b University of Chinese Academy of Sciences, Beijing 100190, China;
c Department of Orthopedics, The Third Affiliated Hospital of Southern Medical University, Guangzhou 510665, China
Bone defects and damage caused by trauma, tumor or diseases are one of the most troubling problems in human health. Bone repair materials, including autografts, allografts, xenografts and artificial biomaterials are widely used in clinic [1-3]. However, autografts, allografts and xenografts have some specific drawbacks such as limited availability, potential risks of transmitting disease and inducing immune responses, etc. [1, 4]. For these reasons, artificial bone substitutes are in high demand.
Poly(1, 8-octanediol-co-citrate) (POC) represents a new promising biocompatible and biodegradable polyester developed recently for tissue engineering. This elastomer is easy to synthesize through a simple, catalyst-free polycondensation reaction with non-toxic monomers and its mechanical and degradation properties can be tuned by controlling the synthesis conditions [5]. Furthermore, the pendant carboxyl and hydroxyl groups make it possible to design novel POC-based materials with antimicrobial, fluorescent, adhesive or other properties [6]. To date, POC has been extensively investigated for soft tissue repairing and engineering, such as cardiovascular graft and scaffold, cartilage, nerves or dermal tissue repair materials [6, 7]. However, pure POC is a soft and viscoelastic material. The low mechanical strength and poor bioactivity retard its use in bone tissue regeneration.
The combination of bioactive inorganic materials and organic elastomers is a prevailing strategy to overcome the low mechanical strength problem and to bring about the required bioactivities. Bioactive glasses (BG) seem to be one of the most promising candidates for the inorganic materials, due to their excellent biocompatibility, osteoconductivity, bioactivity and controllable chemical properties [8-11]. When implanted, they could stimulate osteoprogenitor cells at the genetic level by their dissolution products (i.e., soluble silica and calcium ions) and bond chemically to surrounding bone through the formed bone mineral-like hydroxyapatite (HA) layers on their surfaces [12]. It has been demonstrated that BG could stimulate more bone regeneration and degrade faster than HA [12-15]. Moreover, BG allows for the facile incorporation of dopants, such as Ag, Ga or Sr, thereby providing additional functions for bone regeneration or bactericidal action [9, 16]. In view of this, the incorporation of BG into POC seems likely to improve the mechanical and biological properties of POC simultaneously.
A few studies have been carried out on POC/BG composites. It was found that the incorporation of BG into POC matrix could significantly improve the bioactivity, stimulate osteogenic differentiation of mesenchymal stem cells and improve its mechanical performance (i.e., with 30 wt% BG, the Young's modulus of POC/BG composites increased to ×6.78 Mpa from 0.31-2 MPa) [16-18]. Nevertheless, these POC/BG composites are still mechanically far below the requirement for bone regeneration applications, especially for the load-bearing situations. Therefore, further improvements in mechanical performance are still needed. Generally, mechanical performance of composites can be improved either by increasing the content of inorganic materials before severe phase separation takes place, or by increasing the interactions between organic and inorganic moieties [19]. It is worth noting that the enhanced interactions between organic and inorganic moieties also help increasing the inorganic phase content which can be filled into the polymer matrix without causing severe macroscopic phase separation, for example, the strong chelating effect between Ca2+ and -COOH in POC allows for some inorganic particles, such as HA and calcium silicate, to be incorporated up to 65-70 wt% in POC-based composites [20, 21]. Because of the abundant calcium content in BG, it might also be possible to achieve a high BG content in POC/BG composites, and thus improving their mechanical performance.
In this study, a series of POC/BG composites were developed and evaluated. A novel phytic acid-derived BG with the composition of 10.8% P2O5-54.2% SiO2-35% CaO (mol%; referred to as PSC) was chosen because it shows excellent bioactivity and can maintain a stable pH when reacting with physiological solution [22]. The typical BG, 45S5 Bioglass®, was also used for comparison. The effects of BG on the chemical structure, mechanical properties and biological effect (both in vitro and in vivo) of these composites were studied.
POC pre-polymers were prepared by a polycondensation reaction between citric acid and 1, 8-octanediol (Scheme 1) [17, 20]. 45S5 Bioglass® was purchased from Schoot AG (Germany). PSC were prepared through a sol-gel method as described in our previous work [22]. The POC/BG composites were fabricated by incorporating BG with POC pre-polymers at different mass ratios (Table S1 in Supporting information) and curing under high temperature. As shown in Table S1, the POC/BG composites with different composition were referred to as P(10-x)BG(x). The detailed experimental methods to prepare and evaluate these materials are described in Supporting information. Regarding the possible reactions between the metal ions in BG and the carboxylic groups in pre-polymers, both ester bonds and calcium dicarboxylate bridges may form in these systems, and thus synergistically improving the mechanical properties.
|
Download:
|
| Scheme 1. Schematic diagram for design route and illustration of the POC/BG composites. | |
Firstly, the chemical structures of POC pre-polymers were initially investigated by 1H NMR and FTIR (Fig. S1 and Fig. S2 in Supporting information). As shown in the 1H NMR spectrum, the peak at δ 1.25 (s, 16H, Ha, Hb) was related to the methylene (a, b) from 1, 8-octanediol and the multiple peaks at δ 2.64-2.89 (m, 8H, He) were associated to citric acid. The δ 1.53 (m, 6H, Hc) and δ 3.96 (m, 6H, Hd) were assigned to methylene protons (c, d) of the prepolymers. Corresponding FTIR studies showed the presence of intense C=O stretch at 1735 cm-1 (Fig. S2), confirming the formation of ester bond (-COO-) in the pre-polymers. Consistent with previous reports [23, 24], these results indicated the formation of POC pre-polymers.
The elemental composition on the surface of 45S5 and PSC were examined by XPS. As expected, Si, O and Ca peaks were observed in both of them. The Na peaks were observed in 45S5 but not in PSC (Fig. 1A and Table S2 in Supporting information). According to the Ca2p photoelectron spectra (Fig. 1B), the binding energy of Ca2p (347.4 eV) in PSC is larger than that (346.8 eV) in 45S5, suggesting the higher electronegativity of Ca in PSC (i.e., higher binding energy to electrons or negatively charged species). The difference may affect the interactions between the BG and POC pre-polymers and ultimately the performance of POC/BG composites.

|
Download:
|
| Fig. 1. (A-B) XPS survey spectra of 45S5 and PSC. (C)The FTIR spectra of (a) PSC, (b) 45S5, (c) pure POC, (d) P5PSC5 and (e) P(5)45S5(5). | |
In the preliminary experiment, both 45S5 and PSC were used to composite with POC pre-polymers. Fig. S3 (Supporting information) represents the SEM evaluation of the composite surface morphology. It revealed a distribution of particles and aggregates incorporated into POC matrix. Correspondingly, the characteristic peaks of PSC, 45S5 and POC were apparent in FTIR spectra of the corresponding composites. While, new absorption bands at 1520-1680 cm-1 were present in the FTIR spectra of the composites but not present in the pure PSC, 45S5 or POC (Fig. 1C), which is assigned to the metallic carboxylate peaks of the calcium or sodium carboxylates [17, 25]. The results confirmed that there were strong interactions between the metal oxides in PSC or 45S5 and the carboxylic groups in the POC pre-polymer. Therefore, PSC or 45S5 was not simply a reinforcing filler, but also may act as ionic crosslinkers by forming the metallic carboxylate pairs in these composites.
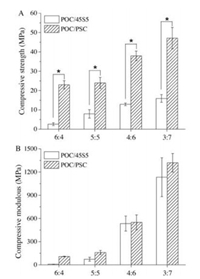
The mechanical performances of POC/BG are summarized in Fig. 2 and Fig. S4 (Supporting information). As expected, the compressive strength (Sc) and modulus (Ec) increased with BG content for both POC/PSC and POC/45S5. The Sc could be higher than 4 MPa and Ec higher than 100 MPa even for P6PSC4 or P(4) 45S5(6), fulfilling the requirements for human cancellous bone substitutes (Sc: 4-12 MPa; Ec: 0.1-2 GPa) [26, 27]. In the case of P3PSC7, the Sc was further improved to approximately 50 MPa and Ec 1.3 ± 0.1 GPa, which allows it for potential application in medium-load-bearing situations or to be used as bone fixation devices [20, 28]. It is interesting to note that at the same BG content, POC/PSC exhibited significantly higher mechanical strength compared with POC/45S5 (Fig. 2). This is presumably related to the metallic carboxylate groups formed in the composites, as observed in the FTIR spectra, because the former has higher calcium content on the surface.
|
Download:
|
| Fig. 2. (A) Compressive strengths and (B) modulus of the POC/BG composites (*P < 0.01). | |
The pure POC system is cross-linked by ester groups. While for POC/BG composites, besides the ester groups, the metallic carboxylate may also act as ionic crosslinkers, and thus synergistically improving the mechanical strength of the composites. The similar phenomena were also reported when POC was composited with HA, β-tricalcium phosphate and some other inorganic fillers [17, 29, 30]. According to XPS analysis, in the case of PSC, the Ca content on the surface was higher (Table S2, PSC: 6.8%, 45S5: 4.6%, about 50% higher) and the electronegativity of Ca also higher compared to 45S5, which may lead to more and stronger calcium dicarboxylate bridges in POC/PSC system. On the contrary, 45S5 has relatively less content of Ca and more importantly, large content of Na, which could interact with carboxylic acid groups and thus competing with the formation of the calcium dicarboxylate bridges. These may be the main reasons why the samples composited with PSC exhibited better mechanical strength than those with 45S5. In addition, previous studies have shown that when HA, an inorganic filler with even higher content of Ca (~21.7 mol%), was used, the POC/HA composites showed high compressive strength (i.e., the Sc of POC/HA composite with 50 wt% HA was 64 ± 9 Mpa) [20], further confirmed the effect of the calcium dicarboxylate bridges.
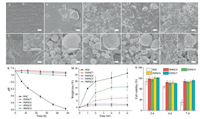
Based on these results, POC/PSC composites were chosen for further biological studies, because high mechanical strength of the substitutes are often expected to share load with surrounding bone tissues. It is generally accepted that the HA-formation ability is a significant characteristic for bioactive materials [31, 32], because through which the materials could bond with the surrounding bone tissues. It can be seen from Fig. 3 that no mineralization of HA was observed on the surface of pure POC after immersion in SBF (Fig. 3F). While, for the POC/PSC composites, hemispherical and needle like HA crystals appeared on their surface (Fig. 3G-J) [33]. Consistent with the SEM observation, new peaks at 607 cm-1 and 567 cm-1 appeared on the FTIR spectra after incubation in SBF (Fig. S5 in Supporting information), which could be the evidence of crystalline phosphate [32], further supporting the formation of HA. Therefore, POC/PSC composites were in vitro bioactive.
|
Download:
|
| Fig. 3. SEM images of surface morphologies of POC/PSC composites before (A-E) and after (F-J) immersion in SBF for 7 d. (A, F) pure POC; (B, G) P6PSC4; (C, H) P5PSC5; (D, I) P4PSC6; (E, J) P3PSC7; Scale bar = 2 μm; (K) The pH change of PBS during the samples immersion within 48 h; (M) the weight loss of them as a function of soaking time. (N) MC3T3-E1 cell viability after culturing with extracts of the samples for 2 d, 4 d and 7 d. | |
The pH changes of the PBS solution during the samples immersion under static conditions are shown in Fig. 3K. For pure POC, the pH value of the solution significantly decreased within 48 h. Interestingly, the pH of POC/PSC composites in the medium all remained relatively constant compared to pure POC, especially for the composites with ≥ 50 wt% PSC (fluctuation of pH < 0.15). This kind of phenomena is not unexpected, since the alkaline ions released by PSC could counteract the acidity provoked through POC degradation and keep the PBS solution neutralized. Noteworthy, the pH environment resulted from the degradation of biomaterial may exert a significant effect on cells or tissues. Too low pH may be detrimental to their biocompatibility.
Fig. 3M shows the weight loss profiles of POC/PSC composites over time. The data clearly revealed that the degradation rate decreased with PSC content in the composites. This could be mainly attributed to the relatively slow dissolution of PSC. Furthermore, the presence of PSC affected the type of crosslinking of the composites and pH of the medium, as shown above, and thus probably affecting the degradation rate.
MC3T3-E1 cells were used to evaluate biocompatibility of the composites in vitro. As shown in Fig. 3N, good cell viability was observed for all the samples after culturing for 2 d. However, at 4 d and 7 d, the cell viability of the composites was significantly higher in comparison with POC. The improved biocompatibility may be associated with the pH stability and the ions (i.e., silica and calcium ions) released by PSC, which has been reported to stimulate osteogenesis-related gene expressions and promote osteoblast proliferation [12].
According to what have mentioned above, P5PSC5 samples were selected for further study of the in vivo biocompatibility. Representative histological sections of the P5PSC5 samples after post-operation are depicted in Figs. S6 and S7 (Supporting information). No evidence of inflammation around the implant was found at all time-points. After 4 w, newly formed bone tissue was observed in the defect edge and the sample showed partial degradation (Fig. S6-8 in Supporting information). After implantation for longer time, more trabecular bone formed and some of bone trabecular grow into the implanted materials, connecting compactly with the materials. The results suggest that P5PSC5 integrated well with surrounding tissues. Moreover, the trabecular bone formation of P5PSC5 group was significantly higher than that of the control group, indicating the good potential of POC/PSC composites to stimulate bone regeneration. The capability of inducing bone generation may partly be attributed to the dissolution products (i.e., soluble silica and calcium ions) of PSC, which has been proved to simulate bone regeneration by many previous studies [8-10, 12]. According to Fig. S6, it can be seen that the samples degraded with implantation time, which provided more and more space for the growth of new bone, and thus also contribute to the bone regeneration.
In summary, POC/BG composites were successfully prepared by using 45S5 Bioglass® and PSC. It was found that at the same BG content, the POC/PSC composites exhibited significantly better mechanical properties in comparison with POC/45S5 composites. This is presumably owing to the strong interactions between calcium in BG and -COOH in POC pre-polymer, which resulted in calcium dicarboxylate bridges in POC/PSC systems. in vitro assays revealed that all POC/PSC composites showed much better in vitro bioactivity and cell viability compared to pure POC. Histological analysis from POC/PSC samples implanted in femoral condyle defects of Sprague-Dawley rats suggested that it integrated well with surrounding tissues and supported the regeneration of bone tissue. Altogether, POC/PSC composites are promising materials for bone repairing in terms of mechanical property and biomedical performance.
AcknowledgmentsThis work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB12020300) and National Natural Science Foundation of China (No. 31370985).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.014.
| [1] |
G.M. Calori, E. Mazza, M. Colombo, C. Ripamonti, Injury 42(2011) S56-S63. |
| [2] |
J.Q. Wei, Y. Liu, X.H. Zhang, et al., Chin. Chem. Lett. 28(2017) 845-850. DOI:10.1016/j.cclet.2017.01.008 |
| [3] |
R. De Santis, A. Russo, A. Gloria, et al., J. Biomed. Nanotechnol. 11(2015) 1236-1246. DOI:10.1166/jbn.2015.2065 |
| [4] |
M. Yang, G. Zhou, H. Castano-Izquierdo, Y. Zhu, C. Mao, J. Biomed. Nanotechnol. 11(2015) 447-456. DOI:10.1166/jbn.2015.2038 |
| [5] |
J. Yang, A.R. Webb, G.A. Ameer, Adv. Mater. 16(2004) 511-516. DOI:10.1002/(ISSN)1521-4095 |
| [6] |
R.T. Tran, J. Yang, G.A. Ameer, Annu. Rev. Mater. Res. 45(2015) 277-310. DOI:10.1146/annurev-matsci-070214-020815 |
| [7] |
M.C. Serrano, E.J. Chung, G.A. Ameer, Adv. Funct. Mater. 20(2010) 192-208. DOI:10.1002/adfm.v20:2 |
| [8] |
J.R. Jones, Acta Biomater. 9(2013) 4457-4486. DOI:10.1016/j.actbio.2012.08.023 |
| [9] |
E. Zeimaran, S. Pourshahrestani, I. Djordjevic, et al., Mater. Sci. Eng. C 53(2015) 175-188. DOI:10.1016/j.msec.2015.04.035 |
| [10] |
Y.S. Sun, A.L. Li, F.J. Xu, D. Qiu, Chin. Chem. Lett. 24(2013) 170-172. DOI:10.1016/j.cclet.2013.01.009 |
| [11] |
Y. Li, Q. Hu, G. Miao, et al., J. Biomed. Nanotechnol. 12(2016) 863-877. DOI:10.1166/jbn.2016.2235 |
| [12] |
L.L. Hench, J. Mater. Sci.:Mater. Med. 17(2006) 967-978. DOI:10.1007/s10856-006-0432-z |
| [13] |
A.R. Boccaccini, M. Erol, W.J. Stark, et al., Compos. Sci. Technol. 70(2010) 1764-1776. DOI:10.1016/j.compscitech.2010.06.002 |
| [14] |
S.K. Ghosh, S.K. Nandi, B. Kundu, et al., J. Biomed. Mater. Res. Part B 86(2008) 217-227. |
| [15] |
D.L. Wheeler, M.J. Montfort, S.W. McLoughlin, J. Biomed. Mater. Res. 55(2001) 603-612. DOI:10.1002/(ISSN)1097-4636 |
| [16] |
E. Zeimaran, S. Pourshahrestani, I. Djordjevic, et al., J. Mater. Sci.:Mater. Med. 27(2016) 18. DOI:10.1007/s10856-015-5620-2 |
| [17] |
E. Zeimaran, S. Pourshahrestani, B. Pingguan-Murphy, et al., J. Mater. Sci. 50(2015) 2189-2201. DOI:10.1007/s10853-014-8782-2 |
| [18] |
E. Zeimaran, S. Mohan, S. Pourshahrestani, et al., Mater. Des. 109(2016) 434-442. DOI:10.1016/j.matdes.2016.07.096 |
| [19] |
A. Li, H. Shen, H. Ren, et al., J. Mater. Chem. B 3(2015) 1379-1390. DOI:10.1039/C4TB01776E |
| [20] |
H. Qiu, J. Yang, P. Kodali, J. Koh, G.A. Ameer, Biomaterials 27(2006) 5845-5854. DOI:10.1016/j.biomaterials.2006.07.042 |
| [21] |
F.S. Shirazi, E. Moghaddam, M. Mehrali, et al., J. Biomed. Mater. Res. Part A 102(2014) 3973-3985. DOI:10.1002/jbm.a.v102.11 |
| [22] |
A.L. Li, D. Qiu, J. Mater. Sci.:Mater. Med. 22(2011) 2685-2691. DOI:10.1007/s10856-011-4464-7 |
| [23] |
J. Yang, A.R. Webb, S.J. Pickerill, G. Hageman, G.A. Ameer, Biomaterials 27(2006) 1889-1898. DOI:10.1016/j.biomaterials.2005.05.106 |
| [24] |
Y. Du, J. Ge, Y. Shao, et al., J. Mater. Chem. B 3(2015) 2986-3000. DOI:10.1039/C4TB02089H |
| [25] |
S.L. Liang, W.D. Cook, G.A. Thouas, Q.Z. Chen, Biomaterials 31(2010) 8516-8529. DOI:10.1016/j.biomaterials.2010.07.105 |
| [26] |
S. Bose, M. Roy, A. Bandyopadhyay, Trends Biotechnol. 30(2012) 546-554. DOI:10.1016/j.tibtech.2012.07.005 |
| [27] |
C. Wang, H. Shen, Y. Tian, et al., ACS Appl. Mater. Interfaces 6(2014) 13061-13068. DOI:10.1021/am5029582 |
| [28] |
E. Saiz, E.A. Zimmermann, J.S. Lee, U.G. Wegst, A.P. Tomsia, Dent. Mater. 29(2013) 103-115. DOI:10.1016/j.dental.2012.08.001 |
| [29] |
Y. Jiao, D. Gyawali, J.M. Stark, et al., Soft Matter. 8(2012) 1499-1507. DOI:10.1039/C1SM05786C |
| [30] |
F. Chen, Z. Song, L. Gao, H. Hong, C. Liu, J. Mater. Chem. B 4(2016) 4198-4205. DOI:10.1039/C6TB00435K |
| [31] |
Y.S. Sun, A.L. Li, H.H. Ren, et al., Chin. Chem. Lett. 27(2016) 579-582. DOI:10.1016/j.cclet.2016.02.018 |
| [32] |
A.J. Salinas, M. Vallet-Regí, RSC Adv. 3(2013) 11116-11131. DOI:10.1039/c3ra00166k |
| [33] |
Y. Zhang, W.L. Gao, Z.Y. Liu, et al., Chin. Chem. Lett. 27(2016) 1091-1096. DOI:10.1016/j.cclet.2016.03.035 |
 2017, Vol. 28
2017, Vol. 28 


