Liquid crystalline polymers (LCPs) are materials that possess both the anisotropy property of the liquid crystals and the entropy elasticity of polymer networks [1]. The most attractive property of these materials is the reversibleactuations (due to transition from isotropy to anisotropy), which requires macroscopic orientation of liquid-crystal order. Researchers have reported numerous methods (such as two-step crosslinking method [2, 3], aligning by external fields or surface anchoring [4, 5], soft lithography [6, 7], inkjetprinting [8-10] and microfluidics [11-13]) to obtained the aligned monodomain LCPs in recent decades. However, all above methods have some inborn disadvantages such as delicate procedures, limited size and difficulty to obtain complex shapes, which restrict the practical applications of LCPs. To overcome these disadvantages, in 2013, our group put forward the concept of liquid crystalline vitrimers (LC-vtirmers) [14].Vitrimers are polymer networks with exchageable links, which endows them the features of both thermoplastics and thermosets [15-17]. They are covalently crosslinked like thermosets (insoluble and infusible), but they can be repeatly processed like thermoplastics at temperature above Tv (the topology-freezing transition temperature [18]). LC-vtirmers allow us to prepare the monodomain LCPs by simply stretching the cured material at the high temperature. In the past 5 years, different kinds of LC-vitrimers have been reported [19-23]. Light responsive LC-vitrimers are of particular interest. As we show previously, the incorporation of carbon nanotubes or polydopamine nanoparticles as photo-thermal agents, the LC-vitrimers can be used to fabricate reconfigurable 3D dynamic structures by light [22, 23]. However, it is easy for nanoscale materials to aggregate. Even though polydopamine has a better compatibility than carbon nanotubes, to a certain extent, small aggregates are still exist in polydopamine nanoparticle dispersed LC-vitrimers. It should be pointed out that aggregates of photothermal agents may result in elevated local temperature much higher than the average temperature of the whole material, which will lead to the loss of local alignment and ultimately do harm to actuation performance of the materials. Besides photothermal agents, azobenzene, which can transform the trans form to the cis form upon light irradiation, has also been introduced to LC-vitrimers [20, 21]. Owing to the exchangeable links, the samples can be reshaped into various monodomain 3D structures with flexible motions. However, on one hand, deformation induced by azobenzene is mainly bending. On the other hand, the film must be thin enough (only tens of micrometers), which is difficult to achieve large mechanical force.
In this paper, a new photo-responsive LC-vitrimer is presented. The polymer was synthesized via simply introducing a small amount of photo-thermal agent amino-capped aniline trimer (ACAT) into the LC-vitrimer we reported previously [14]. The resultant polymer is named ACAT-LC-vitrimer in the following parts. Since ACAT can dissolve well in common organic solvents and the amino groups can react with epoxy groups of the liquidcrystalline monomer, ACAT can be homogeneously incorporated in the material as a curing agent. We have previously shown that ACAT can efficiently convert light into heat [24]. The ACAT-LC-vitrimer obtained has cherished properties such as welding, healing and triple shape memory. Moreover, according to the method our group reported previously [14], aligned monodomain LC actuators can be prepared, of which the actuation performance (such as response mechanism and strain) is close to carbon nanotubes or polydopamine nanoparticles containing LC-vitrimer.
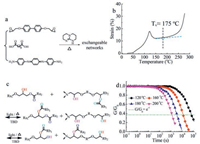
The ACAT-LC-vitrimer was processed via reacting DGE-DHBP, SA and ACAT under the catalysis of TBD (Fig. 1a), where the functionality of DGE-DHBP and cross-linkers (SA and ACAT) was 1:1 to ensure a complete network topology formation. Like the CNT and polydopamine nanoparticle dispersed LC-vitrimers, ACAT-LC-vitrimer is black. The FTIR spectra shows that the epoxy groups at 909 cm-1 and terminal amine groups at 3302 cm-1 and 3194 cm-1 have disappeared. Meanwhile, characteristic peaks of hydroxyl (3385 cm-1) and ester (1731 cm-1) generate, all above confirming the completeness of the curing reaction (Figs. S1–S3 in Supporting information). According to DSC (Fig. S4 in Supporting information), the glass transition temperature Tg and isotropic transition temperature Ti are about 58 ℃ and 105 ℃ upon heating, respectively. The storage modulus of the films with and without ACAT are about1.38 GPa and 1.40 GPa, respectively, justifying that the small amount of ACAT makes little difference to the mechanical properties of the films (Fig. S5 in Supporting information). Owing to the transesterification catalyst TBD, ester groups can exchange with hydroxyl groups to generate new ester groups and hydroxyl groups at temperature sufficiently higher than Tv (the topology freezing transition temperature [25]) (Fig. 1b). Vitrimers can be reprocessed at temperatures above Tv. Tv obtained from dilatometry is about 175 ℃ (Fig. 1c). The Tv is about 15 ℃ higher than the CNT or polydopamine nanoparticles dispersed LC-vitrimers. This may because, on one hand, contents of catalyst TBD (2.5 mol% to COOH groups) wasonly half of the previous reported ones. On the other hand, Tg of this material is also higher. As previously disclosed by Qi's group [26], Tv always increases with the increasing of Tg. As to the higher Tg of this material, it is easy to understand as the ACAT molecules are rigid, which can increase the stiffness of the backbone. The stress relaxation analyses were conducted on the films in linear viscoelastic range at different temperatures of 120, 160, 180 and 200 ℃ (Fig. 1d). In accordance with the previous reports, the exchange reaction is quite slow below Tv and the relaxation time τ* (i.e., the time needed for the film to relaxe to 1/e of its initial stress relaxation modulus) is about 68182 s at 120 ℃. However, the transesterification rate increases exponentially above Tv and τ* at 180 ℃ decreased to 3590 s. Before talking about the actuation performance of the aligned monodomain ACAT-LC-vitrimer, we investigated the properties of the non-aligned polydomain ACAT-LC-vitrimer. First of all, we measured the temperaturesof the LC-vitrimer with and without ACAT using an infraredthermal imager, where the samples were exposed to near-infrared (IR) laser (808 nm) for about 10 s to get an equilibriumtemperature at different light intensities. As shown in Fig. 2a and b, the temperatures of the ACAT-LC-vitrimer are much higher than that of the neat-LC-vitrimer at each intensity. For example, at light intensities of 0.19 W/cm2, 0.39 W/cm2 and 0.66 W/cm2, the temperatures of the ACAT-LC-vitrimer are about 75.7, 128 and 181 ℃, while that of theneat-LC-vitrimer areonly about26.1, 27.6 and 29.8 ℃, respectively. As a result, the material is endowed with the photo-controlled functions such as welding, healing and shape memory.
|
Download:
|
| Fig. 1. Polymer synthesis and characterization. (a) Synthesis of the ACAT–LC-vitrimer. (b) An illustration of reversible transesterification. (c) Dilatometry test of the ACAT–LC-vitrimer with a ramp rate of 5 ℃/min. (d) Stress relaxation experiments of the ACAT–LC-vitrimer at different temperatures. | |

|
Download:
|
| Fig. 2. Photo-responsive properties of the non-aligned polydomain ACAT-LC-vitrimer. (a) The IR temperature images of the ACAT–LC-vitrimer and neat–LC-vitrimerfilms upon light irradiation for 10 s. (b) The temperature changes with the light intensities. (c) Stress–strain curves of the blank film and films welded for 15 s and 30 s (with a thickness of about 0.2 mm) with a ramp rate of 0.4 N/min (inset: an illustration of the welding process). (d-e) Stress–strain curves of the undamaged blank film, damaged film and healed film with a ramp rate of 0.4 N/min with a scratch (d) and needle pierced hole (e), which were healed with a light intensity of 0.66 W/cm2 for 10 s. (f) A shape memory demonstration of the ACAT-LC-vitrimer. | |
Firstly, similar to the effect of CNTs and polydopamine nanoparticles, ACAT enables welding and healing by light. As is shown in Fig. 2c, the connection of the two films becomes more and more robust with time increasing and finally the welded film reaches approximately the same mechanical strength andelongation at break when radiated for 30 s (0.66 W/cm2, T ≈ 180 ℃). The resultant material is also optically healable. Toconfirm the healing efficient, we conducted two sets ofexperiments, where one film was scratched perpendicular to the loading direction (with a width of about 90.67 μm) and the other one was punctured with a hole (with a diameter of 183.94 μm). After exposing the damaged films to IR laser (0.66 W/cm2) for 10 s, the films were completely healed as observed by the optical microscopy (POM) (insets of Fig. 2d and e). The stress-strain results on the blank, damaged and healed films of the two sets are similar. The damaged films fractured quickly, but the healed ones have almost the identical mechanical properties with the blank ones, which proves that the healing is effective and robust (Fig. 2d and e).
Secondly, owing to the glass transition Tg and isotropic transition Ti, which is about 50 ℃ higher than Tg, the material shows an excellent triple shape memory effect. It is worth noting that, since light can be operated locally, a number of temporary shapes can be fixed to meet the practical applications. Taking Fig. 2f for a demonstration example, a flat film (1) was first crinkled into shape (2) via 808 nm IR laser (0.19 W/cm2, T ≈ 75 ℃) then cooled to room temperature, which was further deformed into shape (3) via IR laser (0.39 W/cm2, T ≈ 128 ℃). Then it was reversed to the permanent shape (8) via IR laser companied with the appearance of another four temporary shapes (4–7).
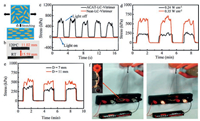
Although the non-aligned polydomain ACAT-LC-vitrimer displays a number of excellent properties, it is intriguing to exploit the properties of the aligned monodomain ACAT-LC-vitrimer, which can covert the external stimuli into large macroscopic strain. Using the method that we have reported previously (Fig. 3a) [14], we obtained a well-aligned monodomain LC actuator that processes a strain of about 40%-45% when the film transforms from isotropic to anisotropic phase (Fig. 3b). To study the stress generated quantitatively, we used DMA to monitor the stress generated by the film during the cycle of light on-off. As shown in Fig. 3c, on one hand, it reveals that when radiated with light 0.35 W/cm2 (T ≈ 120 ℃) the film contracts to generate a stress more than 500 kPa and almost totally releases the stress when light is switched off. On the other hand, it shows that during the cycle the stress produced is relatively stable and the stress of the neat-LC-vitrimer shows little change. Moreover, the light intensity can conveniently modulate the stress generated for isotropic transition is within a wide temperature range (about 90 ℃–115 ℃ for the ACAT-LC-vitrimer). As shown in Fig. 3d, with the same light radiation area, the stress generated with the light intensity of 0.35 W/cm2 (T ≈ 120 ℃) is about 2 times of that generated by 0.24 W/cm2 (T ≈ 105 ℃). Furthermore, to justify the advantage oflight over direct heating, we designed two sets of experiments with the same light intensity (0.35 W/cm2) where the only difference is the light radiation area. As shown in Fig. 3e, it demonstrates that the stress increases with the light radiation area, which reveals that light can be controlled locally and the stress can be modulated easily.
|
Download:
|
| Fig. 3. Performance of the aligned momodomain ACAT-LC-vitrimer. (a) An illustration of the alignment of the momodomain ACAT-LC-vitrimer. (b) Reversible thermal actuation of the aligned momodomain ACAT-LC-vitrimer. (c) Photo-induced actuation of the aligned momodomain ACAT–LC-vitrimer and neat–LC-vitrimer films in constantstrain geometry. (d) Photo-induced actuation of the aligned momodomain ACAT–LC-vitrimer with different light intensities. (e) Photo-induced actuation of the aligned momodomain ACAT–LC-vitrimer with different light areas. (f) A demonstration of utilizing the actuation of the aligned momodomain ACAT-LC-vitrimer to control a circuit. | |
As an example of a practical application, we utilized the actuation of the aligned monodomain ACAT-LC-vitrimer to control a circuit by switching on-off the IR light. As shown in Fig. 3f, without light, the film is in its anisotropic state and circuit is disconnected. However, once the light is switched on (0.35 W/cm2), the temperature of the film rises quickly above Ti, as a result of which the film contracts to raise the copper sheet up and connect the circuit. Then, the light is switched off and the lampbulb goes off. To investigate the stability of the reversible strain, a cycle experiment of the light switch on-off was carried out on the system, which showed a good reproducibility up to our expectations. Moreover, the circuiti can be flexibly controlled by light intensity. In accordance to the aforementioned conclusions from Fig. 3d that different light intensities generate different stress. So it should take longer time for lower light intensity to light the lampbulb. Up to our expectation, it took about 5 s and 7 s to connect the circuit with light intensity 0.35 W/cm2 and 0.24 W/cm2, respectively.
In summary, a photo-responsive LC-vitrimer is synthesized by simply introducing a photo-thermal agent aniline trimer into a LC-vitrimer system. As aniline trimer is covalently linked into the network, there is no need to worry about the aggregation, which is a critical problem when using CNTs or other nanoparticles as photothermal agents. A small amount of aniline trimer has little effect on the mechanical property ofthe LC-vitrimer. Due to the excellent photo-thermal effect of aniline trimer, the resultant polymer can be welded, healed and actuated by light. This strategy used here can also be generated to other kind of LC-vitrimer when proper molecular design is involved.
AcknowledgmentThis research was supported by the National Natural Science Foundation of China (Nos. 20161300524 and 21274075).
| [1] |
C. Ohm, M. Brehmer, R. Zentel, Adv. Mater. 22(2010) 3366-3387. DOI:10.1002/adma.200904059 |
| [2] |
A.R. Tajbakhsh, E.M. Terentjev, Eur. Phys. J. E 6(2001) 181-188. DOI:10.1007/s101890170020 |
| [3] |
J. Küpfer, H. Finkelmann, Macromol. chem. phys. 195(1994) 1353-1367. DOI:10.1002/macp.1994.021950419 |
| [4] |
E.S. Lee, P. Vetter, T. Miyashita, T. Uchida, Jpn. J. Appl. Phys. 32(1993) L1339. DOI:10.1143/JJAP.32.L1339 |
| [5] |
B. Jerome, Rep. Prog. Phys. 54(1991) 391. DOI:10.1088/0034-4885/54/3/002 |
| [6] |
Y. Yu, T. Ikeda, Angew. Chem. Int. Edit. 45(2006) 5416-5418. DOI:10.1002/(ISSN)1521-3773 |
| [7] |
A. Buguin, M.H. Li, P. Silberzan, B. Ladoux, P. Keller, J. Am. Chem. Soc. 128(2006) 1088-1089. DOI:10.1021/ja0575070 |
| [8] |
M. Camacho-Lopez, H. Finkelmann, P. Palffy-Muhoray, M. Shelley, Nat. Mater. 3(2004) 307-310. DOI:10.1038/nmat1118 |
| [9] |
C.L. Van Oosten, C.W.M. Bastiaansen, D.J. Broer, Nat. Mater. 8(2009) 677-682. DOI:10.1038/nmat2487 |
| [10] |
H. Minemawari, T. Yamada, H. Matsui, et al., Nature 475(2011) 364-367. DOI:10.1038/nature10313 |
| [11] |
C. Ohm, C. Serra, R. Zentel, Adv. Mater. 21(2009) 4859-4862. DOI:10.1002/adma.200901522 |
| [12] |
C. Ohm, N. Kapernaum, D. Nonnenmacher, et al., J. Am. Chem. Soc. 133(2011) 5305-5311. DOI:10.1021/ja1095254 |
| [13] |
C. Ohm, E.K. Fleischmann, I. Kraus, C. Serra, R. Zentel, Adv. Funct. Mater. 20(2010) 4314-4322. DOI:10.1002/adfm.v20.24 |
| [14] |
Z. Pei, Y. Yang, Q. Chen, et al., Nat. Mater. 13(2014) 36-41. DOI:10.1038/nmat3812 |
| [15] |
W. Zou, J. Dong, Y. Luo, Q. Zhao, T. Xie, Adv. Mater. 29(2017) 1606100. DOI:10.1002/adma.v29.14 |
| [16] |
W. Denissen, J.M. Winne, F.E. Du Prez, Chem. Sci. 7(2016) 30-38. DOI:10.1039/C5SC02223A |
| [17] |
M. Capelot, D. Montarnal, F. Tournilhac, L. Leibler, J. Am. Chem. Soc. 134(2012) 7664-7667. DOI:10.1021/ja302894k |
| [18] |
M. Capelot, M.M. Unterlass, F. Tournilhac, L. Leibler, ACS Macro Lett. 1(2012) 789-792. DOI:10.1021/mz300239f |
| [19] |
M.K. McBride, M. Hendrikx, D. Liu, et al., Adv. Mater. 29(2017) 1606509. DOI:10.1002/adma.v29.17 |
| [20] |
X. Lu, S. Guo, X. Tong, H. Xia, Y. Zhao, Adv. Mater.(2017), 1606467. |
| [21] |
T. Ube, K. Kawasaki, T. Ikeda, Adv. Mater. 28(2016) 8212-8217. DOI:10.1002/adma.v28.37 |
| [22] |
Z. Li, Y. Yang, Z. Wang, et al., J. Mater. Chem. A 5(2017) 6740-6746. DOI:10.1039/C7TA00458C |
| [23] |
Y. Yang, Z. Pei, Z. Li, Y. Wei, Y. Ji, J. Am. Chem. Soc. 138(2016) 2118-2121. DOI:10.1021/jacs.5b12531 |
| [24] |
Q. Chen, X. Yu, Z. Pei, et al., Chem. Sci. 8(2017) 724-733. DOI:10.1039/C6SC02855A |
| [25] |
M. Capelot, M.M. Unterlass, F. Tournilhac, L. Leibler, ACS Macro Lett. 1(2012) 789-792. DOI:10.1021/mz300239f |
| [26] |
K. Yu, P. Taynton, W. Zhang, M.L. Dunn, H.J. Qi, Rsc Advances 4(2014) 48682-48690. DOI:10.1039/C4RA06543C |
 2017, Vol. 28
2017, Vol. 28 


