friendly resources, and facile synthesis method for high-performance supercapacitor is still an ongoing challenge.
Most recently, biomass has received substantial attention as precursors to synthesize carbon materials toward supercapacitors (such as rice [22], pawn shell [23], eggshell membranes [24]) due to its unique properties including low cost, wide abundance and good environmental compatibility [20, 21]. Sodium alginate is a natural polysaccharide with a large number of hydroxyl and carboxyl groups extracted from brown algae [25], and has been widely used in the many research fields (such as medicine, food, and biology) [26, 27]. In this work, the synthesis of nitrogen doped carbon with porous architecture was proposed by directly pyrolyzing sodium alginate and urea. The resulting NPC at an annealing temperature of 700 ℃ exhibited superior performance for supercapacitors with a specific capacitance of 180.2 F/g at a current density of 1 A/g and extraordinary cycling durability with 96% performance retention over 5000 cycles at 3 A/g in 6 mol/L KOH media.
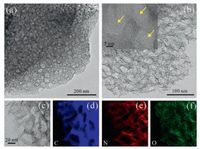
The synthetic procedure of NPC nanomaterials was developed by direct carbonization the mixture of sodium alginate and urea. Typical for material calcined at 700 ℃, the morphology was characterized via TEM. As can be seen in Figs. 1a and b, NPC0.5-700 exhibits interconnected porous sheet structure with a crumpled feature. HRTEM in the inset of Fig. 1b displays that the sample consists of numerous small crystalline randomly exist in the carbon structure, and meso-pores could be observed (see yellow arrows) [28]. The unique porous morphology could be attributed to the release of NH3 and CO2 derived from the decomposition of urea and sodium alginate during pyrolysis [29, 30]. Besides, the elemental mapping images (Figs. 1d-f) indicate that C, N, and O elements were effectively incorporated into the carbon structure and also distributed uniformly in NPC at the nanoscale.
|
Download:
|
| Fig. 1. (a) TEM image of NPC prepared at 700 ℃; (b) The enlarged TEM image of NPC0.5-700 and the HRTEM image (Insert); (c-f) TEM images of NPC0.5-700 and the corresponding elemental mapping images of C, N and O. | |
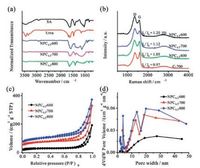
The chemical structure of NPC nanomaterials obtained at different pyrolysis temperature was examined by FTIR spectroscopy. As shown in Fig. 2a, sodium alginate exhibits characteristic peaks of carboxylate salt groups (~1625 and ~1420 cm-1) and C-O structure (~1030 cm-1), and urea exhibits characteristic peaks of N-H bending vibration, C-N stretching vibration and N-H rocking vibration (1683, 1627, 1465 and 1153 cm-1, respectively) [31]. It can be seen that most of those featured peaks of sodium alginate and urea could not be detected after pyrolysis, suggesting the decomposition of these groups. In addition, NPC0.5-700 and NPC0.5-800 both exhibit wide peak at around 1100 cm-1, which could be attributed to the generation of C-N bonds and the remaining C-O groups [32].
|
Download:
|
| Fig. 2. (a) FIRT spectra of sodium alginate, urea, and NPCs obtained at 600, 700 and 800 ℃; (b) Raman spectra of C-700 and NPCs obtained at 600, 700 and 800 ℃; Nitrogen adsorption/desorption isotherms (c) and the corresponding pore size distribution curves (d) of NPCs obtained at 600, 700, 800 ℃. | |
The doping effects of NPC nanomaterials were evaluated by Raman spectroscopy. Fig. 2b illustrates that three peaks at 1350 cm-1, 1585 cm-1, and 2750 cm-1 were observed, assigning to D-band, G-band, and 2D-band, respectively [33]. The D-band is related to the A1g mode and could be used to illustrate the defects and disorder feature of carbon material [34]. The G-band, originating from first-order scattering of the E2g mode, was associated with graphitic carbon with sp2-hybridized carbon atoms [35]. As displayed in Fig. 2b, compared to C-700, peaks of Gband for NPC0.5-700 slightly shifted toward the lower frequency because of nitrogen incorporation [32]. Besides, the defects and disorder degree generated in the formation of the as-synthesized nanomaterials were examined by the intensity ratio of D-band to G-band (ID/IG). Fig. 2b presented that the ID/IG value of NPC0.5-700 was slightly higher than C-700, illustrating the generation of defects due to nitrogen doping which could be favorable for the enhanced supercapacitor performance by increasing active sites and pseudocapacitance. In addition, the ID/IG value of NPC increased as the annealing temperature elevated from 600 ℃ to 800 ℃, revealing the enhanced graphitic degree [31].
The porous structure and the specific area of NPCs at different temperature were then examined through nitrogen absorptiondesorption determination. As depicted from the nitrogen absorption-desorption isotherms in Fig. 2c, the three products prepared at 600, 700 and 800 ℃ all presented the type-IV profile featuring an H2 hysteresis loop, suggesting the existence of mesopores [36]. The clear tails displayed in Fig. 2c demonstrated the macropores existed in the three samples. The BET specific surface area of NPCs 700 and 800 ℃ were determined to be 425 and 517 m2/g, respectively, higher than NPC0.5-600 (192 m2/g), which could be mainly due to the release of amount of gases (NH3 and CO2). Besides, pore size distribution curves (Fig. 2d) based on Barrett-Joyner-Halenda (BJH) method also manifested the mesoporous structure of NPCs, in consistent with the TEM results. The 3D porous architecture with the large surface area of NPC0.5-700 and NPC0.5-800 could facilitate the fast mass transfer and electrolyte diffusion [31], which are beneficial to the supercapacitor performance.
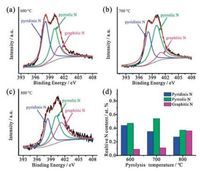
In order to further explore the chemical composition and the element bonding type, XPS of NPC nanomaterials prepared at different temperature was evaluated due to the important role of the content and type of doped nitrogen in supercapacitor applications [37]. It can be seen from the XPS survey spectra in Fig. S1 in Supporting information that C, N, and O were detected in the NPCs obtained at 600, 700 and 800 ℃, consistent with the TEM elemental mapping, demonstrating the effective doping of nitrogen in carbon structure. As shown in Fig. S2 in Supporting information, C 1s spectrum of NPC0.5-700 can be deconvoluted into four peaks, corresponding to C=C (284.5 eV), C-N (285.6 eV), C-O (286.5 eV), and O-C=O (289.0 eV), respectively, suggesting that the successful incorporation of nitrogen [38, 39]. Figs. 3a-c displays the N 1s spectra of NPC at the different temperature. Four peaks located at ~398.2, ~400.0, ~401.0 eV could correspond to pyrrole, pyridine, graphitic nitrogen groups [40], respectively. It was reported that nitrogen doping could enhance the wettability of carbon materials in the electrolyte to improve the mass transfer efficiency [41]. Additionally, the pyrrole-N and pyridine-N are conducive to the enhancement of the capacitance performance by triggering the huge pseudocapacitance [42, 43]. Thus, the relative nitrogen content in NPC obtained at 600, 700 and 800 ℃ was calculated as shown in Fig. 3d. It turned out that both NPC0.5-600 and NPC0.5-700 possess relatively high pyrrole-N and pyridine-N. Combined with the results of Raman spectra and the BET specific surface area, it can be concluded that NPC0.5-700 possessing high surface area with high pyrrole-N and pyridine-N, and also exhibiting higher graphitic degree, was expected to exhibit high supercapacitor performance [41, 44].
|
Download:
|
| Fig. 3. High-resolution N 1s XPS spectra of (a) NPC0.5-600, (b) NPC0.5-700, and (c) NPC0.5-800; (d) Relative percentage contents of different nitrogen types of NPCs synthesized at different temperature. | |
Electrochemical performance of the as-prepared nanomaterials was conducted to obtain the further insight of the significance of nitrogen incorporation and annealing temperature in the supercapacitor applications. Cyclic voltammetry measurement was firstly carried out with a three-electrode system in 6.0 mol/L KOH solution in the potential window from -1.0 V to 0 V (vs. Hg/ HgO). It can be seen from Fig. 4a that CV curves of NPC0.5-700 and C-700 both exhibit slightly distorted rectangular shapes without remarkable redox peaks, realizing the ideal double layer capacitor feature in the charging and discharging process. Besides, the current density of NPC0.5-700 was larger than that of C-700, demonstrating the excellent supercapacitor performance due to N doping. Consistent with CV curves, the galvanostatic curves in Fig. S3 in Supporting information also demonstrate that the specific capacitance of NPC0.5-700 is far higher than C-700. The higher specific capacitance of NPC0.5-700 than C-700 suggests that the incorporation of nitrogen plays a significant role in the enhancement capacitance due to the pseudocapacitive contribution. In addition, impedance results shown in Fig. S4 in Supporting information demonstrate that NPC0.5-700 exhibits good capacitive behavior featuring with the nearly vertical slope at the lowfrequency region and also possesses lower charge transfer resistance with smaller semi-circle loop compared with C-700 [41]. The above results indicate that the N doping could also effectively improve the wettability and the conductivity of carbon material. The effect of pyrolysis temperature to capacitive performance was then investigated. As shown in Fig. S5 in Supporting information, NPC0.5-700 possesses larger CV curves and higher specific capacitance compared with NPC0.5-600 and NPC0.5-800. CV detection and galvanostatic measurement in Fig. S6 in Supporting information illustrate that the resulting NPC possesses much better specific capacitor when added 0.5 g of urea. NPC obtained at 700 ℃ via the addition of 0.5 g of urea exhibits optimal performance with a higher specific capacitance of 180.2 F/g at a discharged current density of 1 A/g than other NPCs (Fig. S7 in Supporting information). Combined the electrochemical experiment data with Raman investigation and XPS analysis, it can be concluded that pyrolysis temperature could generate different active sites and tune the content and type of nitrogen in carbon materials and then affect the capacitance performance. In addition, the relatively high percentage of pyrrolic-N and pyridinic-N is conducive to the enhancement of capacitance.

|
Download:
|
| Fig. 4. (a) CV curves at the scan rate of 5 mV/s; (b) CV curves of NPC0.5-700 at different scan rates from 5 mV/s to 50 mV/s between -1 V and 0 V (vs. Hg/HgO); (c) Galvanostatic charge-discharge curves NPC0.5-700 with different current densities of 0.3, 0.5, 0.7, 1.0, 3.0, 5.0, 10.0 A/g; (d) Cycling life of NPC0.5-700 at 3.0 A/g. | |
In order to demonstrate the rate capability of NPC0.5-700, CV curves at different scan rates were collected, which can be seen in Fig. 4b. The CVs of NPC0.5-700 exhibited typical quasi-rectangular and symmetric shapes without any redox peaks at scan rates varying from 5 mV/s to 50 mV/s, suggesting the excellent rate capability of NPC0.5-700. The electrochemical capacitance performance of NPC0.5-700 was further investigated with the galvanostatic charge/discharge experiments. Consistent with the results shown by the CV curves, the galvanostatic curves of NPC0.5-700 in Fig. 4c exhibited small deviation from linearity, indicating a pseudocapacitive contribution occurring during the charge/discharge processes [45]. The charge-discharge curves of NPC0.5-700 exhibited a nearly isosceles triangular shape without obvious voltage drop at different current densities, implying good electrochemical reversibility of this electrode. The variation of specific capacitance at different current density is presented in Fig. S8 in Supporting information. The decrease of the capacitance with increasing the current density could be ascribed to the sufficient time for the electrolyte ions to enter and diffuse into the porosity at lower current densities, thereby resulting in higher specific capacitance [46].
The long cycle life of the electrode is a crucial parameter for evaluating supercapacitors performance in practical application. To examine the electrochemical durability of NPC0.5-700, galvanostatic charge-discharge detection were carried out at a current density of 3 A/g. It is obviously depicted in Fig. 4d that the specific capacitance of the NPC0.5-700 slightly decreased during the first 200 cycles but was constant thereafter, and more than 96% capacitance remaining even after 5000 cycles. The results indicated that NPC0.5-700 exhibits exceptional long-term stability, presenting great potential for energy storage applications.
In conclusion, nitrogen doped porous carbon material has been successfully prepared via simple pyrolysis of environmentally friendly sodium alginate and urea. The nitrogen content and type as well as the material structure could be effectively tuned by controlling the annealing temperature. NPC obtained at the optimum pyrolysis temperature of 700 ℃ possesses porous feature with mesopore and micropore structure, moderate graphitization, and suitable N doping. Electrochemical detection demonstrates that NPC0.5-700 exhibits high specific capacitance of 180.2 F/g at 1.0 A/g, excellent rate capability and cycling stability with 96% capacitance maintenance after 5000 cycles determination in 6 mol/L KOH media. The sodium alginate derived NPCs show great potential for large-scale production of electrode materials for supercapacitor and extend the applications of biomass based earth-abundant natural resources.
AcknowledgmentsThis work was supported by the National Natural Science Foundation (No. 21573083), the Program for New Century Excellent Talents in Universities of China (No. NCET-13-0237), the Fundamental Research Funds for the Central University (Nos. 2013TS136, 2014YQ009), 1000 Young Talent (to Deli Wang), and initiatory financial support from Huazhong University of Science and Technology (HUST). The authors thank the Analytical and Testing Center of HUST for TEM and Raman measurements.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.009.
| [1] |
A.M. Abioye, F.N. Ani, Renew. Sustain. Energy Rev. 52(2015) 1282-1293. DOI:10.1016/j.rser.2015.07.129 |
| [2] |
J. Wang, H.L. Xin, D. Wang, Part. Part. Syst. Charact. 31(2014) 515-539. DOI:10.1002/ppsc.v31.5 |
| [3] |
Z. Ma, X. Huang, S. Dou, et al., J. Phys. Chem. C 118(2014) 17231-17239. DOI:10.1021/jp502226j |
| [4] |
S. Wang, R.A. Dryfe, J. Mater. Chem. A 1(2013) 5279-5283. DOI:10.1039/c3ta10436b |
| [5] |
L.L. Zhang, X. Zhao, Chem. Soc. Rev. 38(2009) 2520-2531. DOI:10.1039/b813846j |
| [6] |
M. Wang, Y.X. Xu, Chin. Chem. Lett. 27(2016) 1437-1444. DOI:10.1016/j.cclet.2016.06.048 |
| [7] |
M. Liu, L. Chen, D. Zhu, et al., Chin. Chem. Lett. 27(2016) 399-404. DOI:10.1016/j.cclet.2015.12.026 |
| [8] |
A. Pandolfo, A. Hollenkamp, J. Power Sources 157(2006) 11-27. DOI:10.1016/j.jpowsour.2006.02.065 |
| [9] |
E. Frackowiak, Phys. Chem. Chem. Phys. 9(2007) 1774-1785. DOI:10.1039/b618139m |
| [10] |
W. Lei, L. Han, C. Xuan, et al., Electrochim. Acta 210(2016) 130-137. DOI:10.1016/j.electacta.2016.05.158 |
| [11] |
T. Lin, I.W. Chen, F. Liu, et al., Science 350(2015) 1508-1513. DOI:10.1126/science.aab3798 |
| [12] |
Y. Wen, B. Wang, C. Huang, et al., Chem. Eur. J. 21(2015) 80-85. DOI:10.1002/chem.201404779 |
| [13] |
M. Seredych, T.J. Bandosz, J. Mater. Chem. A 1(2013) 11717-11727. DOI:10.1039/c3ta12252b |
| [14] |
D.W. Wang, F. Li, Z.G. Chen, et al., Chem. Mater. 20(2008) 7195-7200. DOI:10.1021/cm801729y |
| [15] |
Y. Deng, Y. Xie, K. Zou, et al., J. Mater. Chem. A 4(2016) 1144-1173. DOI:10.1039/C5TA08620E |
| [16] |
Y. Li, G. Wang, T. Wei, et al., Nano Energy 19(2016) 165-175. DOI:10.1016/j.nanoen.2015.10.038 |
| [17] |
W. Fan, Y.Y. Xia, W.W. Tjiu, et al., J. Power Sources 243(2013) 973-981. DOI:10.1016/j.jpowsour.2013.05.184 |
| [18] |
Y. Zhao, J. Liu, Y. Hu, et al., Adv. Mater. 25(2013) 591-595. DOI:10.1002/adma.201203578 |
| [19] |
F. Miao, C. Shao, X. Li, et al., J. Mater. Chem. A 4(2016) 4180-4187. DOI:10.1039/C6TA00015K |
| [20] |
J. Wang, P. Nie, B. Ding, et al., J. Mater. Chem. A 5(2017) 2411-2428. DOI:10.1039/C6TA08742F |
| [21] |
J. Deng, M. Li, Y. Wang, Green Chem. 18(2016) 4824-4854. DOI:10.1039/C6GC01172A |
| [22] |
S. Gao, Y. Chen, H. Fan, et al., J. Mater. Chem. A 2(2014) 3317-3324. DOI:10.1039/c3ta14281g |
| [23] |
F. Gao, J. Qu, Z. Zhao, et al., Electrochim. Acta 190(2016) 1134-1141. DOI:10.1016/j.electacta.2016.01.005 |
| [24] |
Z. Li, L. Zhang, B.S. Amirkhiz, et al., Adv. Energy Mater. 2(2012) 431-437. DOI:10.1002/aenm.v2.4 |
| [25] |
M. Latorre-Sánchez, A. Primo, H. García, Angew. Chem. Int. Ed. 52(2013) 11813-11816. DOI:10.1002/anie.v52.45 |
| [26] |
Y. Song, L. Liu, H. Shen, et al., Food Control 22(2011) 608-615. DOI:10.1016/j.foodcont.2010.10.012 |
| [27] |
N. Manabe, K. Haruma, M. Ito, et al., Dis. Esophagus 25(2012) 373-380. DOI:10.1111/des.2012.25.issue-5 |
| [28] |
K. Ai, Y. Liu, C. Ruan, et al., Adv. Mater. 25(2013) 998-1003. DOI:10.1002/adma.v25.7 |
| [29] |
P.M. Schaber, J. Colson, S. Higgins, et al., Thermochim. Acta 424(2004) 131-142. DOI:10.1016/j.tca.2004.05.018 |
| [30] |
F. Pan, J. Jin, X. Fu, et al., ACS Appl. Mater. Interfaces 5(2013) 11108-11114. DOI:10.1021/am403340f |
| [31] |
C. Xuan, Z. Wu, W. Lei, et al., ChemCatChem 9(2017) 809-815. DOI:10.1002/cctc.v9.5 |
| [32] |
Z. Lin, G. Waller, Y. Liu, et al., Adv. Energy Mater. 2(2012) 884-888. DOI:10.1002/aenm.201200038 |
| [33] |
J. Wang, Z.X. Wu, L.L. Han, et al., Chin. Chem. Lett. 27(2016) 597-601. DOI:10.1016/j.cclet.2016.03.011 |
| [34] |
D. Deng, X. Pan, L. Yu, et al., Chem. Mater. 23(2011) 1188-1193. DOI:10.1021/cm102666r |
| [35] |
D. Weingarth, M. Zeiger, N. Jäckel, et al., Adv. Energy Mater. 4(2014) 1400316. DOI:10.1002/aenm.201400316 |
| [36] |
X. Liu, Y. Zhou, W. Zhou, et al., Nanoscale 7(2015) 6136-6142. DOI:10.1039/C5NR00013K |
| [37] |
Z. Wu, J. Wang, R. Liu, et al., Nano Energy 32(2017) 511-519. DOI:10.1016/j.nanoen.2017.01.014 |
| [38] |
Y. Li, Y. Zhao, H. Cheng, et al., J. Am. Chem. Soc. 134(2011) 15-18. |
| [39] |
M. Wu, J. Wang, Z. Wu, et al., J. Mater. Chem. A 3(2015) 7727-7731. DOI:10.1039/C4TA06323F |
| [40] |
J. Wang, Z. Wu, L. Han, et al., J. Mater. Chem. A 4(2016) 5678-5684. DOI:10.1039/C6TA00490C |
| [41] |
Y. Tan, C. Xu, G. Chen, et al., ACS Appl. Mater. Interfaces 5(2013) 2241-2248. DOI:10.1021/am400001g |
| [42] |
D. Hulicova-Jurcakova, M. Seredych, G.Q. Lu, et al., Adv. Funct. Mater. 19(2009) 438-447. DOI:10.1002/adfm.v19:3 |
| [43] |
Y.H. Lee, K.H. Chang, C.C. Hu, J. Power Sources 227(2013) 300-308. DOI:10.1016/j.jpowsour.2012.11.026 |
| [44] |
X. Yang, D. Wu, X. Chen, et al., J. Phys. Chem. C 114(2010) 8581-8586. |
| [45] |
H. Zhu, J. Yin, X. Wang, et al., Adv. Funct. Mater. 23(2013) 1305-1312. DOI:10.1002/adfm.v23.10 |
| [46] |
G. Ma, Q. Yang, K. Sun, et al., Bioresour. Technol. 197(2015) 137-142. DOI:10.1016/j.biortech.2015.07.100 |
 2017, Vol. 28
2017, Vol. 28 


