b Department of Hepatobiliary-Pancreatic Surgery, China-Japan Union Hospital of Jilin University, Changchun 130033, China;
c Department of Biomedical Engineering, Southern University of Science and Technology, Shenzhen 510855, China
Photodynamic therapy (PDT) has been accepted as an effective cancer treatment modality [1, 2]. It involves the photosensitizer administration and local irradiation with appropriate excitation wavelength to activate the photosensitizer. The activated photosensitizer transfers the energy to molecular oxygen to generate singlet oxygen (1O2) through type Ⅱ reaction mechanism [3], which can lead to tumor cells ablation and blood vessels destruction [4, 5]. As PDT exploits the localized oxidative damage to implement the cancer treatment, making it much milder than conventional treatment modalities including surgery, chemotherapy and radiotherapy [6]. In the past few years, PDT has been demonstrated for treatment of different cancers such as skin cancer [7], bladder cancer [8], lung cancer [9], head and neck cancer [10]. Along with the development and practice of PDT, a variety of nanoparticle based photosensitizers have been developed to enhance the photodynamic effect, including polymeric nanoparticles [11], upconversion nanoparticles [12-14], gold nanoparticles [15], mesoporous silica nanoparticles [16, 17] and quantum dots [18, 19].
Recently, semiconducting polymer dots (Pdots) have attracted considerable attention in biomedicine field [20-22]. The rapid development of Pdots is owing to their outstanding properties, such as high brightness, excellent photostability, low cytotoxicity and good water solubility, etc. [23]. Meanwhile, Pdots with photosensitizer loading have been widely used for PDT to kill cancer cells [24]. For PDT applications, Pdots can be used as carrier to load hydrophobic photosensitizer, and served as energy donor for amplified 1O2 generation. However, in vitro cellular uptake of Pdots is conducted by nonspecific endocytosis [25], which leads to low penetration speed and efficiency of Pdots in cancer cells, and required relatively long incubation time (more than 8 h) [24]. In addition, carboxyl Pdots with negative charge were difficult to be internalized by cancer cells (cell membrane was negatively charged) due to electrostatic repulsion, resulting in unsatisfactory therapeutic efficiency [26]. These issues need to be addressed for practical application of Pdots.
Here we utilized a semiconducting polymer as matrix to prepare photosensitizer-doped Pdots (carboxyl Pdots), and modified with cell penetrating peptides (CPPs). CPPs have a strong penetration ability to speed the carrier transfer into cells, thus they are used to promote the cellular uptake and increase the penetration efficiency [27, 28]. In vitro experiments indicated that the peptide coated-Pdots promoted the cellular uptake and increased the penetration efficiency in fairly short time, and enhanced the photodynamic effect at low incubation concentration, in contrast with carboxyl Pdots. In vivo experiments indicated that the peptide coated-Pdots could suppress tumor growth and enhance the photodynamic effect.
We designed and prepared multifunctional photosensitizer-doped Pdots with CPPs modification.Zincphthalocyanine (ZnPc)as a second-generation photosensitizer is widely used in PDT. However, photosensitizer ZnPc generally suffers severe aggregation in physiological environment due to poor water-solubility, which limits its practical application in cancer therapy [29]. Thus we employed the polymer do-PFDTBT as carrier to load hydrophobic ZnPc to overcome the problem of aggregation. Furthermore, we employed the polymer do-PFDTBT as energy donor for ZnPc to generate efficient 1O2 generation [24]. The synthetic route of doPFDTBT was described in our previous study [30], and the chemical structures of required materials were shown in Scheme 1. The ZnPc-doped Pdots (carboxyl Pdots) in aqueous solution were prepared by the nanoprecipitation method [20]. The resulting carboxyl Pdots were further used for preparation of peptide coated-Pdots.
|
Download:
|
| Scheme 1. Preparation and photodynamic effect of the peptide coated-Pdots. | |
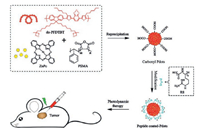
Fluorescence spectroscopy indicates the efficient energy transfer from the polymer to photosensitizer. Fig. 1a showed the reasonable spectral overlap between the absorption spectra of ZnPc and the emission spectra of do-PFDTBT, which indicated the possibility for energy transfer to occur inside the Pdots. To maximize the energy transfer efficiency, we investigated the spectroscopic properties of ZnPc-doped Pdots (carboxyl Pdots). Fig. S1a (Supporting information) showed the absorption spectra of Pdots doping with different fractions of ZnPc. As the doping fraction was increased (0–5wt%), the absorption intensity of ZnPc at 680nm gradually increased, which indicated that ZnPc was doped into Pdots successfully. Fig. S1b (Supporting information) showed the emission spectra of Pdots doping with different fractions of ZnPc. At the doping fraction of 5wt%, the energy transfer efficiency of ZnPc-doped Pdots reached the maximum, which was the prerequisite for efficient 1O2 generation. Fig. 1b showed the absorption and emission spectra of ZnPc-doped Pdots at the doping fraction of 5wt%. As expected, the ZnPc-doped Pdots presented broad emission spectra with a dominant emission peak of 685nm, which indicated the efficient energy transfer between do-PFDTBT and ZnPc.
|
Download:
|
| Fig. 1. Preparation and characterization of ZnPc-doped Pdots. (a) Spectral overlap between the absorption spectra of ZnPc and the emission spectra of do-PFDTBT. (b) Absorption and emission spectra of ZnPc-doped Pdots at 5% doping fraction. (c) Absorption spectra changes of ADMA mixed with ZnPc-doped Pdots. (d) Absorption spectra quenching of ADMA mixed with RB, ZnPc-doped Pdots, and ADMA alone. | |
1O2 generation yield of pure conjugated polymer is generally low [31, 32]. Incorporation of photosensitizer into the Pdots makes energy transfer occur inside the Pdots, which can significantly enhance the 1O2 generation yield. 9, 10-Anthracenediylbis(methylene)dimalonic acid (ADMA) is widely used to detect the 1O2 generation, and the photodegradation of ADMA is revealed by its absorption spectral bleaching. Therefore, the 1O2 generation can be indirectly measured by monitoring the absorbance changes of ADMA [33]. In the present of ZnPc-doped Pdots, the absorption spectra of ADMA at 259nm occurred obvious bleaching due to a large amount of 1O2 generation (Fig. 1c). Meanwhile, we utilized Rose Bengal (Ф (1O2)=0.76) as a standard substance to precisely calculate the 1O2 quantum yield of ZnPc-doped Pdots [34]. The 1O2 quantum yield of ZnPc-doped Pdots was determined to be ~0.2, and the 1O2 quantum yield of pure Pdots was ~0.1 according to our previous study [30], which indicated the efficient 1O2 generation of ZnPc-doped Pdots (Fig. 1d).
CPPs can speed the carrier transfer into cell membrane [35]. Octa-arginine (R8) as a kind of CPPs, is composed of eight amino acids and positively charged. To promote the cellular uptake and increase the penetration efficiency, carboxyl Pdots with negative charge were modified with CPPs through electrostatic adsorption (Scheme 1). The resulting R8-Pdots (peptide coated-Pdots) were further characterized for PDT applications. To obtain stable R8-Pdots, it was necessary to explore the optimal molar ratio of R8 and carboxyl Pdots. Figs. S2a and b (Supporting information) showed the zeta potential and particle size distribution of carboxyl Pdots mixed with different molar ratios of R8. When the molar ratio of R8/carboxyl Pdots was increased to 600:1, the R8-Pdots tended to be stable, thus the optimal molar ratio of R8/carboxyl Pdots was set as 600:1. For carboxyl Pdots, Fig. S2c (Supporting information) showed the average zeta potential was about -31 mV, and most particles possessed diameters in the range of 21 ±10 nm and were uniformly spherical. For R8-Pdots (the molar ratio was 600:1), Fig. S2d (Supporting information) showed the average zeta potential was about +28 mV, most particles possessed diameters in the range of 24 ±10 nm and were uniformly spherical, indicating that the carboxyl Pdots were successfully modified by R8 through electrostatic adsorption. Based on the above experimental results, we concluded that carboxyl Pdots modified with R8 could form stable and multifunctional Pdots for further experiments in vitro and in vivo.
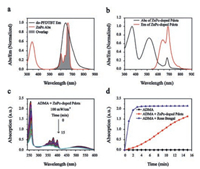
Besides the photodynamic effect, it is greatly beneficial to explore the cellular uptake and cytotoxicity of carboxyl Pdots and R8-Pdots. The cellular uptake was evaluated in SGC-7901 cells by fluorescence imaging and flow cytometry. Fig. S3a (Supporting information) showed fluorescence imaging of cells incubated with 10 mg/mL carboxyl Pdots for 0–8 h and 10 mg/mL R8-Pdots for 1 h. As compared to carboxyl Pdots, brighter intracellular fluorescence was observed in cells incubated with R8-Pdots. In addition, Fig. S3b (Supporting information) showed intracellular fluorescence intensity measured by flow cytometry, which was in accordance with the results of fluorescence imaging. Meanwhile, cellular uptake percentage (Fig. S3c in Supporting information) and mean fluorescence intensity (Fig. S3d in Supporting information) were analyzed by flow cytometry. The cellular uptake percentages of cells incubated with carboxyl Pdots for 8 h and R8-Pdots for 1 h both reached 99.8%, however, the mean fluorescence intensity of cells incubated with R8-Pdots for 1 h was more than 5.4-fold of cells incubated with carboxyl Pdots for 8 h. Therefore, we indicated that carboxyl Pdots modified with R8 could efficiently shorten the incubation time and promote the cellular uptake. To evaluate the cytotoxicity of carboxyl Pdots and R8-Pdots, we carried out MTT assay to measure the cell viability. Fig. 2a showed the cell viability of SGC-7901 cells incubated with 0–10 mg/mL carboxyl Pdots and R8-Pdots for 24 h respectively, indicating the negligible cytotoxicity of carboxyl Pdots and R8-Pdots. Besides the cytotoxicity, the photodynamic effect induced by carboxyl Pdots and R8-Pdots were explored comparatively. SGC-7901 cells were incubated with 0–10 mg/mL carboxyl Pdots for 1 h and 8 h, and incubated with 0–10 mg/mL R8-Pdots for 1 h, then cells were irradiated by green light. As seen from Fig. 2a, the cell viability of cells incubated with R8-Pdots for 1 h was lower than cells incubated with carboxyl Pdots for 8 h, indicating that R8 efficiently promoted the cellular uptake in relatively short time, and significantly enhanced the photodynamic effect at low incubation concentration. To explore the photodynamic effect of R8-Pdots in detail, cells were incubated with 0–10 mg/mL R8-Pdots for 1 h and irradiated by green light for 5–15 min. As seen from Fig. 2b, with the increase of incubation concentration and irradiation time, the cell viability was apparently decreased, indicating the remarkable photodynamic effect induced by the R8-Pdots.

|
Download:
|
| Fig. 2. Cytotoxicity and photodynamic effect of carboxyl Pdots and R8-Pdots in vitro. (a) Cytotoxicity and photodynamic effect of SGC-7901 cells incubated with carboxyl Pdots and R8-Pdots. (b) Photodynamic effect of SGC-7901 cells incubated with 0–10 μg/mL R8-Pdots and irradiated for 5–15 min. (c) Calcein-AM/PI staining of SGC-7901 cells incubated with 10 mg/mL R8-Pdots and irradiated for 15 min. | |
To investigate the photodynamic effect induced by R8-Pdots, Calcein-AM/PI staining was carried out to distinguish live and dead cells. SGC-7901 cells were incubated with 10 mg/mL R8-Pdots for 1 h and irradiated by green light. As shown in Fig. 2c, control cells (without irradiation) produced strong green fluorescence, indicating that only incubated with R8-Pdots (without irradiation) did not lead to cell death. As for cells with irradiation, almost all the cells produced strong red fluorescence, indicating that the excellent photodynamic effect induced by R8-Pdots. To quantitatively evaluate the photodynamic effect of carboxyl Pdots and R8-Pdots, Annexin-V/PI staining was carried out to measure the cell death by flow cytometry. As seen in Fig. S4a (Supporting information), cells were incubated with 0–10 mg/mL R8-Pdots for 1 h and irradiated by green light. When the incubation concentration was increased to 10 mg/mL, the proportion of late apoptotic cells and necrotic cells reached 98.8%. As seen in Fig. S4b (Supporting information), cells were incubated with 10 mg/mL carboxyl Pdots for 0–8 h and irradiated by green light. When the incubation time was increased to 8 h, the proportion of late apoptotic cells and necrotic cells reached 61.5%. These results indicated that with the same incubation concentration, the photodynamic effect induced by R8-Pdots (incubated for 1 h) was more remarkable than carboxyl Pdots (incubated for 8 h). On the basis of the results above, we concluded that modification with R8 could significantly enhance the photodynamic effect in vitro.
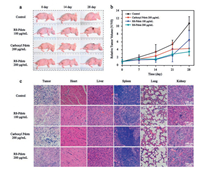
Before the operation of PDT, it is important to explore the biodistribution of R8-Pdots in tumor-bearing nude mice. Tumorbearing nude mice were intravenously or intratumorally injected with R8-Pdots, and were observed by a small animal imaging system after 24 h. As shown in Fig. S5a in Supporting information, the tumor tissue of mice with intratumoral injection presented strong fluorescence as compared to the control group. Meanwhile, fluorescence signal of R8-Pdots could not be detected for mice with intravenous injection, due to the limited tissue penetration depth of exciting light. To precisely explore the distribution of R8-Pdots, fluorescence imaging and fluorescence intensity of tumor tissues and major organs collected from mice were shown in Figs. S5b and c in Supporting information. Results indicated that the majority of R8-Pdots were accumulated in tumor tissue via intratumoral injection, and the majority of R8-Pdots were accumulated in liver via intravenous injection, which was possibly related to reticuloendothelial system (RES) of liver. In addition, we evaluated the photodynamic effect of carboxyl Pdots and R8-Pdots in tumorbearing nude mice. Mice were intratumorally injected with carboxyl Pdots and R8-Pdots, and irradiated by green light for 30 min at the first day and the 7th day. Fig. 3a showed the pictures of representative mice during the process of PDT. Furthermore, we monitored the tumor volume and body weight of mice during the process of PDT. For 200 mg/mL R8-Pdots injection group (Fig. 3b), the effect of inhibiting tumor growth was more remarkable than other groups, which indicated that R8-Pdots had the potential to enhance the photodynamic effect in vivo. As shown in Fig. S6 in Supporting information, in contrast with the control group, the body weight of mice injected with carboxyl Pdots and R8-Pdots showed slight changes, indicating that intratumoral injection with carboxyl Pdots and R8-Pdots did not produce apparent toxicity for mice. Finally, H&E staining of tumor tissues and major organs was carried out to evaluate the toxicity of carboxyl Pdots and R8-Pdots (Fig. 3c). Through observation the pathological sections of tumor tissues, we found that there were more inflammatory cells, more necrotic tissues, and less vascular tissues in carboxyl Pdots and R8-Pdots injection group, as compared to control group. In addition, there was no obvious pathological change in major organs, which indicated that intratumoral injection with carboxyl Pdots and R8-Pdots did not produce apparent toxicity for mice.
|
Download:
|
| Fig. 3. In vivo photodynamic effect of carboxyl Pdots and R8-Pdots in tumor-bearing nude mice. (a) Pictures of representative mice during the process of PDT. (b) Relative tumor volume of mice treated with carboxyl Pdots and R8-Pdots. (c) H&E staining of major organs and tumor tissues collected from mice after PDT. | |
In summary, we developed semiconducting polymer dots with photosensitizer loading and peptide modification for effective PDT. For ZnPc-doped Pdots (carboxyl Pdots), spectroscopic properties displayed the efficient energy transfer and efficient 1O2 generation. After modification with CPPs, the resulting R8-Pdots exhibited good colloidal stability in aqueous solution. Then we evaluated the cellular uptake, cytotoxicity, and photodynamic effect of the carboxyl Pdots and R8-Pdots in SGC-7901 cells through fluorescence imaging, flow cytometry and MTT assay. In vitro results indicated that Pdots modification with R8 could significantly shorten the incubation time, promote the cellular uptake and increase the penetration efficiency. Furthermore, we evaluated the photodynamic effect and toxicity of R8-Pdots in tumor-bearing nude mice, which indicated that intratumoral injection with R8-Pdots could inhibit the tumor growth and enhance the photodynamic effect. Our results indicate that the peptide-coated Pdot photosensitizer holds the great promise for clinical photodynamic treatment of cancers.
AcknowledgmentHong Xu acknowledges financial support from the National Science Foundation of China (No. 81641177).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.015.
| [1] |
D.E. Dolmans, D. Fukumura, R.K. Jain, Nat. Rev. Cancer 3(2003) 380-387. DOI:10.1038/nrc1071 |
| [2] |
M. Triesscheijn, P. Baas, J.H. Schellens, F.A. Stewart, Oncologist 11(2006) 1034-1044. DOI:10.1634/theoncologist.11-9-1034 |
| [3] |
L.L. Rui, H.L. Cao, Y.D. Xue, et al., Chin. Chem. Lett. 27(2016) 1412-1420. DOI:10.1016/j.cclet.2016.07.011 |
| [4] |
T.J. Dougherty, C.J. Gomer, B.W. Henderson, et al., J. Natl. Cancer Inst. 90(1998) 889-905. DOI:10.1093/jnci/90.12.889 |
| [5] |
M. Niedre, M.S. Patterson, B.C. Wilson, Photochem. Photobiol. 75(2002) 382-391. DOI:10.1562/0031-8655(2002)0750382DNILDO2.0.CO2 |
| [6] |
A.P. Castano, P. Mroz, M.R. Hamblin, Nat. Rev. Cancer 6(2006) 535-545. DOI:10.1038/nrc1894 |
| [7] |
K. Kostovic, Z. Pastar, R. Ceovic, et al., Coll. Antropol. 36(2012) 1477-1481. |
| [8] |
N. Yavari, S. Andersson-Engels, U. Segersten, P.U. Malmstrom, Can. J. Urol. 18(2011) 5778-5786. |
| [9] |
R. Allison, K. Moghissi, G. Downie, K. Dixon, Photodiagn. Photodyn. Ther. 8(2011) 231-239. DOI:10.1016/j.pdpdt.2011.03.342 |
| [10] |
B. Green, A.R. Cobb, C. Hopper, Br. J. Oral Maxillofac. Surg. 51(2013) 283-287. DOI:10.1016/j.bjoms.2012.11.011 |
| [11] |
J.P. Wei, X.L. Chen, X.Y. Wang, et al., Chin. Chem. Lett.(2017). |
| [12] |
Y. Liu, Y. Liu, W. Bu, et al., Angew. Chem. Int. Ed. 54(2015) 8105-8109. DOI:10.1002/anie.201500478 |
| [13] |
J. Rieffel, F. Chen, J. Kim, et al., Adv. Mater. 27(2015) 1785-1790. DOI:10.1002/adma.201404739 |
| [14] |
C. Wang, L. Cheng, Y.M. Liu, et al., Adv. Funct. Mater. 23(2013) 3077-3086. DOI:10.1002/adfm.v23.24 |
| [15] |
H.D. Cui, D.H. Hu, J.N. Zhang, et al., Chin. Chem. Lett.(2017). |
| [16] |
F. Chen, H. Hong, S. Goel, et al., ACS Nano 9(2015) 3926-3934. DOI:10.1021/nn507241v |
| [17] |
Z.X. Zhao, Y.Z. Huang, S.G. Shi, et al., Nanotechnology 25(2014) 285701. DOI:10.1088/0957-4484/25/28/285701 |
| [18] |
C.Y. Hsu, C.W. Chen, H.P. Yu, Y.F. Lin, P.S. Lai, Biomaterials 34(2013) 1204-1212. DOI:10.1016/j.biomaterials.2012.08.044 |
| [19] |
J.M. Tsay, M. Trzoss, L. Shi, et al., J. Am. Chem. Soc. 129(2007) 6865-6871. DOI:10.1021/ja070713i |
| [20] |
J. Pecher, S. Mecking, Chem. Rev 110(2010) 6260-6279. DOI:10.1021/cr100132y |
| [21] |
A. Kaeser, A.P. Schenning, Adv. Mater. 22(2010) 2985-2997. DOI:10.1002/adma.v22:28 |
| [22] |
D. Tuncel, H.V. Demir, Nanoscale 2(2010) 484-494. DOI:10.1039/b9nr00374f |
| [23] |
C. Wu, D.T. Chiu, Angew. Chem. Int. Ed. 52(2013) 3086-3109. DOI:10.1002/anie.201205133 |
| [24] |
S. Li, K. Chang, K. Sun, et al., ACS Appl. Mater. Interfaces 8(2016) 3624-3634. DOI:10.1021/acsami.5b07995 |
| [25] |
C. Wu, B. Bull, C. Szymanski, K. Christensen, J. McNeill, ACS Nano 2(2008) 2415-2423. DOI:10.1021/nn800590n |
| [26] |
T. Feng, X. Ai, G. An, P. Yang, Y. Zhao, ACS Nano 10(2016) 4410-4420. DOI:10.1021/acsnano.6b00043 |
| [27] |
L. Feng, J. Zhu, Z. Wang, ACS Appl. Mater. Interfaces 8(2016) 19364-19370. DOI:10.1021/acsami.6b06642 |
| [28] |
L. Zhang, F. Liu, G. Li, Y. Zhou, Y. Yang, J. Pharm. Sci. 104(2015) 4185-4196. DOI:10.1002/jps.24649 |
| [29] |
X. Ma, S. Sreejith, Y. Zhao, ACS Appl. Mater. Interfaces 5(2013) 12860-12868. DOI:10.1021/am404578h |
| [30] |
Y. Tang, H. Chen, K. Chang, et al., ACS Appl. Mater. Interfaces 9(2017) 3419-3431. DOI:10.1021/acsami.6b14325 |
| [31] |
S. Chemburu, T.S. Corbitt, L.K. Ista, et al., Langmuir 24(2008) 11053-11062. DOI:10.1021/la8016547 |
| [32] |
J.L. Grimland, C.F. Wu, R.R. Ramoutar, J.L. Brumaghim, J. McNeill, Nanoscale 3(2011) 1451-1455. DOI:10.1039/c0nr00834f |
| [33] |
H.F. Shi, X. Ma, Q. Zhao, et al., Adv. Funct. Mater. 24(2014) 4823-4830. DOI:10.1002/adfm.v24.30 |
| [34] |
R.W. Redmond, J.N. Gamlin, Photochem. Photobiol. 70(1999) 391-475. DOI:10.1111/php.1999.70.issue-4 |
| [35] |
A. Komin, L.M. Russell, K.A. Hristova, P.C. Searson, Adv. Drug Deliv. Rev.110- 111(2016) 52-64. |
 2017, Vol. 28
2017, Vol. 28 


