With the increasing global energy demand and environmental problems caused by fossil-fuel combustion, photoelectrochemical (PEC) water splitting has been attracting increasing attentions and considered as a promising route to convert solar energy to clean hydrogen energy [1]. Undoubtedly, in a PEC cell, semiconductor photoelectrodes are the essential components, which absorb solar light and where water redox reactions happen [2]. Since the pioneering study of PEC by Fujishima and Honda [3], booming researches have been conducted for semiconducting photoelectrode materials for solar water splitting, including oxides, sulfides, (oxy)nitrides, and silicon-based photoelectrodes [4-6]. Despite a successful development of PEC performance for these materials, there is not yet a single system that integrates high solar-tohydrogen (STH) efficiency, durability, and low cost for practical solar-hydrogen production.
Hematite (α-Fe2O3), a n-type semiconductor with band gap of ~2.1 eV, appears a couple of advantages on PEC water splitting, such as excellent stability, good visible light absorption, nontoxicity, earth-abundant resource and low cost [4]. These merits of α-Fe2O3 make it a promising photoelectrode for PEC water splitting. However, as a photoanode, α-Fe2O3 still suffers from the low conductivity, high charge combination rate, short hole diffusion length, and sluggish surface water oxidation kinetics, which greatly limit its PEC performances [7]. In the past decades, several approaches have been implemented to improve PEC performance for water splitting over α-Fe2O3 [4, 8] by (a) nanostructure design for efficient charge collection, (b) metal ion doping for improved electrical conductivity, (c) heterojunction for charge separation, and (d) surface modification for accelerated surface water oxidation reaction.
Since the aqueous solution growth successfully demonstrated by Vayssieres et al. [9], α-Fe2O3 nanorods with efficient charge transfer directed in the one-dimension pathway have been extensively investigated as a typical photoanode for further modifications. Started with α-Fe2O3 nanorods, a number of foreign elements (e.g., Cr [10], Ta [11], Nb [12]) could be doped into the nanorods for improved charge transfer ability, different cocatalysts (e.g., Co3O4 [13], Co-Pi [14]) and passivation layer (e.g., Al2O3 [15], P [16]) have been deposited on the surface to accelerate surface water oxidation reaction and reduce surface charge recombination, which resulted in considerable PEC enhancement. In recent years, there have been more expectations to simultaneously improve the charge transfer ability and the surface reaction kinetics for further enhancement in PEC performances, and of course, significant advances have been achieved. For example, Shen et al. [17] obtained α-Fe2O3/AgxFe2-xO3 core/shell nanorod films via ultrasonication treatment of solution-based β-FeOOH nanorods in Ag precursor solution followed by high temperature annealing, which achieved an improved incident photon-to-current efficiency (IPCE) from 2.2% to 8.4% at 400 nm due to increased carrier density and accelerated surface oxidation reaction kinetics. Gong et al. [18] reported a TiO2/Ti:Fe2O3/FeOOH photoanode with an improved photocurrent of approximately 3.1 mA/cm2 at 1.23 V (vs. reversible hydrogen electrode, RHE), in which the atomic layer deposition (ALD) grown TiO2 interlayer suppressed charge recombination at the substrate/hematite interface, the doped Ti4+ increased the hematite bulk conductivity, and the loaded FeOOH served as an oxygen evolution reaction (OER) cocatalyst to accelerate water oxidation kinetics. Lee et al. [19] modified high-temperature annealed single-crystalline hematite photoanodes by platinum doping to improve the charge transfer characteristics in bulk and Co-Pi cocatalyst to enhance the OER on surface, resulting in a stable and excellent performance of 4.32 mA/cm2 PEC water oxidation current at 1.23 V (vs. RHE) under simulated 1-sun irradiation.
Motivated by these advances, in this study α-Fe2O3 nanorods grown on a conductive substrate were modified with cobalt oxide (CoOx) and carbon (C) by ultrasonic dipping in a cobalt nitrate/ glucose aqueous solution. In comparison to the CoOx or C singly modified counterpart, the CoOx/C modified α-Fe2O3 nanorods showed further increased PEC activity for water splitting. It was evidenced that the improved carrier transfer ability both in the bulk and on the surface, was synergistically contributed by the coexistence of CoOx and C on the surface, resulting in the facilitated charge transfer from the bulk to the surface reactions across the photoanode-electrolyte interface and thus the enhanced PEC performance. This study could be a good example of using a simple method to achieve a multi-functional modification for improved PEC performances.
The β-FeOOH films were synthesized through hydrothermal method at 100 ℃ and transferred to α-Fe2O3 films by annealing at 750 ℃ in air [20], which were finally modified by CoOx and C. The morphology of CoOx/C modified α-Fe2O3 is shown in Fig. 1a. It could be clearly observed that the nanorod arrays are perpendicular to the fluorine-doped tin oxide (FTO) substrate and have a diameter range from 50 nm to 70 nm, with an average length of about 600 nm as reported before [11]. It is evident that the modification process has no influence on surface morphology of α-Fe2O3, as shown in Fig. S1 in Supporting information. Considering the short hole diffusion length (2 -4 nm) and low electrical conductivity of α-Fe2O3 [21, 22], this nanorod-array configuration has been believed to improve the photo-induced charge carrier separation [23]. With the rhombohedral crystal structure, α-Fe2O3 has a high degree of anisotropy in the direction of charge carrier mobility, and the conductivity in the (001) basal planes (e.g., in the [110] direction) has been measured up to 4 orders of magnitude higher than that in the perpendicular direction [4, 24]. As evidenced by X-ray diffraction (XRD) patterns in Fig. 1b, α-Fe2O3 phase exits in all samples and no other phases can be identified, in addition to substrate phase (i.e., FTO). Interestingly, the (110) peak intensity at 35.61° of α-Fe2O3 film is much higher than that of (104) peak at 33.15°, while the (110) peak has lower intensity in natural isotropic powder [24]. Transmission electron micro-scope (TEM) image in the inset of Fig. S1a clearly indicates the (001) basal planes vertical to (110) planes formed in [110] growth direction [25]. Together with Fig. 1a and b, it could be evidenced that there is a strong preferential orientation of [110] axis [26] and the relatively high-conductivity (001) planes are parallel to the nanorod lengthwise direction.

|
Download:
|
| Fig. 1. (a) Scanning electron microscope (SEM) image of CoOx/C modified α-Fe2O3 nanorods (taking Co0.02G0.001 as the example). (b) XRD patterns of CoOx/C modified α-Fe2O3 nanorods, in which star represents XRD peaks of hematite as indexed by PDF card (#33-0664). (c) UV-vis absorption spectra of CoOx/C modified α-Fe2O3 nanorods. (d) Co 2p3/2 binding energy spectra of Co0.02 and Co0.02G0.001. (e) HAADF image of CoOx/C modified α-Fe2O3 nanorods (taking Co0.02G0.001 as the example) and (f-h) element mappings of Fe, Co, C, respectively. | |
UV-visible and Raman spectra were used to explore the possible change in light absorption and structure of CoOx/C modified α-Fe2O3 films, as induced by surface modification. As shown in Fig. 1c, in the region from 300 nm to 800 nm, both α-Fe2O3 and CoOx/C modified α-Fe2O3 nanorod films exhibited almost the same optical absorption properties, with absorption onset at around 600 nm. Tauc plots [27] further confirmed that the pristine and CoOx/C modified α-Fe2O3 nanorod films have very close band gaps of 2.08 eV (Fig. S3 in Supporting information), within the reported values ranging from 2.0 eV to 2.1 eV for α-Fe2O3 [28]. Raman spectra recorded from 100 cm-1 to 2000 cm-1 (Fig. S2 in Supporting information) demonstrated that there is no obvious difference in the crystal structure for all the nanorod films. All these observations indicated that the CoOx/C modification has no obvious influence on optical property and crystal structure of α-Fe2O3 nanorods.
Given the ignorable difference among surface morphology, crystal structure and optical property, it then comes to be of great importance to figure out the valance states and distribution of CoOx and C on α-Fe2O3. Fig. 1d plots the Co 2p3/2 binding energy spectra of CoOx and C modified α-Fe2O3 nanorods (taking Co0.02 and Co0.02G0.001 as examples) from 795 eV to 775 eV. Both profiles can be deconvoluted into three species, in which peaks in range of 790-785 eV represent shake up satellites and others can be considered as Co2+ and Co3+ peaks [29]. With only CoOx modification, the obtained Co0.02 sample has a Co3+ peak centered at 780.4 eV and a Co2+ peak at 781.7 eV, while with CoOx and C modification, the obtained Co0.02G0.001 sample has Co3+ and Co2+ peaks centered at 780.8 eV and 783.0 eV respectively. By comparing the binding energies of Co2+ and Co3+ in these two films, it is evident that after the addition of C, both Co3+ and Co2+ peaks were shifted to higher binding energies, which implies a strong interaction between CoOx and C [29], causing a positively charged Co ions of a high oxidation state for the enhancement of the oxidizing ability of cobalt oxide species [30]. High angle annular dark filed (HAADF) image and element distribution are shown in Fig. 1e-h. The nanorod has a uniform diameter of about 55 nm and Fe/Co/C elements are well distributed on the nanorod, demonstrating the homogeneous dispersion of CoOx and C species in CoOx/C modified α-Fe2O3 nanorods.
To investigate the PEC water splitting performance, potentialdependent photocurrent densities were measured in a conventional three-electrode PEC cell. As shown in Fig. 2a and Fig. S4 in Supporting information, for all the CoOx and/or C modified α-Fe2O3 films, with the applied potential increasing, the photocurrent densities increased while the current spikes at the time of light on or off decreased. The instantaneous current spike when light is switched on is a record of the flux of holes to surface and a subsequent exponential decay is observed, rooting in the recombination of build-up holes at surface states with an electron flux [31]. The cathodic current spikes observed when light is off should be resulted from the recombination of a continuing flux of electrons to surface with holes remaining in surface states [31, 32]. The decreasing current spikes indicated the reduced charge recombination, depending on the increase in bias voltages. Obviously, the CoOx/C modified α-Fe2O3 nanorods has significantly improved photocurrent densities compared to that of pristine α-Fe2O3, especially, the Co0.02G0.001 film exhibited the highest photocurrent density of 0.37 mA/cm2 at 1.6 V, while the pristine α-Fe2O3 film obtained a photocurrent density of only 0.10 mA/cm2. The much higher photocurrent densities achieved over the CoOx/C modified α-Fe2O3 nanorods as compared to the CoOx or C modified nanorods (Fig. 2a), implied a synergistic effect in the PEC enhancement, as introduced by the coexistence of CoOx and C species on the surface of α-Fe2O3 nanorods. The i-t test of the Co0.02G0.001 films demonstrated a slight decay in photocurrent density during 1200 s PEC reaction (inset in Fig. 2a), confirming the good stability for the hematite-based photoanodes [4].

|
Download:
|
| Fig. 2. (a) Photocurrents of CoOx/C modified α-Fe2O3 nanorods, (b) IPCE profiles of α-Fe2O3 and Co0.02G0.001. | |
As an important diagnostic figure of merit for PEC water splitting, IPCE describes the ability of photoelectrode for photocurrent conversion [27]. Herein, the IPCE profiles of the pristine α-Fe2O3 nanorods and the CoOx/C modified α-Fe2O3 nanorods (taking the Co0.02G0.001 film as example) were collected in Fig. 2b. The IPCE values of both films decrease with wavelength extending to longer region and are close to zero at the wavelength of 600 nm. The Co0.02G0.001 film has an IPCE of 3.8% at 310 nm, which is much higher than that of 2.2% for the pristine α-Fe2O3 film. An illustration is shown in Fig. 2b which schemes the photo-excited charge carrier transfer processes in the α-Fe2O3 nanorod photoanodes, including photoexcitation of charge carriers, electron transfer to the FTO substrate and hole transfer to the surface of nanorods, and holes induced water oxidation reaction. Due to the almost same optical property, the different IPCEs of α-Fe2O3 and Co0.02G0.001 should be caused by the different efficiencies of charge carrier transfer to the photoanode/electrolyte interface. Thus, the PEC improvement of the CoOx/C modified α-Fe2O3 nanorods can be explained by the more efficient charge transfer to the photoanode/ electrolyte interface, as induced by the synergistic effect of CoOx and C modification, which will be further evidenced by electrochemical characterization results.
To further disclose the reasons of the PEC enhancement, photoanode/electrolyte interface impedances were measured by a single-frequency alternating-current (AC) impedance method at a range of bias potential and the results are transferred to MottShottky (M-S) plots as shown in Fig. 3a. Positive slopes observed of all samples indicate a n-type semiconductor and thus holes serve as minority carriers, implying that photo-generated holes play the main role for photo-induced property. The CoOx and/or C modified α-Fe2O3 films have similar slope values that are smaller than that of pristine α-Fe2O3, implying higher carrier concentrations which are calculated shown in the inserted table in Fig. 3a. The improvement of carrier concentration for CoOx or C modified α-Fe2O3 may ascend to charge collection effect by C [33] or CoOx [34] species. It is evident that after CoOx/C modification, the Co0.02G0.001 film has the near-surface carrier concentration increased by about one order of magnitude, which would contribute to of the increased electrical conductivity and thus the improved carrier transfer ability [17].
To reveal the effect of the CoOx/C modification on the charge transfer at the photoanode/electrolyte interface, surface injection efficiency was determined from Eq. (1) [35, 36]. The water splitting photocurrent (Jp, W) is the product of the rate of photon absorption expressed as a current density (Ja), the charge transfer efficiency of photo-generated carriers (Pt), and the surface injection efficiency (Ps):

|
(1) |
Pt includes the process of the transfer to electrode/electrolyte interface for photo-generated carriers (i.e., the fraction of holes that does not recombine with electrons in the bulk). Ps includes the process of hole injection to electrolyte to oxidize the water on the surface. On the other hand, the photocurrent measured by adding H2O2 to the electrolyte (Jp, H) is a product of Ja and Pt only for the surface injection efficiency equals 100% in the presence of H2O2:
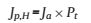
|
(2) |
Therefore, the surface injection efficiency could be reasonably calculated by dividing Jp, W by Jp, H, with results shown in Fig. 3b. It can be clearly observed that the CoOx and/or C modified α-Fe2O3 films have improved surface efficiencies, in comparison to the pristine α-Fe2O3 film. Especially, the Co0.02G0.001 film yielded the highest surface charge injection efficiency of about 60% at 1.6 V. The improvement in surface efficiency indicates the accelerated surface water oxidation reaction kinetics for the Co0.02G0.001 film, as induced by the introduction of CoOx and C species. It was believed that the CoOx species acting as the water oxidation catalyst could promote surface charge carrier transfer [37], while the C species as the surface passivation layer could reduce surface charge recombination [38], which synergistically contributed to the enhancement in PEC water splitting performances.

|
Download:
|
| Fig. 3. (a) M-S plots and (b) surface injection efficiencies of CoOx/C modified α-Fe2O3 nanorods. (c) Nyquist plots and (d) fitting parameters of α-Fe2O3 and Co0.02G0.001 | |
Nyquist plots of pristine α-Fe2O3 and CoOx/C modified α-Fe2O3 (taking Co0.02G0.001 as the example) were also collected from the impedances in a range of AC frequencies under illumination at a fixed bias. As shown in Fig. 3c, two semicircles could be observed for both films. The resistance parameters were fitted from the equivalent circuit (Fig. 3c, inset) [28, 39-41], in which Rctss represents a surface charge transfer resistance, Rrec represents the central role of a surface state acting as a recombination center trapping electrons from the conduction band and holes from the valence band, R represents external resistance, Qsc and Qss are constant phase elements (CPE) for space-charge region of bulk hematite and surface chemical state, respectively, with relevant data collected in Fig. 3d. Both Rrec and Rctss for Co0.02G0.001 are much smaller than those for α-Fe2O3, which implies the decreased charge carrier recombination rate and the increased interface charge transfer rate after the CoOx/C modification. The numerical Qss value of Co0.02G0.001 was fitted to be 5.899 × 10-4, which is more than 3 times the value of α-Fe2O3. Given the fact that the surface hole density is proportional to the surface state capacitance [41], which is analogous to CPE [42], and the increased surface hole density could contribute to the accelerated water oxidation rate [43], the Co0.02G0.001 film had a higher surface hole density as compared to the pristine α-Fe2O3 film, and therefore the CoOx/C modification increased the surface hole collection efficiency and then benefited the surface water oxidation reaction.
To ascertain the percentage of charge carriers transferred to photoanode/electrolyte interface for water oxidation, the charge transfer efficiency (ηtrans) can be estimated as the following equation [44-47]:
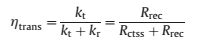
|
(3) |
where kt is the charge transfer rate constant, while kr is the surface charge recombination rate constant. The efficiency ηtrans for Co0.02G0.001 calculated from the parameters in Fig. 3d is 60.7%, which is higher than 34.5% for the pristine α-Fe2O3 nanorods, indicating the greater hole flux into surface to be consumed in oxygen evolution [45]. Thus, it could be deduced that the coexistence of CoOx and C species synergistically promoted charge transfer ability and accelerated surface water oxidation reaction kinetics, contributing to the great enhancement in PEC water splitting performances.
In conclusion, CoOx and C modified α-Fe2O3 nanorod arrays have been successfully synthesized through ultrasonic impregnation of α-Fe2O3 in a cobalt nitrate/glucose aqueous solution, following an annealing process in N2 atmosphere. The PEC water splitting performance had a great improvement, with photocurrent density increased from 0.10 mA/cm2 of α-Fe2O3 to 0.37 mA/cm2 of CoOx/C modified α-Fe2O3 nanorods at 1.6 V and the IPCE increased from 2.2% to 3.8% at 310 nm. From the electrochemical measurements, the improvement in PEC performance was due to a synergy of the improved charge transfer ability and the accelerated electrode/electrolyte water oxidation reaction kinetics, as introduced by CoOx and C species. This design of surface modification by earth-abundant elements via a facile solution method provides an inspiration to the development of low cost and high efficiency photoelectrodes for solar hydrogen generation.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51672210, 51323011, 51236007), the Natural Science Foundation of Shaanxi Province (No. 2014KW07-02), the Foundation for the Author of National Excellent Doctoral Dissertation of China (No. 201335), the National Program for Support of Top-notch Young Professionals, and the Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.037.
| [1] |
K. Sivula, R. Van De Krol, Nat. Rev. Mater. 1(2016) 15010.
|
| [2] |
H.M. Chen, C.K. Chen, R.S. Liu, et al., Chem. Soc. Rev. 41(2012) 5654-5671. DOI:10.1039/c2cs35019j |
| [3] |
A. Fujishima, K. Honda, Nature 238(1972) 37-38. DOI:10.1038/238037a0 |
| [4] |
S.H. Shen, S.A. Lindley, X.Y. Chen, et al., Energy Environ. Sci. 9(2016) 2744-2775. DOI:10.1039/C6EE01845A |
| [5] |
Y. Na, B. Hu, Q.L. Yang, et al., Chin. Chem. Lett. 26(2015) 141-144. DOI:10.1016/j.cclet.2014.09.011 |
| [6] |
Y.F. Shen, C. Zhang, C.G. Yan, et al., Chin. Chem. Lett. 28(2017) 1312-1317. DOI:10.1016/j.cclet.2017.04.004 |
| [7] |
C.J. Sartoretti, B.D. Alexander, R. Solarska, et al., J. Phys. Chem. B 109(2005) 13685-13692. DOI:10.1021/jp051546g |
| [8] |
S.H. Shen, J. Mater. Res. 29(2014) 29-46. DOI:10.1557/jmr.2013.310 |
| [9] |
L. Vayssieres, N. Beermann, S.E. Lindquist, et al., Chem. Mater. 13(2001) 233-235. DOI:10.1021/cm001202x |
| [10] |
S.H. Shen, J.G. Jiang, P.H. Guo, et al., Nano Energy 1(2012) 732-741. DOI:10.1016/j.nanoen.2012.05.013 |
| [11] |
Y.M. Fu, C.L. Dong, Z.H. Zhou, et al., Phys. Chem. Chem. Phys. 18(2016) 3846-3853. DOI:10.1039/C5CP07479G |
| [12] |
Y.M. Fu, C.L. Dong, W.Y. Lee, et al., ChemNanoMat 2(2016) 704-711. DOI:10.1002/cnma.v2.7 |
| [13] |
L.F. Xi, P.D. Tran, S.Y. Chiam, et al., J. Phys. Chem. C 116(2012) 13884-13889. DOI:10.1021/jp304285r |
| [14] |
Y.R. Hong, Z. Liu, Al-Bukhari S.F.B., et al., Chem. Commun. 47(2011) 10653-10655. DOI:10.1039/c1cc13886c |
| [15] |
Le Formal F., N. Tetreault, M. Cornuz, et al., Chem. Sci. 2(2011) 737-743. DOI:10.1039/C0SC00578A |
| [16] |
D.H. Xiong, W. Li, X.G. Wang, Nanotechnology 27(2016) 375401. DOI:10.1088/0957-4484/27/37/375401 |
| [17] |
S.H. Shen, J.G. Zhou, C.L. Dong, Sci. Rep. 4(2014) 6627. |
| [18] |
Z.B. Luo, T. Wang, J.J. Zhang, et al., Angew. Chem. Int. Edit. 56(2017) 12878-12882. DOI:10.1002/anie.201705772 |
| [19] |
J.Y. Kim, G. Magesh, D.H. Youn, Sci. Rep. 3(2013) 2681. DOI:10.1038/srep02681 |
| [20] |
S.H. Shen, M.T. Li, L.J. Guo, et al., J. Colloid Interface Sci. 427(2014) 20-24. DOI:10.1016/j.jcis.2013.10.063 |
| [21] |
H.W. Tang, W.J. Yin, M.A. Matin, J. Appl. Phys. 11(2012) 73502. |
| [22] |
J.H. Kennedy, K.W. Frese, J. Electrochem. Soc. 125(1978) 709-714. DOI:10.1149/1.2131532 |
| [23] |
L.S. Li, Y.H. Yu, F. Meng, et al., Nano Lett. 12(2012) 724-731. DOI:10.1021/nl2036854 |
| [24] |
M. Cornuz, M. Grätzel, K. Sivula, Chem. Vap. Depos. 16(2010) 291-295. DOI:10.1002/cvde.v16.10/12 |
| [25] |
J. Liu, Y.Y. Cai, Z.F. Tian, et al., Nano Energy 9(2014) 282-290. DOI:10.1016/j.nanoen.2014.08.005 |
| [26] |
A. Kay, I. Cesar, M. Grätzel, J. Am. Chem. Soc. 128(2006) 15714-15721. DOI:10.1021/ja064380l |
| [27] |
Z. B. Chen, H. N. Dinh, E. Miller, Photoelectrochemical Water Splitting: Standards, Experimental Methods, and Protocols, Springer Science & Business Media, New York, 2013.
|
| [28] |
S.C. Riha, B.M. Klahr, E.C. Tyo, et al., ACS Nano 7(2013) 2396-2405. DOI:10.1021/nn305639z |
| [29] |
T.Y. Ma, S. Dai, M. Jaroniec, et al., J. Am. Chem. Soc. 136(2014) 13925-13931. DOI:10.1021/ja5082553 |
| [30] |
C.X. Guo, Y. Zheng, J.R. Ran, et al., Angew. Chem. Int. Edit. 56(2017) 8539-8543. DOI:10.1002/anie.201701531 |
| [31] |
C.Y. Cummings, F. Marken, L.M. Peter, et al., Chem. Commun. 48(2012) 2027-2029. DOI:10.1039/c2cc16382a |
| [32] |
L.M. Peter, Chem. Rev. 90(1990) 753-769. DOI:10.1021/cr00103a005 |
| [33] |
Y. Zhao, R. Nakamura, K. Kamiya, Nat. Commun. 4(2013) 2390. |
| [34] |
D.R. Gamelin, Nat. Chem. 4(2012) 965-967. DOI:10.1038/nchem.1514 |
| [35] |
H. Dotan, K. Sivula, M. Grätzel, et al., Energy Environ. Sci. 4(2011) 958-964. DOI:10.1039/C0EE00570C |
| [36] |
J.H. Kim, J.H. Kim, J.W. Jang, Adv. Energy Mater. 5(2015) 1401933. DOI:10.1002/aenm.201401933 |
| [37] |
H.Y. Jin, J. Wang, D.F. Su, et al., J. Am. Chem. Soc. 137(2015) 2688-2694. DOI:10.1021/ja5127165 |
| [38] |
Y.W. Phuan, M.N. Chong, O. Satokhee, Part. Part. Syst. Charact. 34(2017) 1600216. DOI:10.1002/ppsc.v34.1 |
| [39] |
L. Bertoluzzi, J. Bisquert, J. Phys. Chem. Lett. 3(2012) 2517-2522. DOI:10.1021/jz3010909 |
| [40] |
B. Klahr, S. Gimenez, Fabregat-Santiago F., et al., J. Am. Chem. Soc. 134(2012) 4294-4302. DOI:10.1021/ja210755h |
| [41] |
A.J. Abel, A.M. Patel, S.Y. Smolin, et al., J. Mater. Chem. A 4(2016) 6495-6504. DOI:10.1039/C6TA01862A |
| [42] |
A. Lasia, Electrochemical Impedance Spectroscopy and Its Applications, Springer, New York, 2014.
|
| [43] |
Le Formal F., E. Pastor, S.D. Tilley, et al., J. Am. Chem. Soc. 137(2015) 6629-6637. DOI:10.1021/jacs.5b02576 |
| [44] |
D. Monllor-Satoca, M. Bärtsch, C. Fàbrega, et al., Energy Environ. Sci. 8(2015) 3242-3254. DOI:10.1039/C5EE01679G |
| [45] |
C.Y. Cummings, F. Marken, L.M. Peter, et al., Chem. Commun. 48(2012) 2027-2029. DOI:10.1039/c2cc16382a |
| [46] |
K.G.U. Wijayantha, Saremi-Yarahmadi S., L.M. Peter, Phys. Chem. Chem. Phys. 13(2011) 5264-5270. DOI:10.1039/c0cp02408b |
| [47] |
P.Y. Tang, H.B. Xie, C. Ros, et al., Energy Environ. Sci. 10(2017) 2124-2136. DOI:10.1039/C7EE01475A |
 2017, Vol. 28
2017, Vol. 28 


