In recent years, organic optoelectronic materials have attracted considerable attention due to their various applications, such as organic light-emitting diodes, field-effect transistors, photovoltaics, sensors and solid-state lasers [1-8]. As a kind of morphological forms studied for optoelectronic materials, single crystals are featured by the ordered molecular arrangement and well-defined surface, which endow the crystals with diverse and outstanding properties, such as good thermal stability, high charge-carrier mobilities, low light propagation loss and reflecting mirror structures [9-14]. As such, they have attracted increasing research interest in optoelectronics.
Amplified spontaneous emission (ASE) is a phenomenon that has long been studied, which is closely related to pumping laser [15-21]. In general, the ASE is accompanied by the waveguide and emission polarization properties of the active materials. Some organic crystals have been found to possess good waveguide and ASE performance because of their low light scattering, smooth surfaces and high fluorescence quantum yields (ΦF) [22, 23]. To date, the ASE of crystals with blue, green and red colors has all been reported. However, the crystals that can realize red ASE with low energy thresholds and high gain coefficients are still lacking [24-27].
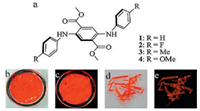
In this work, we have employed a series of 2, 5-diaminoterephthalates, 1–4, for ASE study (Fig. 1). The existence of electrondonating amino and electron-withdrawing ester groups in the molecule is beneficial to the realization of desirable low-energy emission. The substituents on the peripheral phenyl rings are adopted to tune the material properties. The intramolecular hydrogen bonds between the amino and the carbonyl groups are expected, which could lead to a relatively planar and rigid conformation for the central part of the molecules. Such a conformation may enhance the luminescence efficiency in the dispersed state and thus provide a good opportunity for realizing high solid-state ΦF that benefits the ASE [24]. Although compounds 1, 3 and 4 are known [28, 29], their optoelectronic properties have not been disclosed. In addition to the ASE, the waveguide and polarized emission properties have also been explored.
|
Download:
|
| Fig. 1. Molecular structures of 1–4 (a) and the photographs of crystal 1 under daylight (b, d) and UV irradiation (c, e). | |
The syntheses of compounds 1, 3 and 4 have been reported previously, and compound 2 is newly synthesized according to the literature procedure (Scheme S1 in Supporting information) [29]. These compounds can be easily crystalized by slow evaporation of their solutions in the CHCl3/CH3OH mixed solvent (1:2, v/v), and bar-shaped single crystals with a large size and a smooth surface can be obtained. The thermal properties of 1–4 were characterized by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) under a nitrogen atmosphere at a heating rate of 10 ℃/min (Figs. S1 and S2 in Supporting information). 1–4 exhibit high thermal decomposition temperatures (Td, corresponding to 5% weight loss) of 233, 250, 251 and 275 ℃, respectively, as can be seen from the TGA curves. The melting points (Tm) of 1–4 determined by DSC measurements are moderate to high, being 167, 230, 227 and 232 ℃, respectively. Considering the close molecular weights and structures of 1–4, the much lower Tm of 1 may imply relatively weaker intermolecular interactions. The good thermal properties of 1–4 benefit their optoelectronic application.
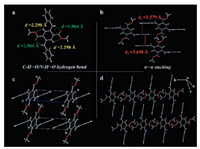
To get insight into molecular conformations and packing features, the crystal structure of 1 was resolved by X-ray diffraction. The crystal belongs to the triclinic system, space group P-1. There is one molecule in the asymmetric unit (Fig. 2a), and no solvation is observed. Within the molecule, there are two kinds of intramolecular hydrogen bonds: The N—H…O hydrogen bond (H…O distance: 1.964 Å, ∠N—H…O: 136.4°) between the N—H group and the carbonyl oxygen; and the C — H…O hydrogen bond (H…O distance: 2.298 Å, ∠C—H…O: 103.48°) between the central benzene ring and the ester group (Fig. 2a). As expected, these interactions lead to a relatively planar conformation for the central part of the molecule. The two peripheral phenyl rings twist out of the plane defined by the central benzene ring by an angle less than 34°. In the packing structure, there are π…π interactions between the peripheral phenyl rings of adjacent molecules (Fig. 2b). Notably, all the molecules in crystal 1 are arranged in the same conformation and orientation (Figs. 2c and d), resulting in the formation of so-called uniaxially oriented crystal, which is beneficial to the waveguide, polarized emission and ASE of organic crystals [30, 31].
|
Download:
|
| Fig. 2. Single-crystal structures of compound 1: (a) molecular structure of 1 in the asymmetric unit; (b) intermolecular π…π interactions; and (c) and (d) crystal packing mode. | |
Fig. 3a shows the absorption and emission spectra of 1–4 measured in CH2Cl2 (10-5 mol/L), and the photophysical data are summarized in Table S1 in Supporting information. All these compounds possess a broad absorption band in the visible-light region. The absorption maxima of 1 and 2 are both located at 469 nm, while those of 3 (479 nm) and 4 (484 nm) are gradually red shifted. 1–4 show a broad and structureless emission spectrum in CH2Cl2, with the emission maxima located at 564, 564, 578 and 594 nm, respectively. Correspondingly, the emission colors range from yellow to orange-red. The bar-shaped single crystals of these compounds display bright orange-red emission. The emission maxima of crystals 1–4 are 597, 604, 597 and 608 nm, respectively (Fig. 3b), which are significantly red shifted compared with those of corresponding emission spectra measured with CH2Cl2 solutions. The ΦF values of crystals 1–4 are 0.22, 0.38, 0.48 and 0.24, respectively. Notably, these values are at a high level as for organic solids with the emission maxima around 600 nm, as the nonradiative decay of the excited state becomes more serious for lowenergy emission according to the energy-gap law. The good ΦF values are favorable for the optoelectronic application of the compounds.

|
Download:
|
| Fig. 3. (a) Absorption and emission spectra of 1–4 in CH2Cl2 (10-5 mol/L); (b) Emission spectra of crystals 1–4. | |
Interestingly, when the bar-shaped crystals of 1–4 are irradiated with UV light, the two ends of crystal emit brighter light than the body, suggesting that these crystals are optical waveguides and may be useful for ASE or lasing [32-35]. To evaluate the waveguide performance, optical loss coefficients were studied with crystal 1. The method adopted is to irradiate different positions of a single crystal using the same excitation light and collect, at one end of the crystal, the emission spectrum corresponding to each irradiation position (Fig. 4a) [36, 37]. As can be seen in Fig. 4b, the emission intensity at the end decreases gradually when the distance between the end and the irradiated position is increased, due to a longer propagation distance of light. In addition, a spectral redshift can be observed with increased propagation distance. The optical loss coefficients are estimated to be 0.361 dB/mm and 0.058 dB/mm at 617 nm and 638 nm, respectively, by fitting the data of Fig. 4b according to literature procedures [38, 39]. Notably, these values are significantly smaller than those of many organic crystals ever reported [32], suggesting the good optical waveguide performance of crystal 1. The relatively larger loss coefficient for the light of 617 nm should be related to the reabsorption, as deduced from the overlap between the emission and absorption spectra of crystal 1 in the range of ca. 525–620 nm (Fig. S4 in Supporting information). Upon increasing the propagation distance, more light within 525–620 nm is reabsorbed, which is responsible for the observed spectral redshift of long-distance propagation.

|
Download:
|
| Fig. 4. (a) Fluorescence microscopy images collected upon excitation of the identical crystal of 1 at five different positions; (b) Emission spectra measured at the end of a single crystal of 1 with the distance between the end and the excitation point in the range of 1–8 mm. | |
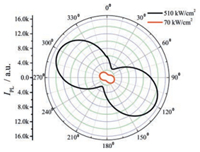
In addition to the waveguide property, the polarization property of the light emitted from the bar-shaped crystal of 1 was also studied. A polarization analyzer (polarizer) was set between the end of a crystal and a CCD spectrometer [40, 41]. Upon irradiating the crystal with a laser (λex = 355 nm) with the energy of 510 kW/cm2 or 70 kW/cm2, the light emitted from the crystal transmitted through the analyzer and was detected by the CCD spectrometer. As can be seen in Fig. 5, the detected light intensity shows dependence on the rotation angle of the analyzer. The minimum intensities of emission appear at ca. 30° and 210°, and the maximum intensities occur at ca. 120° and 300°, which are approximately perpendicular to the direction in which the maximum emission intensity is detected. Moreover, for both powers of the pumping laser, the emission intensity can be well fitted by a cosine quadratic (cos2 θ) function, where θ is the angle between the transmission axes of the analyzer and the crystal. In addition, the polarization contrast calculated with literature procedures [42] is as high as 0.70. These results suggest that the light emitted from crystal 1 is well linearly polarized [22].
|
Download:
|
| Fig. 5. Dependence of the intensity of polarized light from crystal 1 on the rotation angle of the polarization analyzer situated between the end of crystal 1 and the CCD spectrometer. 510 kW/cm2 and 70 kW/cm2 is the energy of the pumping laser irradiated on the crystal. | |
Finally, the ASE properties of these crystals were explored. A single bar-shaped crystal of each compound was selected and excited with pulsed laser beams (λex = 355 nm) with different energy, and the emission spectrum for each energy was collected at one end of the crystal using a CCD spectrometer. For crystal 1, the emission spectrum displays a broad band with the full width at half maximum (FWHM) of ca. 58 nm when the laser power is low (57 kW/cm2), as can be seen in Fig. 6a and b. The rapid narrowing of emission spectra and the nonlinear increase of the emission intensity, caused by the increase of laser power, can be observed when the laser power is over ca. 143 kW/cm2, suggesting the ASE behavior of crystal 1 with the energy threshold of ca. 143 kW/cm2. The narrowest spectrum with the FWHM of ca. 5.9 nm is achieved at 792 kW/cm2. Crystals 2–4 also display the ASE property, with the energy thresholds of ca. 198, 72 and 98 kW/cm2 and the smallest FWHM of ca. 6.1, 8.6 and 6.1 nm, respectively (Figs. S5–S7 in Supporting information). Notably, crystals 1 (lem: ca. 615 nm) and 4 (λem: ca. 613 nm) display the red ASE. Moreover, they possess the energy thresholds comparable to those of the reported organic crystals [43]. The gain coefficient of crystal 1 was measured using a successively varied pump stripe method. Fig. 6c shows the curve of emission-spectra intensities (at 615 nm) versus pump stripe lengths at three different intensities of the pulsed laser. Upon enlarging the pump strip, there is a nonlinear increase in the emission intensity, consistent with a higher gain at a higher laser intensity for ASE [44-47]. As shown in Fig. 6d, the net gain coefficients at 615 nm are 48 cm-1 at a pump intensity of 548 kW/cm2 and up to 62 cm-1 at a pump intensity of 668 kW/cm2.

|
Download:
|
| Fig. 6. ASE property of crystal 1: (a) emission spectra as a function of the energy of pump laser; (b) dependences of peak intensity and FWHM of the emission spectra on the energy of pump laser; (c) emission peak intensity as a function of the pump stripe length at different pump energy; (d) the net gain coefficient as a function of wavelength at different pump energy. | |
In summary, a series of structurally simple 2, 5-diaminoterephthalates were readily synthesized, and high-quality single crystals of these compounds were prepared through simple solventevaporation method. These compounds possess good thermal properties, and their crystals display bright orange-red emission. The crystals can realize ASE with appreciable energy thresholds. More importantly, red ASE with good performance is achieved by the crystals of 1 and 4. Crystal 1 was also selected to explore the waveguide and polarized emission properties, and the waveguide featured by low optical loss coefficients, as well as the linear polarization of the light emitted from crystal 1, have been demonstrated. The uniaxially oriented packing of 1, as observed in the single-crystal structure, could be a reason for the good performance of crystal 1. The success in adopting structurally simple and easily synthesized organic compounds to realize lowenergy emissive organic crystals with high material performance is of guiding significance for the further development and practical application of organic optoelectronic materials.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51622304, 51603082) and Open Research Fund of State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences (No. 201631).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.045.
| [1] |
S. Park, O. Kwon, S. Kim, et al., J. Am. Chem. Soc. 127(2005) 10070-10074. DOI:10.1021/ja0508727 |
| [2] |
H. Mizuno, I. Ohnishi, H. Yanagi, F. Sasaki, S. Hotta, Adv. Mater. 24(2012) 2404-2408. DOI:10.1002/adma.201104182 |
| [3] |
D. Li, H. Zhang, Y. Wang, Chem. Soc. Rev. 42(2013) 8416-8433. DOI:10.1039/c3cs60170f |
| [4] |
Z. Xie, C. Chen, S. Xu, et al., Angew. Chem. Int. Ed. 54(2015) 7181-7184. DOI:10.1002/anie.201502180 |
| [5] |
H. Lu, Y. Zheng, X. Zhao, et al., Angew. Chem. Int. Ed. 55(2016) 155-159. DOI:10.1002/anie.201507031 |
| [6] |
Y. Zhang, Y. Fu, D. Zhu, et al., Chin. Chem. Lett. 27(2016) 1429-1436. DOI:10.1016/j.cclet.2016.05.019 |
| [7] |
Y. Xie, L. Han, C. Ge, Y. Cui, J. Gao, Chin. Chem. Lett. 28(2017) 285-292. DOI:10.1016/j.cclet.2016.06.042 |
| [8] |
W. Liu, J. Yao, C. Zhan, Chin. Chem. Lett. 28(2017) 875-880. DOI:10.1016/j.cclet.2017.01.013 |
| [9] |
H. Yanagi, T. Ohara, T. Morikawa, Adv. Mater. 13(2001) 1452-1455. DOI:10.1002/1521-4095(200110)13:19<>1.0.CO;2-S |
| [10] |
L. Ji, X. Wang, S. Luo, et al., Sci. China Ser. B:Chem. 51(2008) 661-668. DOI:10.1007/s11426-008-0025-4 |
| [11] |
J. Tok, Z. Bao, Sci. China-Chem. 55(2012) 718-725. DOI:10.1007/s11426-012-4503-3 |
| [12] |
C. Wang, Y. Gong, W. Yuan, Y. Zhang, Chin. Chem. Lett. 27(2016) 1184-1192. DOI:10.1016/j.cclet.2016.05.026 |
| [13] |
Y. Zhen, H. Dong, L. Jiang, W. Hua, Chin. Chem. Lett. 27(2016) 1330-1338. DOI:10.1016/j.cclet.2016.06.023 |
| [14] |
Q. Li, S. Liu, H. Chen, H. Li, Chin. Chem. Lett. 27(2016) 1421-1428. DOI:10.1016/j.cclet.2016.06.027 |
| [15] |
H. Yanagi, T. Morikawa, Appl. Phys. Lett. 75(1999) 187-189. DOI:10.1063/1.124314 |
| [16] |
M. McGehee, A. Heeger, Adv. Mater. 12(2000) 1655-1668. DOI:10.1002/(ISSN)1521-4095 |
| [17] |
M. Ichikawa, R. Hibino, M. Inoue, et al., Adv. Mater. 17(2005) 2073-2077. DOI:10.1002/(ISSN)1521-4095 |
| [18] |
K. Shimizu, Y. Mori, S. Hotta, J. Appl. Phys. 99(2006) 063505. DOI:10.1063/1.2181278 |
| [19] |
L. Heng, X. Wang, D. Tian, et al., Adv. Mater. 22(2010) 4716-4720. DOI:10.1002/adma.v22:42 |
| [20] |
C. Zhang, Y. Zhao, J. Yao, Phys. Chem. Chem. Phys. 13(2011) 9060-9073. DOI:10.1039/c0cp02376k |
| [21] |
S. Ma, J. Zhang, J. Qian, et al., Adv. Opt. Mater. 3(2015) 763-768. DOI:10.1002/adom.v3.6 |
| [22] |
J. Chen, S. Ma, J. Zhang, et al., ACS Photonics 2(2015) 313-318. DOI:10.1021/ph5004384 |
| [23] |
Q. Chen, H. Fang, B. Xu, et al., Appl. Phys. Lett. 94(2009) 201113. DOI:10.1063/1.3142383 |
| [24] |
J. Gierschner, S. Varghese, S. Park, Adv. Optical Mater. 4(2016) 348. DOI:10.1002/adom.201500531 |
| [25] |
M. Ichikawa, K. Nakamura, M. Inoue, et al., Appl. Phys. Lett. 87(2005) 221113. DOI:10.1063/1.2138361 |
| [26] |
D. Fichou, S. Delysse, J.M. Nunzi, Adv. Mater. 9(1997) 1178. DOI:10.1002/(ISSN)1521-4095 |
| [27] |
X. Cheng, K. Wang, S. Huang, et al., Angew. Chem. Int. Ed. 54(2015) 8369. DOI:10.1002/anie.201503914 |
| [28] |
J. Shi, S. Zheng, Macromolecules 34(2001) 6571-6576. DOI:10.1021/ma010666t |
| [29] |
M. Shimizu, Y. Asai, Y. Takeda, A. Yamatani, T. Hiyama, Tetrahedron Lett. 52(2011) 4084-4089. DOI:10.1016/j.tetlet.2011.05.087 |
| [30] |
T. Yamao, Y. Okuda, Y. Makino, S. Hotta, J. Appl. Phys. 110(2011) 053113. DOI:10.1063/1.3634117 |
| [31] |
S. Varghese, S. Yoon, E. Calzado, et al., Adv. Mater. 24(2012) 6473-6478. DOI:10.1002/adma.v24.48 |
| [32] |
Y. Xu, H. Zhang, F. Li, et al., J. Mater. Chem. 22(2012) 1592-1597. DOI:10.1039/C1JM14815J |
| [33] |
B. Tang, H. Liu, F. Li, Y. Wang, H. Zhang, Chem. Commun. 52(2016) 6577-6580. DOI:10.1039/C6CC02616H |
| [34] |
X. Wang, H. Li, Y. Wu, Z. Xu, H. Fu, J. Am. Chem. Soc. 136(2014) 16602-16608. DOI:10.1021/ja5088503 |
| [35] |
W. Zhang, Y. Yan, J. Gu, J. Yao, Y. Zhao, Angew. Chem. Int. Ed. 54(2015) 7125-7129. DOI:10.1002/anie.201502684 |
| [36] |
K. Guo, Q. Zhang, F. Wang, et al., Phys. Status. Solid A 211(2014) 2372-2377. DOI:10.1002/pssa.v211.10 |
| [37] |
X. Zhao, Z. Wu, S. Ning, et al., Optics Exp. 19(2011) 16126-16131. DOI:10.1364/OE.19.016126 |
| [38] |
Y. Li, Z. Ma, A. Li, et al., ACS Appl. Mater. Interfaces 9(2017) 8910-8918. DOI:10.1021/acsami.7b00195 |
| [39] |
H. Luo, S. Chen, Z. Liu, et al., Adv. Funct. Mater. 24(2014) 4250-4258. DOI:10.1002/adfm.v24.27 |
| [40] |
H. Fang, J. Yang, R. Ding, et al., Appl. Phys. Lett. 97(2010) 101101. DOI:10.1063/1.3486683 |
| [41] |
Y. Li, F. Shen, H. Wang, et al., Chem. Mater. 20(2008) 7312-7318. DOI:10.1021/cm801427s |
| [42] |
H. Fang, Q. Chen, J. Yang, et al., Optics Lett. 35(2010) 441-443. DOI:10.1364/OL.35.000441 |
| [43] |
B. Tang, H. Zhang, X. Cheng, K. Ye, H. Zhang, ChemPlusChem 81(2016) 1320-1325. DOI:10.1002/cplu.v81.12 |
| [44] |
X. Cheng, F. Li, S. Han, et al., Sci. Rep. 5(2015) 9140. DOI:10.1038/srep09140 |
| [45] |
H. Wang, F. Li, I. Ravia, et al., Adv. Funct. Mater. 21(2011) 3770-3777. DOI:10.1002/adfm.201100783 |
| [46] |
K. Wang, H. Zhang, S. Chen, et al., Adv. Mater. 26(2014) 6168-6173. DOI:10.1002/adma.201401114 |
| [47] |
J. Zhang, B. Xu, J. Chen, et al., Adv. Mater. 26(2014) 739-745. DOI:10.1002/adma.201303639 |
 2017, Vol. 28
2017, Vol. 28 


