Organic semiconducting polymers gathered enormous attentions in developing polymer solar cells (PSCs) with characteristic features, i.e., light-weight, flexibility and solution processabilities[1-5].Backbone of conjugated polymers comprise of alternatively arranged push-pull configuration of electron rich donatingmoieties (D) and electron deficient accepting units (A), whichfacilitates the charge transfer over the entire back-bone.Thus, theoptoelectronic properties of conjugated polymers can be finelytuned, which plays a vital role in obtaining good photovoltaic performance [6-9].Over the past few years, the PSC powerconversion efficiencies (PCEs) have been boosted over 12% [10-13].
Among the large numbers of molecular entities documented forPSCs, DPP pigment has attracted attentions of researcher due to itsversatile properties, such as strong absorption, chemical andthermal stabilities, outstanding charge transporting capability etc.[14-26].
Considerable amount of responses have been rewarded todevelop low bandgap polymers with benzo[1, 2-b:4, 5-b']dithiophene (BDT) electron rich unit and DPP, due their strongabsorption in near IR region and excellent hole-transportingability [27-31].However, one of drawbacks for DPP-basedpolymers has shown relatively strong aggregation in solution[32], which require various side chains being introduced into DPP polymers for good solution processibilities.Solid state features ofpolymers are influenced from the nature of various functionalities being introduced on the DPP lactam groups [33-37].Besides of thestraight and branched alkyl chains, it is interesting to note thatdormant substituents (i.e., tert-butoxycarbonyl, t-Boc) have beenintroduced to DPP units.Such t-Boc functionalities can beeliminated at elevated temperature, resulting in the possibleformation H-bonding (N-H…O=C) between DPP units [38, 39].Asresults, it dramatically alters the solubility and packing behaviorsof the correspondent materials, before and after removal of t-Boc group, to generate robust film.
Herein, we designed narrow band gap regio-random terpolymers through partial replacement of solubilizing alkyl chainswith t-Boc group on the DPP units.The resultant low bandgappolymers are tuned by yielding various properties, such assolubility and energy levels.The as-synthesized polymers showreasonable solubility in common organic solvents and thin filmscan be easily cast.In addition, the thermal gravimetric analysis(TGA) analysis showed that the dormant t-Boc groups can beremoved at ~190 ℃ form the t-BocDPP based polymers.These twonew semiconducting polymers, P2 and P3 show PCEs of 1.96% and 2.69% in conventional PSC devices with PC71BM acceptor.
All polymers were synthesized via Stille cross-couplingreactions and their structures are illustrated in Scheme 1.These polymers P1, P2 and P3 exhibited reasonable solubility in commonorganic solvents including chloroform, chlorobenzene and dichlorobenzene.However, P4 is insoluble which could be attributed to high concentration of t-BocDPP.The molecularweights (Mw) and polydispersity index (PDI, Mw/Mn) of thecopolymers are measured through gel permeation chromatography (GPC) with average molecular weights (Mn) of 72.9, 47.3, 46.7 kDa for P1, P2 and P3, respectively.
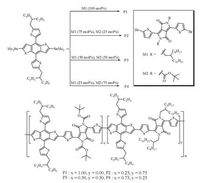
|
Download:
|
| Scheme 1. Synthetic scheme of the polymers. | |
All reagents, unless otherwise specified, are obtained fromAldrich, Acros and TCI Chemical Co., and used as received.Solventsare freshly distilled prior to use.Reactions are carried out underinert atmosphere and column chromatography is performed withthe use of silica gel 200–300 mesh.Monomers (M1 and M2) aresynthesized according to the reported procedure [40, 41].M1 isobtained as dark purple solid (0.5 g, in 78% yield).1H NMR(300 MHz, CDCl3): δ 0.86 (t, 12H), 1.10–1.47 (m, 64H), 1.88 (s, 2H), 3.98 (d, 4H), 7.52 (d, 2H), 7.22 (d, 2H), 8.63 (d, 2H).M2 is obtained asdark purple solid (0.49 g, in 75% yield).1H NMR (300 MHz, CDCl3): δ8.08 (d, 2H), 7.17 (d, 2H), 1.62 (s, 18H).
Synthesis of polymers is accomplished via Stille cross-coupling polymerization.Monomer M1, M2 and BDTT are charged in a 10 mL Schlenk tube with the presence of catalyst Pd2(dba)3, P(o-tol)3 and dry toluene.After degassing with successive freeze-pump-thawcycles, the reaction mixture is stirred for 24 h at 110 ℃ under nitrogen atmosphere.Then, the mixture is cooled to room temperature and precipitated in 50 mL methanol.The precipitateis filtered and washed with methanol and hexane successively in a Soxhlet apparatus.Finally, the polymer is extracted with chloroform and dried in a vacuum oven at 40 ℃ overnight.Thepolymers are characterized by GPC with polystyrene as standard in tetrahydrofuran as eluent.P1 (PBDTT-DPP): BDTT (100 mg, 0.11 mmol), M1 (112 mg, 0.11 mmol), P(o-tolyl)3 (6.5 mg, 0.022 mmol) and Pd2(dba)3 (5 mg, 0.005 mmol) were used.P1 is obtained as dark green solid (92% yield).1H NMR (600 MHz, CDCl3):δ 9.16–9.0 (br, 5H), 7.36–7.26 (br, 6H), 7.02 (br, 5H), 3.04 (br, 7H), 1.9–1.8 (br, 6H), 1.7–1.0 (br, 83H), 0.9–0.7 (br, 16H); GPC: Mn = 72.9, PDI = 3.6; P2 (PBDTT-DPP0.75-t-BocDPP0.25): BDTT (100 mg, 0.11 mmol), M1 (84.6 mg, 0.083 mmol), M2 (18.2 mg, .027 mmol), P(o-tolyl)3 (6.5 mg, 0.022 mmol) and Pd2(dba)3 (5 mg, 0.005 mmol)were used.P2 is obtained as dark green solid (87% yield).1H NMR (600 MHz, CDCl3): δ 7.4–7.3 (br, 5H), 6.97–6.9 (br, 5H), 3.73–3.41(br, 8H), 3.18–2.78 (br, 8H), 2.34–1.66 (br, 18H), 1.6–1.5 (br, 18H), 1.5–0.6 (br, 87H); GPC: Mn = 47.3, PDI = 3.0; P3 (PBDTT-DPP0.50-t-BocDPP0.50): BDTT (100 mg, 0.11 mmol), M1 (56.3 mg, 0.055 mmol), M2 (36.4 mg, 0.055 mmol), P(o-tolyl)3 (6.5 mg, 0.022 mmol) andPd2(dba)3 (5 mg, 0.005 mmol) were used.P3 is obtained as darkgreen solid (89% yield).1H NMR (600 MHz, CDCl3): δ 7.4–7.3 (br, 5H), 6.97–6.9 (br, 5H), 3.73–3.41 (br, 8H), 3.18–2.78 (br, 8H), 2.34–1.66 (br, 18H), 1.6–1.5 (br, 18H), 1.5–0.6 (br, 87H); GPC:Mn = 46.7, PDI = 5.2; P4 (PBDTT-DPP0.25-t-BocDPP0.75): BDTT(100 mg, 0.11 mmol), M1 (28.2 mg, 0.027 mmol), M2 (54.6 mg, 0.083 mmol), P(o-tolyl)3 (6.5 mg, 0.022 mmol) and Pd2(dba)3(5 mg, 0.005 mmol) were used.P4 is obtained as dark green solid(98% yield), and is insoluble in common solvent which cannot befurther characterized.
The UV–vis absorption properties of the obtained polymers are measured both in dilute CHCl3 solutions and thin film spin-coatedon quartz slides.The three polymers show broad absorption andtypical peaks as described in Figs.1a and b, of which the intenseabsorption peaks 750 nm for P1, 755 nm for P2 and 757 nm for P3are assigned to intramolecular charge transfer (ICT) characteristics.The absorption edges of the three polymers are determined as 925 nm (P1), 910 nm (P2) and 971 nm (P3), respectively, through which the optical band gaps are calculated as 1.34, 1.36 and 1.28 eV, respectively for three polymers, according to the equation:opt = 1240/λonset.The absorption profiles of pristine polymer filmsare slightly broadened with respect to those in chloroformsolutions, which can be attributed to polymer aggregation insolid films.Furthermore, the blend absorption of P3:PC71BM (1:2, w/w) and P2:PC71BM (1:2, w/w) are shown in Fig.1c, exhibitingbroad absorption in the wavelength range of 300–900 nm.

|
Download:
|
| Fig. 1. UV–vis absorption of (a) polymer solution and (b) films of neat polymers, as well as (c) polymer:PC71BM blends; (d) TGA analysis of the polymers. | |
Thermal decomposition temperatures (Td) (about 5% weight loss) are determined by TGA under nitrogen atmosphere at a heating rate of 10 ℃/min, to be 306 ℃, 191 ℃ and 190 ℃ for P1, P2 and P3, respectively (Fig.1d).It indicates that the dormant functional groups can be easily eliminated at elevated temperatures.
Electrochemical properties are investigated by cyclic voltammetry (CV) and the HOMO and LUMO (lowest unoccupiedmolecular orbital) values are determined from the onset potentialof the first oxidation and reduction peaks, with the ferrocene standard is 4.35 eV below the vacuum level.The HOMO of P2 is -5.26 eV and its LUMO is 3.93 eV (calculated from optical bandgap), HOMO for P3 is -5.46 eV and the LUMO is -4.19 eV, respectively.The deepening of HOMO and LUMO energy levels are due to the increase in concentration of t-Boc moieties which acts aselectron withdrawing units.
As polymer P1 is well documented in literature [27-31], wefurther choose P2 and P3 to investigate the photovoltaic propertieswith PC71BM acceptor (Fig.2 and Table 1), with a conventional architecture of ITO/PEDOT:PSS/active-layer/PFN/Al, where theactive-layer is use of polymer:PC71BM blends, cast from DCB with 3% DIO (1, 8-diiodooctane).PSC devices are fabricated on the glasssubstrates pre-coated with a layer of indium tin oxide (ITO).Priorto spinning a layer of poly(3, 4-ethylendioxythiophen): poly(styrenesulphonate) (35 nm, PEDOT:PSS), the substrate weresubsequently cleaned by using detergent, deionized water, acetone, isopropanol for 15 min, the substrate were transferredto the glove box.The BHJ active-layer was spin cast solution ofpolymer and PC71BM in dichlorobenzene (DCB) 16 mg/mL.Thethickness of the active-layer was tuned by adjusting the solution concentration and spin speed.The poly[(9, 9 bis(3-N-N-dimethylamino)propyl) 2, 7-fluorene-alt-2, 7-(9, 9-dioctylfluorene)] (PFN)interlayer material was dissolved in methanol and its solution(2 mg/mL) was spin-coated on the top of active-layer.Then thesamples were loaded into the vacuum deposition chamber(background pressure ≈ 5 ×10-4 Pa) to deposit 120 nm thick aluminum cathode with a shadow mask (device area 11.5 mm2).The photocurrent was measured under a calibrated solar simulator(Abet 300 V) at 100 mW/cm2, and the light intensity was calibratedwith a standard silicon photovoltaic reference cell.

|
Download:
|
| Fig. 2. Conventional PSC (a) architecture and (b) energy levels of the each materialin device. | |
|
|
Table 1 Photovoltaic performance of the PSCs with a conventional structure of ITO/PEDOT:PSS/active-layer/PFN/Al under illumination of AM 1.5G (100 mW/cm2) with average PCE of20 devices given in parentheses. |
The J–V curves are plotted in Fig.3a, and relevant photovoltaic parameters are summarized in Table 1.After a few device efforts, the PCE of 1.96% is obtained in P2/PC71BM blend (1:2, w/w), with an open-circuit voltage (VOC) of 0.73 V, a short-circuit current density(JSC) of 5.07 mA/cm2, and a fill factor (FF) of 0.53.And P3 shows the PCE of 2.71% (VOC = 0.71 V, JSC = 7.15 mA/cm2 and FF = 0.53) withdonor/acceptor blend ratio of 1:2 (w/w).The average PCE form 20 devices based on P2/PC71BM and P3/PC71BM blends (1:2, w/w)Exhibits 1.85% and 2.68%, respectively.The best PCE obtained inconventional device is for P3 based conventional device shows PCEof 2.92% (VOC = 0.71 V, JSC = 7.09 mA/cm2, and FF = 0.58) by tuningthe ratio of P3:PC71BM to 1:2.5 (w/w), and the average PCE of2.88%.The external quantum efficiency (EQE) spectra of thepolymers-based devices are shown in Fig.3b.Compared to the P2:PC71BM based device, the P3:PC71BM BHJ (1:2, w/w) exhibitssignificant improvement photo response in EQE spectra (Fig.3b), with a maximum EQE value of 44% at 450 nm, which suggests thatmore efficient photoelectron conversion and hence benefits for theimprovement of JSC.

|
Download:
|
| Fig. 3. (a) J–V curve of the devices for P2 and P3, (b) external quantum efficiency, (c) hole-only device and (d) electron-only devices. | |
For exploring the structure-carrier mobility correlation ofthese terpolymers based blends, we employ the space-chargelimited current (SCLC) method to measure the hole and electronmobilities of two blends.Hole-only devices with a structure ofITO/PEDOT:PSS/active-layer/MoO3/Ag and electron-only deviceswith a structure of ITO/ZnO/active-layer/PFN/Al are fabricated respectively.J–V characteristics under dark of the hole-only andelectron-only devices are shown in Fig.3c and d, the corresponding mobilities are summarized in Table 2.The hole-mobilities for P2 and P3 blend are 1.54 ×10-5 and 1.58 ×10-5cm2 V-1 s-1, respectively.It indicates that the increase of t-BocDPP concentration in polymer backbone is not affecting hole mobilities of blend.Similar trend is found in electron only devices for both of thepolymers exhibiting, 1.79 ×10-4 and 2.02 ×10-4 cm2 V-1 s-1, asgiven in Table 2.Hence, the introduction of t-BocDPP moiety inpolymer back-bone is seemingly not affecting the mobilities ofblends.As for the P3/PC71BM blend (1:2.5, w/w), the SCLCmeasurements show slightly improvements for both electron(7.01 ×10-5 cm2 V-11 s-1) and hole (5.50 ×10-4 cm2 V-1 s-1) mobilities, consisting with the improvement of device FF and PCE.
|
|
Table 2 Hole and electron mobilities of the polymers based on P2 and P3/PC71BM. |
Atomic force microscopy (AFM) is used to investigate the thinfilm surface morphologies of these polymer/PC71BM blends.Andthe corresponding 3D height images are presented in Fig.4.Theyshow surface morphologies with root-mean-square (RMS) valuesof 8.3 nm for P2 blend and 2.12 nm P3 blend.The P3 with 50% t-BocDPP exhibit smoother surface than that of P2 with 25% t-Boc, which may lead to better interface property, thus slightly increased PCE for P3 based blend.

|
Download:
|
| Fig. 4. AFM images: 3D height images for P2 blend (a) and P3 blend (b), respectively.All the images are 5 × 5 mm. | |
In summary, a series of terpolymers comprising BDTT and DPP with varied solubilizing groups (t-Boc and 2-octyldodecyl), aresynthesized and investigated for their photovoltaic properties inconventional PSCs.These polymers possess narrow band gaps of1.36 (P2) and 1.27 (P3), respectively.Solar cells using P3:PC71BM blend exhibit moderate power conversion efficiencies of 2.92%, with JSC of 7.09 mA/cm2, VOC of 0.71 V and FF of 0.58.To best of ourknowledge this is the highest efficiency reported for t-BocDPP based polymers.Further studies may subject to multilayer fabrication for thick layers [11], through transforming solublepolymer into insoluble film, by removal of dormant substituents.
AcknowledgmentsThis research was funded by the National Natural ScienceFoundation of China (Nos.21674093 and 51620105006), 973program (No.2014CB643503) and International Science andTechnology Cooperation Program of China (No.2016YFE0102900).C.-Z.Li thanks the support by Zhejiang NaturalScience Fund for Distinguished Young Scholars (No.LR17E030001), the Young 1000 Talents Global Recruitment Program of China, and100 Talents Program of Zhejiang University.
| [1] |
L. Lu, T. Zheng, Q. Wu, et al., Chem. Rev. 115(2015) 12666-12731. DOI:10.1021/acs.chemrev.5b00098 |
| [2] |
Y. Li, Acc. Chem. Res. 45(2012) 723-733. DOI:10.1021/ar2002446 |
| [3] |
L. Dou, Y. Liu, Z. Hong, G. Li, Y. Yang, Chem. Rev. 115(2015) 12633-12665. DOI:10.1021/acs.chemrev.5b00165 |
| [4] |
H. Yao, L. Ye, H. Zhang, et al., Chem. Rev. 116(2016) 7397-7457. DOI:10.1021/acs.chemrev.6b00176 |
| [5] |
M. Zhang, X. Guo, W. Ma, H. Ade, J. Hou, Adv. Mater. 27(2015) 4655-4660. DOI:10.1002/adma.v27.31 |
| [6] |
T.Y. Chu, J. Lu, S. Beaupré, et al., J. Am. Chem. Soc. 133(2011) 4250-4253. DOI:10.1021/ja200314m |
| [7] |
X.P. Xu, Y. Li, M.M. Luo, Q. Peng, Chin. Chem. Lett. 27(2016) 1241-1249. DOI:10.1016/j.cclet.2016.05.006 |
| [8] |
X.W. Zhu, K. Lu, H. Li, R.M. Zhou, Z.X. Wei, Chin. Chem. Lett. 27(2016) 1271-1276. DOI:10.1016/j.cclet.2016.06.015 |
| [9] |
P. He, X.L. Qiao, Q. Qian, H.X. Li, Chin. Chem. Lett. 27(2016) 1277-1282. DOI:10.1016/j.cclet.2016.06.032 |
| [10] |
S. Li, L. Ye, W. Zhao, et al., Adv. Mater. 28(2016) 9423-9429. DOI:10.1002/adma.201602776 |
| [11] |
J. Huang, H. Wang, K. Yan, et al., Adv. Mater. 29(2017) 1606729. DOI:10.1002/adma.v29.19 |
| [12] |
F. Zhao, S. Dai, Y. Wu, et al., Adv. Mater. 29(2017) 1700144. DOI:10.1002/adma.201700144 |
| [13] |
W. Zhao, S. Li, S. Zhang, X. Liu, J. Hou, Adv. Mater. 29(2017) 1604059. DOI:10.1002/adma.v29.2 |
| [14] |
L. Biniek, B.C. Schroeder, C.B. Nielsen, I. McCulloch, J. Mater. Chem. 22(2012) 14803-14813. DOI:10.1039/c2jm31943h |
| [15] |
H. Bronstein, Z. Chen, R.S. Ashraf, et al., J. Am. Chem. Soc. 133(2011) 3272-3275. DOI:10.1021/ja110619k |
| [16] |
V.S. Nair, J. Sun, P. Qi, et al., Macromolecules 49(2016) 6334-6342. DOI:10.1021/acs.macromol.6b00954 |
| [17] |
Y. Liu, G. Li, Z. Zhang, et al., J. Mater. Chem. A 4(2016) 13265-13270. DOI:10.1039/C6TA05471D |
| [18] |
S. Wen, W. Chen, M. Fan, et al., J. Mater. Chem. A 4(2016) 18174-18180. DOI:10.1039/C6TA08130D |
| [19] |
M. Chen, W. Fu, M. Shi, et al., J. Mater. Chem. A 1(2013) 105-111. DOI:10.1039/C2TA00148A |
| [20] |
S.Y. Liu, W.Q. Liu, J.Q. Xu, et al., ACS Appl. Mater. Interfaces 6(2014) 6765-6775. DOI:10.1021/am500522x |
| [21] |
A. Tang, C. Zhan, J. Yao, E. Zhou, Adv. Mater. 29(2017) 1600013. DOI:10.1002/adma.v29.2 |
| [22] |
H. Song, Y. Deng, Y. Gao, et al., Macromolecules 50(2017) 2344-2353. DOI:10.1021/acs.macromol.6b02781 |
| [23] |
S.Y. Liu, W.F. Fu, J.Q. Xu, et al., Nanotechnology 28(2014) 014006. |
| [24] |
E. Zhou, S. Yamakawa, K. Tajima, C. Yang, K. Hashimoto, Chem. Mater. 21(2009) 4055-4061. DOI:10.1021/cm901487f |
| [25] |
E. Zhou, Q. Wei, S. Yamakawa, et al., Macromolecules 43(2010) 821-826. DOI:10.1021/ma902398q |
| [26] |
E. Zhou, J. Cong, K. Hashimoto, K. Tajima, Energy Environ. Sci. 5(2012) 9756-9759. DOI:10.1039/c2ee23383e |
| [27] |
J. Yu, B. Zhao, X. Nie, et al., New J. Chem. 39(2015) 2248-2255. DOI:10.1039/C4NJ02192D |
| [28] |
L. Dou, J. Gao, E. Richard, et al., J. Am. Chem. Soc. 134(2012) 10071-10079. DOI:10.1021/ja301460s |
| [29] |
L. Dou, W.H. Chang, J. Gao, et al., Adv. Mater. 25(2013) 825-831. DOI:10.1002/adma.v25.6 |
| [30] |
W. Li, W.S.C. Roelofs, M.M. Wienk, R.A.J. Janssen, J. Am. Chem. Soc. 134(2012) 13787-13795. DOI:10.1021/ja305358z |
| [31] |
L. Ye, X. Jiao, S. Zhang, et al., Adv. Energy Mater. 7(2017) 1601138. DOI:10.1002/aenm.201601138 |
| [32] |
Z. Hao, A. Iqbal, Chem. Soc. Rev. 26(1997) 203-213. DOI:10.1039/cs9972600203 |
| [33] |
J.J. van Franeker, G.H.L. Heintges, C. Schaefer, et al., J. Am. Chem. Soc. 137(2015) 11783-11794. DOI:10.1021/jacs.5b07228 |
| [34] |
K.H. Hendriks, G.H.L. Heintges, V.S. Gevaerts, M.M. Wienk, R.A.J. Janssen, Angew. Chem. Int. Ed. 52(2013) 8341-8344. DOI:10.1002/anie.v52.32 |
| [35] |
K.H. Hendriks, W. Li, G.H.L. Heintges, et al., J. Am. Chem. Soc. 136(2014) 11128-11133. DOI:10.1021/ja505574a |
| [36] |
W. Li, K.H. Hendriks, M.M. Wienk, R.A.J. Janssen, Acc. Chem. Res. 49(2016) 78-85. DOI:10.1021/acs.accounts.5b00334 |
| [37] |
Y. Wang, F. Yang, Y. Liu, et al., Macromolecules 46(2013) 1368-1375. DOI:10.1021/ma3025738 |
| [38] |
J.S. Zambounis, Z. Hao, A. Iqbal, Nature 388(1997) 131-132. DOI:10.1038/40532 |
| [39] |
J. Lee, A.R. Han, J. Hong, et al., Adv. Funct. Mater. 22(2012) 4128-4138. DOI:10.1002/adfm.v22.19 |
| [40] |
Y. Li, S.P. Singh, P. Sonar, Adv. Mater. 22(2010) 4862-4866. DOI:10.1002/adma.201002313 |
| [41] |
K. Yang, T. He, X. Chen, S.Z.D. Cheng, Y. Zhu, Macromolecules 47(2014) 8479-8486. DOI:10.1021/ma501960t |
 2017, Vol. 28
2017, Vol. 28 




