Organic solar cells (OSCs) have attracted considerable attention because of their easy fabrication of low-cost, light-weight, flexible and large-area devices with a wet process, such as spin-coating and roll-to-roll printing technologies, etc. [1, 2]. Traditionally, the active layer of OSCs consists of a polymer or small molecule as the donor material and a fullerene derivative as the acceptor material, respectively. In the past decades, high-performance donor materials were deeply studied by fine-tuning the molecular and electronic properties to match the requirements of fullerene acceptors well. The commonly used acceptor materials were mainly based on phenyl-C61-butyric acid methyl ester (PC61BM), phenyl-C71-butyric acid methyl ester (PC71BM), indene-C60 bisadduct (ICBA) and indene-C70 bisadduct (IC70BA), which had strong electron affinity, ultrafast photo-induced separation and transfer and high isotropic electron mobility [3-6]. The power conversion efficiency (PCE) of fullerene-based OSCs has already exceeded 10% both in single-junction and tandem cells [7, 8]. However, fullerene acceptors have some intrinsic drawbacks, such as low absorption in the UV-visible region, difficult to modify their chemical structures and energy levels, and expensive for largescale production and purification. Therefore, non-fullerene acceptors (NFAs) have been put forward recently to overcome the limitations of fullerene derivatives. With the rapid development in the past few years, the PCE of NFA-based OSCs have reached more than 12%, which implied that NFAs can replace fullerene derivatives as a new generation of efficient acceptor materials [9].
Similar to donor materials, NFAs could also be divided into two types: polymers (P-NFAs) and small molecules (SM-NFAs), which should have the strong electron-deficient units in the conjugated backbones. Some good candidates of P-NFAs were reported typically with the acceptor building blocks of naphthalene diimide (NDI), perylene diimide (PDI), B N bridged bipyridine (BNBP) and so on [10-13]. In addition, PDI, NDI, fluoranthene-fused imide, diketopyrrolopyrrole (DPP), and 3, 9-bis[2-(3-oxo-2-vinyl-2, 3-dihydroinden-1-ylidene)-malononitrile have also been used to design the high-performance SM-NFAs [14-17]. From the literatures, PDI derivatives are the most popular acceptor materials, since Tang et al. reported the first non-fullerene OSC in 1986 using a single PDI derivative (PV) as the acceptor material, which exhibited a relatively low PCE of 1% [18]. PDI has a large and rigid planar π-conjugated plane, leading to excellent structural stability, strong electron-accepting ability and high electron mobility (>1 cm2 V-1 s-1) in their functional materials [19-21]. The structure of PDI can be modified by introducing alkyl chains at the N-positions to enhance the solubility and introducing aryl substituents at its α/β-positions to adjust the electronic and optical properties. However, the device performance of PDI-based OSCs is still lagging behind that of fullerene-based counterparts, which is mainly limited by its strong self-aggregation to form microscale or sub-microscale phase separation in its blend films. Many strategies have been used to resolve this issue. For monomeric PDI derivatives, introducing aryl substituents at the α/β-positions would break the self-aggregation to some extent. For PDI dimers with a conjugated bridge, variable core units, such as benzene, thiophene, selenophene and their fused structures, were inserted between two PDI groups to form a twisted conformation and suppress the self-aggregation of PDI efficiently. Another way to suppress the self-aggregation of PDI is to develop threedimensional (3D) molecular structures. For example, Zhan et al. reported a star-shaped PDI acceptor of S(TPA-PDI), which exhibited a quasi-3D nonplanar structure and isotropic optical and charge transfer properties [22]. By using this design strategy, more and more 3D high-performance PDI derivatives have pushed forward the PCEs of NFA-based OSCs to over 9% [23-25].
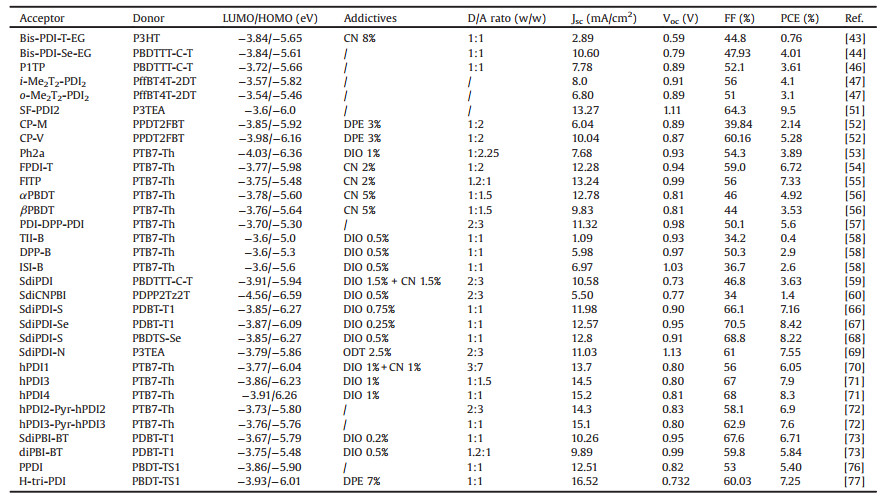
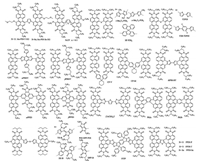
Here, we review the research activities of PDI-based small molecular acceptors for OSC applications, by the sequence of monomeric PDI, linear PDI dimers, trimers and oligomers with/ without a conjugated bridge, and PDI trimers and tetramers with a 3D structure. We also highlighted different structural modification effect on the inherent properties and the final device performance of PDI derivatives, which would help us to understand deeply the structure-property relationships of PDI-based SM-NFAs for realizing highly efficient OSCs. The chemical structures of the related donor materials are listed in Fig. 1.
|
Download:
|
| Fig. 1. Chemical structures of the related donor materials in this review | |
2. Monomeric PDI derivatives as the acceptors
In fact, PDI was first used as an acceptor material in OSCs in 1986 by Tang et al. [18]. Double-junction OSCs fabricated with PV as the acceptor layer and copper phthalocyanine (CuPc) as the donor layer had achieved a PCE of 1%. As mentioned above, the serious self-aggregation of PDI induced the low efficiency in that work. In 2000, Dittmer et al. used MEH-PPV as the donor material and a soluble PDI as the electron acceptor to fabricate OSC devices for exploring the formation of crystal network [26]. PDI was blended later with HBC-PhC12, delivering a higher PCE of 1.95% with a short-circuit current density (Jsc) of 7.07 mA/cm2, open-circuit voltage (Voc) of 0.69 V and fill factor (FF) of 40% [27]. In 2009, Xie et al. reported another bulk-heterojunction (BHJ) OSCs using P3HT:PDI blend as the active layer, which exhibited a relatively low PCE of 0.25% due to the strong self-aggregation of PDI and unmatched energy levels between P3HT and PDI [28]. In 2013, Bazan et al. employed p-DTS(FBTTh2)2 as the donor material, which exhibited a good complimentary absorption with PDI, leading to a very broad coverage of visible spectrum within 300-800 nm [29]. Thus, the PCE of 3.0% was finally obtained after device optimization. When adding the processing additive of DIO, the PCEs of p-DTS(FBTTh2)2: PDI devices were further elevated to 3.1% [30]. Subsequently, Zhan et al. optimized the D/A ratio of p-DTS(FBTTh2)2:PDI to 1.3:1, and the much higher PCE of 5.13% was achieved [31]. Recently, Keivanidis et al. used PBDTTT-C-T as the donor material and PDI as the acceptor material, the related OSCs showed a PCE up to 3.71% [32]. Yao et al. synthesized a series of monomeric PDI derivatives (O-PDI-O, B-PDI-O and B-PDI-B) by attaching the 2-methoxylethoxyl side chain and BDT unit in turn on the PDI skeleton [33, 34]. B-PDI-B exhibited a broad absorption ranging from 650 nm to 800 nm in solid state because of the photoinduced intramolecular charge transfer (ICT) from the BDT segments to the PDI core. The enhanced absorption promoted the usage of near IR photons. In addition, the steric hindrance between the PDI and BDT units formed a twisted structural conformation, which effectively suppressed the aggregation tendency of PDI and resulted in a reduced domain size going from 0.5 mm to 20 nm. When using P3HT as the donor material, B-PDI-B devices showed a PCE of 1.66%.
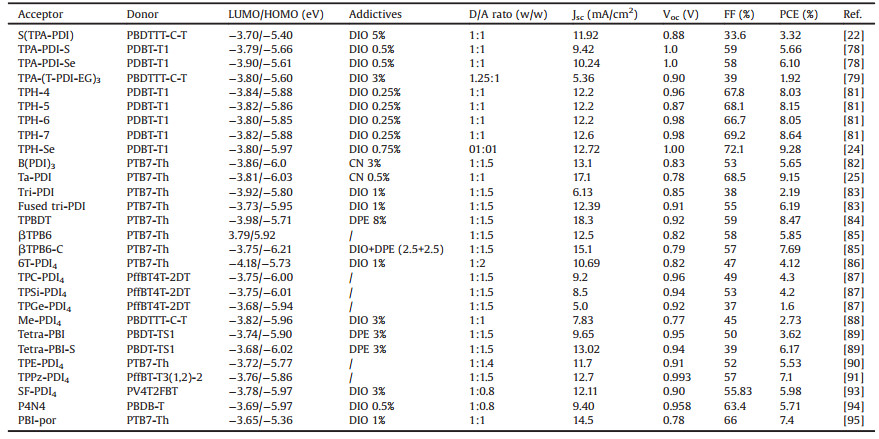
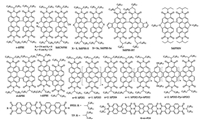
Modification of PDI with aromatic groups at the bay positions was another method to develop efficient monomeric PDI acceptors. Marks et al. synthesized a series of PDI derivatives, hexyl-PDI, phenethyl-PDI, and phenyl-PDI, which were substituted with hexyl, phenethyl and phenyl at the 2, 5, 8, 11-positions of PDI skeleton, respectively [35]. All the molecules exhibited slipstacking in the solid state, thus preventing the coupling necessary for rapid excimer formation. Although these materials underwent efficient charge separation, PBTI3T:phenyl-PDI had the matched energy levels, intermediate crystallinity and the optimized domains. Geminate recombination was observed in phenyl-PDI devices with the highest PCE of 3.67%. Sun et al. synthesized a twisted PDI monomer of TP-PDI, in which four phenyl groups were appended at the bay positions of PDI skeleton [36]. TP-PDI exhibited a weak aggregation in the solid state, a broad but strong absorption in the visible region from 400 nm to 700 nm, and a deep LUMO level of -3.82 eV. The OSCs based on PTB7-Th:TP-PDI achieved a high PCE of 4.1% when adding 1% of CN as the additive. Zheng et al. reported a series of ortho-functionalized PDI acceptors of PDI-4Mes, PDI-4Th, PDI-2S-4Mes and PDI-2S-4Th by 1, 4-addition of aryl Grignard reagents, which provided a new avenue to directly functionalize the 2, 5, 8, 11-positions of PDI unit with different aryl substituents [37]. The PTB7-Th:PDI-2S-4Th devices showed a PCE as high as 5.19%, that was a new record for single PDI acceptors in OSCs. With different functional groups of pyrene and tert-butyl pyrene, Su et al. reported two SMAs of P1 and P2 [38]. These two acceptors possessed a broad absorption covering the wavelength range of 400-650 nm and a relatively high LUMO energy level of -3.77 eV. When using PTB7 as the donor material, the PCE of the OSCs based on P2 was only 0.41%, while a higher PCE of 1.35% was obtained for P1 devices with the same D/A ratio (1:1). Sharma et al. synthesized a symmetrical perylene-anthracene diimide acceptor of P-A with the tert-butylphenoxy side chains at the 1, 7-bay positions of PDI skeleton. A PCE of 2.85% was achieved when using BTD-TNP as the donor material [39]. By replacing anthracene with pyrene, Sharma et al. reported a perylene-pyrene bisimide acceptor of PPI later [40]. A PCE was decreased to 1.87% when the blend ratio was optimized to be 1:3.5 for donor (T) and PPI. However, a thin ZnO layer was inserted between the active layer and the top Al electrode, the corresponding PCE was elevated to 2.46%, which would thank for the enhanced light absorption because of the optical interference between incident light and reflected light at the Al electrode. The PCE was further increased to 3.17% when the device with the ZnO layer was annealed at 100 ℃ for 5 min. Employing acetonaphthopyrazine dicarbonitrile as functional moieties, the same group synthesized another symmetrical PDI acceptor of PERI [41]. The maximum absorption of PERI film peaked at 455 nm and the absorption onset was observed at 583 nm, corresponding to a large optical bandgap of 2.13 eV. PERI:Se-SM devices showed a low PCE of 1.28%, which was improved to 3.88% when the devices were treated with the thermal annealing process. Kozma et al. reported three PDI acceptors of PDI-SF, PDI-BSF and PDI-BT, with the conjugated bithiophene, spirofluorene and bithiophene spirobifluorene groups at the bay positions [42]. The OSC devices based on P3HT and PDI-SF showed the PCE of 1.58%, while the relatively lower PCE values of 1.18% and 0.81% were achieved for the cases from PDI-BSF and PDI-BT. The reason was that the bithiophene units attached on the PDI skeletons in PDI-BSF and PDI-BT lowered the HOMO-LUMO energy gap and thus generated a spectral overlap between the acceptor and the donor materials. The chemical structures of monomeric PDI acceptors are listed in Fig. 2.
|
Download:
|
| Fig. 2. Chemical structures of the monomeric PDI acceptors. | |
3. Linear PDI derivatives as the acceptors 3.1. PDI dimers with a bridged core
Beside the monomeric PDIs, PDI dimers are also received wide attention as acceptor materials in OSCs. Two PDI units in PDI dimers are often separated by the conjugated bridge to decrease the self-aggregation of PDI backbone. Yao et al. reported a PDI dimer of bis-PDI-T-EG bridged with an oligothienyl group [43]. When blending with PBDTTT-CT donor and adding 5% of DIO additive, bis-PDI-T-EG devices showed a PCE of 4.03% [33]. After that, the same group replaced the thiophene bridge with selenophene candidate, and synthesized the similar PDI dimer of bis-PDI-Se-EG [44]. Also using the same polymer donor of PBDTTT-C-T, bis-PDI-Se-EG devices showed a comparable PCE of 4.01%. Later, they further improved the device performance by optimizing the fabrication condition, such as adjusting the additive content, using the solvent vapor annealing (SVA) possess [45]. Finally, the recombination of the devices was suppressed efficiently and the electron mobility was enhanced at the same time, which promoted the PCE from 1.44% to 6.08%. Zhan et al. also reported a series of PDI dimmers (PnTP, n = 0-3) with the oligothiophene as the separated bridge [46]. PnTP showed the maximum absorptions within 538-570 nm in solution, which would be enhanced and red-shifted with increasing the numbers of the thiophene unit. P0TP exhibited the lowest HOMO (-5.74 eV) and LUMO (-3.84 eV) levels. The increase of thiophene numbers could up-shift the HOMO and LUMO energy levels of these PDI dimers, which were attributed to the strong electron-donating feature of the oligothiophene bridge. When blending P(0-3)TP with a low bandgap polymer donor of PBDTTT-C-T, the PSCs exhibited decreased device performance with increasing the number of thiophene units. Thus, PCEs of 3.61%, 0.79% and 0.91% were achieved for P1TP-, P2TP-and P3TP-based OSCs. Yan et al. reported two bithiophene-bridged PDI dimers (iMe2T2-PDI2 and oMe2T2-PDI2), on which two simple methyl groups were attached at different positions of thiophene banckbone [47]. The dihedral angles between the two PDI planes were about 60° for i-Me2T2-PDI2 and 90° for o-Me2T2-PDI2, which caused a smaller domain size of i-Me2T2-PDI2 blend than o-Me2T2-PDI2 counterpart when blending with the same polymer donor of PffBT4T-2DT. Therefore, i-Me2T2-PDI2 devices achieved a higher PCE of 4.1% than that of o-Me2T2-PDI2 devices (3.1%). Jung et al. reported two NF-NFAs of F2BT2PDI and T2PDI linked by the bridge of 2, 5-difluorobenzene (F2B) or bithiophene [48]. Compared with T2PDI, F2B-T2PDI had a more rigid core and enhanced molecular packing property. The fluorination down-shifted the energy levels to prevent backtransfer of holes from the acceptor to the cathode and enhanced the complementary absorption with the donor polymer of PTB7-Th. Because of the geometric and energetic effects from 2, 5-difluorobenzene (F2B) moiety, the PCE of 5.05% was achieved for F2B-T2PDI-based devices, whereas that was 3.63% by using T2PDI. Zhao et al. reported a series of PDI dimers with various arylene linkers [49]. Using P3HT as the donor, the PCE of 2.35% was achieved for spirobifluorene-linked SF-PDI2. Subsequently, Yan et al. employed SF-PDI2 as SM-NFAs and a novel polymer of PffBT4T-2DT as donor material to fabricate more efficient solar cells [50]. The resulting devices showed an impressively large Voc of 0.98 V and a high PCE of 6.3%. The Voc loss of this device was only 0.67 V, which was among the lowest values of Voc losses for OSCs. By fine-tuning the energy levels of polymer donors, the same group synthesized a new polymer donor of P3TEA, which exhibited only 0.05 eV LUMO energy level offset with SF-PDI2, giving rise to a high Voc of 1.11 V and a low Voc loss of 0.61 V. Combining with the high Jsc of 13.27 mA/cm2 and an FF of 64.3%, the PCE was further elevated to 9.5%, which is the best value for PDI-based single-junction OSCs [23]. To improve the light harvesting property, they used P3TEA: SF-PDI2 as the active layer and fabricated a homo-tandem device with a mild thermal annealing treatment. The PCE was finally improved to 10.8% with a high Voc of 2.13 V [51]. Choi et al. reported the M-and V-shaped PDI dimers of CP-M and CP-V with a nonconjugated 1, 1-diphenylcyclohexane bridge [52]. CP-V could mix well with PPDT2FBT and maintained the appropriate domains, which could facilitate exciton dissociation/diffusion and provide suitable percolation pathways for charge transfer. Therefore, in comparison with CP-M cells, the CP-V devices displayed a better performance with a PCE of 5.28%.
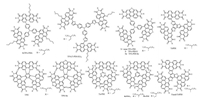
Ph2a was a ring-fused compound with benzene as the central core [53]. The ring-fusion resulted in increased electronic coupling between the two PDI units, which could increase its electron mobility. The blend of Ph2a and PTB7-Th showed a significantly decreased recombination. Thus, the Jsc was increased from 1.54 mA/cm2 to 7.68 mA/cm2 and the PCE was also elevated from 0.23% to 3.89%. When fused with heterocycles at the bay positions of PDI, such as furan, thiophene and selenophene, a series of nonplanar fused PDI analogues of FPDI-F, FPDI-T, and FPDI-Se were prepared by Jen's group [54]. Compared to the non-fused counterparts, the fused PDI dimers showed the reduced reorganization energy and extended π-conjugation, which was helpful for exciton diffusion and charge transfer. In addition, by changing the chalcogen atoms from S to O and Se, FPDI-F and FPDI-Se exhibited the similar absorptions and energy levels with FPDI-T, while their molecular geometries could be optimized by varying the atomic size in the sequence of O < S < Se. With increasing the chalcogen atomic size (O < S < Se), the twist angles between two PDI planes would be enlarged from 15.8× (FPDI-F) to 24.4° (FPDI-T) and 27.2° (FPDI-Se). FPDI-T had a modest twist angle but balanced above mentioned factors, resulting in the highest carrier mobilities and the best PCE of 6.72%. Chen et al. reported another SM-NFA of FITP, in which two PDI units were fused with the indacenodithieno[3, 2-b]thiophene (IDTT) central core [55]. This fully fused backbone made FITP possess superior charge carrier transfer property and elevated LUMO level. As a result, the OSCs based on FITP and PTB7-Th exhibited a high PCE of 7.33% with a high Voc of 0.99 V. Yu et al. reported four PDI dimers of αPPID, αPBDT, βPPID and βPBDT, which were linked by the core of pyrene diimide (PID) or benzo [1, 2-b:4, 5-b']-dithiophene (BDT) at the ortho-or bay-position of PDI skeleton, respectively [56]. Because of the good planarity and strong π-stacking feature of ortho-functionalized PDI, αPBDT and αPPID exhibited a strong aggregation tendency, which endowed these molecules with high electron mobility. When blending with PBT7-Th, the inverted OSCs achieved the PCE of 4.92% for αPBDT and 3.61% for αPPID, which were 39% and 4% higher than those of the βPBDT and βPPID. At the same time, Welch et al. incorporated thienoisoindigo (TII), diketopyrrolopyrrole (DPP) and isoindigo (ISI) to N-annulated PDI, and reported a series of PDI dimers of PDIDPP-PDI, TII-B, DPP-B and ISI-B [57, 58]. It was found that the DPPbased acceptor of DPP-B outperformed the other derivatives, demonstrating an energy loss of less than 0.6 eV and a promising efficiency of 4.1%. Blakey et al. synthesized a new PDI-thiazolebased NFA ((TsCDI)2T) with a thiophene bridge, by KOtBu-initiated aryl C-H iodination [59]. Using PTB7-Th as donor, the resulting OSCs based on (TsCDI)2T showed a PCE of 4.7%. The chemical structures of linear PDI dimers with a bridged core are listed in Fig. 3.
|
Download:
|
| Fig. 3. Chemical structures of linear PDI dimers with a bridged core. | |
3.2. PDI dimers and trimers without a bridged core
In order to obtain the twisted conformation, two PDI units can be covalently linked by just a single carbon-carbon bond to afford the PDI dimer of s-diPBI. Wang et al. first synthesized s-diPBI by the one-step homocoupling of halogenated PDIs in the presence of copper powder as catalyst [60]. It showed a broad absorption in the range of 400 nm to 600 nm with a LUMO level was -3.91 eV. When blending with the donor of PBDTTT-C-T, the PCE was 3.63%. Hou et al. replaced PBDTTT-C-T with PBDTBDD as the donor material, the PCEs could be improved up to 4.39% [61]. The better device performance of PBDTBDD:s-diPBI system was originated from the improved exciton dissociation efficiency, enhanced crystallinity and optimized nanophase separation. Based on the blend of PTB7-Th and s-diPBI, Jen et al. fabricated the inverted device configuration with a SAM-modified ZnO interface, the PCE was further elevated to 5.90% [62]. They also studied the molecular geometry effect of PDI dimers (s-diPBI and PPDI) on blend morphology and photovoltaic performance [63]. The results indicated that the more flexible and twisted structure of s-diPBI could disrupt the π-π stacking of PTB7-Th, leading to the lower electron and hole mobilities than those of PTB7-Th:PPDI. In addition, PPDI acceptor could not only mix well with PTB7-Th, but also maintain appropriate aggregation domains for more efficient exciton dissociation and suitable percolation pathways for faster charge transfer. Thus, the PCE of PTB7-Th:PPDI device was improved finally to 6.41%. In order to improve the performance of PTB7-Th:sdiPBI devices, an amine-functionalized PDI (PDIN) was also used as the electron-transporting layer. PDIN possessed the respectable charge-transporting properties and possible interfacial doping capability to the PDI-based acceptors, which was positive for improving the charge transport and extraction efficiency. Therefore, the PCEs of PTB7-Th:s-diPBI devices were increased from 5.52% to 6.29% [64]. Li et al. introduced the cyan group onto PDI skeleton and synthesized a PDI dimer of SdiCNPBI, which exhibited a deep LUMO of -4.56 eV [65]. When using the PDPP2TzT and PDPP2Tz2T as the donors, the devices showed the relatively low PCE of 1.4% for PDPP2Tz2T:SdiCNPBI and 0.72% for PDPP2TzT: SdiCNPBI, respectively.
Introduction of heteroatoms would enhance the intermolecular interactions through van der Waals and heteroatom-heteroatom interactions, which could improve the device performance of the corresponding semiconducting materials based on fused or extended heteroarenes. Wang et al. modified s-diPBI by inserting S or Se atoms in the bay positions and reported two novel NF-NFAs of SdiPBI-S and SdiPBI-Se [66, 67]. These molecules had a more twisted molecular configuration and a higher LUMO level than sdiPBI. The PDI subunits in SdiPDI-S or SdiPDI-Se were almost perpendicular to each other, with the dihedral angles of 80° and 77°, respectively. When blending with the wide bandgap polymer of PDBT-T1, the devices showed PCEs of 7.16% and 8.4% for SdiPBI-S and SdiPBI-Se. Sun et al. used PBDTS-Se as the donor material and SdiPBI-S as the acceptor material to fabricate another highperformance device with a PCE of 8.22% [68]. Later, Welch et al. found that incorporation of a nitrogen heteroatom (so called "N-annulation") at the bay position of PDI would increase structural diversity through modulation of the N-R functional group, thus allowing for the tailored self-assembly [69]. Through such N-alkyl chain modification, they reported a PDI dimer of S-diPDIN, which obtained a PCE of 3.13% when blended with PTB7. The PCE was further increased to 5.54% and 7.55% by using another two excellent donor polymers of PTB7-Th and P3TEA, respectively.
The adjacent PDI units could be fused with a two-carbon bridge, which was first reported by Nuckolls's group as the highperformance NFA materials. A series of helical PDI molecules (hPDI1, hPDI3 and hPDI4) with different numbers of PDI units were synthesized for comparison [70, 71]. All of them showed a relative high electron mobility, strong absorption within 350 nm and 550 nm, good electron-accepting ability, and suitful LUMO level (ca. -4 eV). The strong aggregation of PDI backbone was efficiently suppressed due to their twisted molecular conformations. Thus, the high PCEs of PTB7:hPDI1, PTB7-Th:hPDI3 and PTB7-Th:hPDI4 devices were obtained as 5.21%, 7.9% and 8.3%, respectively. Subsequently, the same group reported two nanoribbon-like PDI derivatives (hPDI2-Pyr-hPDI2 and hPDI3-Pyr-hPDI3), which were synthesized by fusing two electron-deficient PDI trimers with an electron-rich alkoxy-modified pyrene central core [72]. The rigidity of the molecular backbone yielded a sharply red-shifted absorption edge. The non-fullerene OSCs fabricated with hPDI2-Pyr-hPDI2 and hPDI3-Pyr-hPDI3 showed PCEs of 6.9% and 7.6% without any optimization. Yang et al. reported two novel PDI dimers (SdiPBI-BT and diPBI-BT), with fusing a thienobenzene unit at the bay region of the PDI skeleton [73]. The resulting PDI dimers exhibited a higher-lying LUMO level and an extended conjugation system. As a result, a PCE of 6.71% was achieved with a high Voc of 0.95 V, a Jsc of 10.31 mA/cm2 and a high FF of 68.7% from PDBT-T1: SdiPBI-BT devices.
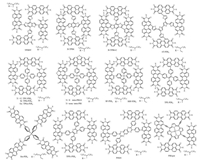
Another effective method to disrupt the planarity and reduce the aggregation of the PDI derivatives is to link the corresponding PDI subunits through the N-N bond at amide position [72-75]. Narayan et al. used TP as the acceptor material and PBDTTT-C-T as the donor material and reported the related non-fullerene OSCs with a PCE of 3%, which was 10-fold increase in Jsc in comparison with the counterpart based on monomeric PDI [75]. Hou et al. reported an N-N-linked PDI derivative (PPDI), which used C5H11 instead of C7H15 in TP [76]. Benefiting from the enhanced interchain π-π stacking of the polymer donor, the PBDT-TS1:PPDI blend showed a relatively higher and symmetric hole and electron mobility. Thus, a high PCE of 5.58% (certificated as 5.40%) was obtained in the fabricated fullerene-free OSCs based on PBDTTS1: PPDI. Jen et al. recently also reported a high-performance OSCs with a PCE of 6.41% by using PTB7-Th:PPDI as the active layer [62]. Later, Hou et al. synthesized a SM-NFA of H-tri-PDI, which composed of three PDI units connected together via N-N linkage [77]. The PBDTTS1:H-tri-PDI devices showed a PCE as high as 7.25%, benefiting from the appropriate energy level alignment and the favorable morphology of the active layer, which was the highest value in this type of PDI derivatives. The chemical structures of linear PDI dimers without a bridged core are listed in Fig. 4. The photovoltaic parameters of the OSCs based on linear PDIs as the acceptors are also provided in Table 1.
|
Download:
|
| Fig. 4. Chemical structures of linear PDI derivatives without a bridged core. | |
|
|
Table 1 The photovoltaic parameters of the OSCs based on linear PDIs as the acceptors. |
4. Three-dimensional (3D) PDI derivatives as the acceptors 4.1. 3D PDI trimers
Apart from linear PDI acceptors, three dimensional (3D) PDI SMAs linked with different cores have also been explored. Lin et al. reported a star-shaped PDI trimer of S(TPA-PDI) with a triphenylamine (TPA) core [22]. S(TPA-PDI) had the isotropic optical and charge-transporting properties due to the sp3 hybrid orbital of nitrogen atom of TPA. In addition, S(TPA-PDI) exhibited weak molecular aggregation, a strong absorption in the visible region and appropriate energy levels, which could be matched well with the used low bandgap polymer donor of PBDTTT-C-T. A maximum PCE of 3.45% was then achieved based on PBDTTTC-T:S(TPA-PDI) (1:1, w/w) blend adding 5% of DIO. By introducing S or Seannulated PDI as the peripheral groups, Yang et al. synthesized two novel star-shaped SMAs of TPA-PDI-S and TPA-PDI-Se, the PCEs were further elevated to 6.10% [78]. Lu et al. also synthesized a TPAbased acceptor of TPA(T-PDIEG)3, which showed edge-to-face type of π-π stacking in the corresponding solid films. The PCE of 1.92% was obtained by adding 3% of DIO when using PBDTTT-C-T:TPA(TPDI-EG)3 as the active layer [79].
Wang et al. reported C3-symmetric PDI trimers of triperylene hexaimides (TPH) and selenium annulated triperylene hexaimides (TPH-Se), which consisted of three PDI subunits or selenium annulated PDI subunits adjoined at a single benzene ring [24]. Both small molecules had a twisted three-bladed propeller configuration with the compact 3D network assembly. They also showed the broad and strong absorption in the region within 300-600 nm, together with suitable LUMO levels of about -3.8 eV. The integration of selenophene onto TPH skeleton endowed TPH-Se with a more distorted propeller configuration and a more compact 3D network assembly because of the Se···O interactions. Using TPH and TPH-Se as the acceptor materials, the related nonfullerene OSCs showed the high PCEs of 8.28% and 9.28%, respectively. The same group further employed TPH as the acceptor material to fabricate all small molecular OSCs using DRCN5T as the donor material, which exhibited a PCE of 6.16% [80]. To study the length effect of branched alkyl side chain on the photovoltaic performance of TPH, Wang et al. designed and synthesized a series of propeller-shaped triperylene hexaimides (TPH-4, TPH-5, TPH-6, TPH-7) as the NFAs for OSC applications [81]. The physical properties, thin-film microstructure, molecular packing, charge transport and resulting photovoltaic properties of these materials have been systematically investigated in that work. When blended with the wide bandgap polymer donor of PDBT-T1, the devices based on TPH-4, TPH-5 and TPH-6 showed the similar device performance, however, OSCs based on TPH-7 with the longest alkyl chain exhibited the highest PCE of 8.64%. Chen et al. reported a simple PDI trimer of B(PDI)3 with a benzene ring as the central core [82]. B(PDI)3 had a highly twisted molecular structure, which could help for reducing the strong aggregation of PDI units. When B(PDI)3 was blended with PTB7-Th donor in OSCs, a good PCE of 5.65% was achieved. Our group reported a novel PDI trimer of Ta-PDI with an electron-withdrawing triazine as the central core [25]. Compared with Ph-PDI with the benzene core, Ta-PDI had the higher cryrtallinity as well as the more suppressed molecular aggregation, yielding a much higher PCE of 9.15%. Recently, Chen et al. reported two PDI trimers based on benzotrithiophene (BTT) as the core, connecting via either single bond (TriPDI) or ring-fusion (Fused-TriPDI) approaches [83]. Compared to TriPDI, the fused TriPDI exhibited the good structural rigidity and planarity as well as the effective conjugation along the whole molecular backbone. Therefore, TriPDI showed up-shifted energy levels, enhanced absorption and charge mobility than TriPDI. The OSCs based on PTB7-Th:Fused-TriPDI blend showed a PCE of 6.19%, which was around three times higher than that of PTB7-Th:TriPDI devices. The chemical structures of 3D PDI trimers are listed in Fig. 5.
|
Download:
|
| Fig. 5. Chemical structures of three dimensional PDI trimers | |
4.2. 3D PDI tetramers
The α-substituted PDI derivatives exhibited superior photovoltaic performance than the corresponding β-isomers because of their better planarity, which induced closer packing of π-conjugated backbone. Yu et al. reported a new α-substituted PDI tetramer of TPBDT with a BDT-Th core, in which four PDI units formed a cross-like molecular conformation [84]. The inverted non-fullerene OSCs based on TPBDT showed a high PCE of 8.47% with the extraordinary large Jsc value (>18 mA cm2) by adding a small amount of DPE as the co-solvent. On the other hand, β-substituted PDI tetramer of βTPB6 with a BDT-Th core was also reported by Yu's groups [85]. Extending the conjugation of βTPB6 by fusing with the thiophene cycle, βTPB6-C possessed a more rigid molecular geometry. The inverted OSCs based on βTPB6-C and PTB7-Th exhibited the larger PCE of 7.69%, which was 31% higher than that of βTPB6-based devices. Wang et al. reported a PDI derivative of 6T-PDI4 with a dendritic sexithiophene (6T) core [86]. 6T-PDI4 had a broad absorption spectra from 400 nm to 600 nm and a LUMO level of -4.1 eV. Using PTB7-Th as the donor material, the 6T-PDI4 devices exhibited a PCE of 4.12%.
Yan et al. reported a series of tetrahedron-shaped PDI tetramers (TPC-PDI4, TPSi-PDI4 and TPGe-PDI4) with the tetraphenylmethane (TPC), tetraphenylsilane (TPSi) and tetraphenylgermane (TPGe) central cores [87]. All of them had a sp3 atom in the middle section, which led to a tetrahedron-shaped molecular configuration. The results indicated that OSCs based on TPC-PDI4 and TPSi-PDI4 showed significantly better performance than TPGe-PDI4. The TPC-PDI4 devices showed the highest PCE of 4.3% with an outstanding Voc of 0.96 V. At the same time, Zhang et al. also used TPC as the central core to link four PDI units and synthesized a 3D NFA of MePDI4. The solution-processed OSCs based on Me-PDI4 showed a PCE of 2.73% [88]. The TPC core was also used by Li's group to synthesize two novel PDI tetramers of tetra-PBI and tetra-PBI-S [89]. Compared with tetra-PBI, tetra-PBI-S had an appropriate LUMO level, balanced carrier mobility and favorable phase separation when blended with PBDT-TS1, which made tetra-PBIS devices show a much higher PCE of 6.2% than that of tetra-PBI devices (3.6%). Yan et al. further studied the structure-property relationship of PDI tetramers by replacing TPC with tetraphenylethylene (TPE) and tetraphenylpyrazine (TPPz) and synthesized another two PDI derivatives of TPE-PDI4 and TPPz-PDI4 [90, 91]. The results indicated that TPC-PDI4 had a more twisted configuration compared to TPE-PDI4. As a result, the device based on TPC-PDI4 showed an inferior performance (PCE = 4.3%) than that of TPE-PDI4 (PCE = 5.5%). Therefore, increasing the twisting extent of the PDI molecules (from TPE-PDI4 to TPC-PDI4) would decrease the overall device performance. Compared to TPE-PDI4, TPPz-PDI4 had a less crowded space, which made the twist extent of molecular backbone reduce further. Thus, OSCs fabricated with TPPz-PDI4 exhibited a higher FF and PCE (7.1%) than those of TPC-PDI4 and TPE-PDI4.
Cho et al. reported a novel 3D SM-NFA of SF-PDI4 based on a spirobifluorene (SF) core [92, 93]. This highly twisted core could suppress molecular aggregation of SF-PDI4 and facilitate excitation energy transfer. Thus, SF-PDI4 showed an intense UV-vis absorption band peaked at 531 nm, which complemented well with the absorption bands of P4T2FBT and PV4T2FBT. Furthermore, the comparative study revealed that replacing P4T2FBT with PV4T2FBT could significantly increase the solar cell efficiency due to the enhanced absorption and more favorable BHJ morphology. The PV4T2FBT:SF-PDI4 devices had a higher Jsc of 12.02 mA/cm2 with a reasonably high Voc and FF values, giving rise to a higher PCE of 5.98%. Importantly, the PV4T2FBT:SF-PDI4 blend exhibited a well mixed interpenetrating BHJ morphology with well-defined nanophase separation, indicating that SF-PDI4 could formed an excellent 3D charge transport network for efficient OSCs. Huang et al. reported another PDI tetramer of P4M4, whose devices based on PDBT-T1:P4M4 active layer showed a PCE of 5.71% [94]. Li et al. designed a 3D PDI derivative of PBI-pro using porphyrin as the central core [83, 95]. PBI-pro had three characteristic absorption bands within 300-600 nm and 700-800 nm, which could perfectly complement with the absorption profile of the polymer donor of PBDB-T. The above advantages facilitated a high PCE of 7.4% from the related OSCs. The chemical structures of 3D PDI tetramers are listed in Fig. 6. The related photovoltaic parameters of the OSCs based on 3D PDI trimers and tetramers as the acceptors are also provided in Table 2.
|
Download:
|
| Fig. 6. Chemical structures of three dimensional PDI tetramers | |
|
|
Table 2 The photovoltaic parameters of the OSCs based on three-dimensional PDI trimers and tetramers as the acceptors. |
5. Conclusion
In this review, we briefly introduced the recent progress of PDI-containing small molecules as the acceptor materials for nonfullerene OSCs. PDI derivatives are attractive alternatives to fullerenes because they have low-lying LUMO levels, high electron mobilities, easy modification of the frontier molecular orbital levels, inexpensive synthesis and purification on a large scale. More importantly, they are chemically and environmentally robust (some cases have been used as automotive paint pigments). Regardless of above advantages, PDIs have historically performed poor performance in BHJ solar cells. One of the main reasons is that PDIs are strongly aggregated in the solid state due to their rigid molecular configurations, which will form seriously large crystallites up to micrometer scale during the solutionprocess. Many efforts for minimizing the domain size of PDI derivatives focused on three major strategies: (1) developing monomeric PDIs by introducing large bulky groups at the imide position and the ortho or bay positions; (2) creating twisted PDI dimmers by inserting spacers at the ortho or bay positions, or directly correcting two PDIs at the imide position; (3) designing twisted PDIs with three dimensional (3D) molecular configurations by different 3D central cores. Although creating twisted or non-planar geometries seems to be a successful design strategy for PDI-based acceptor materials, the reduced π-π stacking feature of PDI backbone will be induced by increasing steric repulsion, which will thus weaken the electron-transporting capability of PDI unit to some extent. To tap into the potential of PDIs as the NFAs, the trade-off between ideal nanophase separation and strong π-π stacking should be properly resolved. Many smart design strategies have already realized the high PCE over 9% in OSCs. However, there is still a large lifting space for improving the device performance of PDI-based acceptor materials further. With the continued striving for understanding the structure-property relationships, developing novel PDI-based NFAs, choosing suitable donor materials and optimizing the morphology of the active layer, we believe that highly efficient PDI-based OSCs can be anticipated in the near future.
AcknowledgmentsThis work was supported by the National Science Foundation of China (NSFC, Nos. 51573107, 91633301 and 21432005) and the Foundation of State Key Laboratory of Polymer Materials Engineering (No. sklpme 2017-2-04).
| [1] |
Y.F. Li, Acc. Chem. Res. 45(2012) 723-733. DOI:10.1021/ar2002446 |
| [2] |
G. Li, R. Zhu, Y. Yang, Nat. Photonics 6(2012) 153-161. DOI:10.1038/nphoton.2012.11 |
| [3] |
Y.H. Liu, J.B. Zhao, Z.K. Li, et al., Nat. Commun. 5(2014) 1-8. |
| [4] |
Y.F. Li, Chem. Asian J. 10(2013) 2316-2328. |
| [5] |
Y.J. He, H.Y. Chen, J.H. Hou, Y.F. Li, J. Am. Chem. Soc. 132(2010) 1377-1382. DOI:10.1021/ja908602j |
| [6] |
Y.J. He, G.J. Zhao, B. Peng, Y.F. Li, Adv. Funct. Mater. 20(2010) 3383-3389. DOI:10.1002/adfm.201001122 |
| [7] |
S.Q. Zhang, L. Ye, J.H. Hou, Adv. Energy Mater. 6(2016) 1502529. DOI:10.1002/aenm.201502529 |
| [8] |
J.B. Zhao, Y.K. Li, G.F. Yang, et al., Nat. Energy 1(2016) 1-7. |
| [9] |
S.S. Li, L. Ye, W.C. Zhao, et al., Adv. Mater. 28(2016) 9423-9429. DOI:10.1002/adma.201602776 |
| [10] |
B.B. Fan, L. Ying, Z.F. Wang, et al., Energy Environ. Sci. 10(2017) 1243-1251. DOI:10.1039/C7EE00619E |
| [11] |
S.S. Li, H. Zhang, W.C. Zhao, et al., Adv. Energy Mater. 6(2015) 1501991. |
| [12] |
C.D. Dou, X.J. Long, Z.C. Ding, et al., Angew. Chem. Int. Ed. 55(2016) 1436-1440. DOI:10.1002/anie.201508482 |
| [13] |
X.J. Long, Z.C. Ding, C.D. Dou, et al., Adv. Mater. 28(2016) 6504-6508. DOI:10.1002/adma.201601205 |
| [14] |
Y.Z. Lin, J.Y. Wang, Z.G. Zhang, et al., Adv. Mater. 27(2015) 1170-1174. DOI:10.1002/adma.201404317 |
| [15] |
G.J. Zhang, G.F. Yang, H. Yan, et al., Adv. Mater. 29(2017) 1606054. DOI:10.1002/adma.201606054 |
| [16] |
S.X. Li, W.Q. Liu, M.M. Shi, et al., Energy Environ. Sci. 9(2016) 604-610. DOI:10.1039/C5EE03481G |
| [17] |
H.Y. Li, Y.J. Hwang, B.A.E. Courtright, et al., Adv. Mater. 27(2015) 3266-3272. DOI:10.1002/adma.v27.21 |
| [18] |
C.W. Tang, Appl. Phys. Lett. 48(1986) 183. DOI:10.1063/1.96937 |
| [19] |
Z. An, J. Yu, S.C. Jones, et al., Adv. Mater. 17(2005) 2580-2583. DOI:10.1002/(ISSN)1521-4095 |
| [20] |
C. Li, H. Wonneberger, Adv. Mater. 24(2012) 613-636. DOI:10.1002/adma.201104447 |
| [21] |
Z.T. Liu, Y. Wu, Q. Zhang, X. Gao, J. Mater. Chem. A 4(2016) 17604-17622. DOI:10.1039/C6TA06978A |
| [22] |
Y.Z. Lin, Y.F. Wang, J.Y. Wang, et al., Adv. Mater. 26(2014) 5137-5142. DOI:10.1002/adma.201400525 |
| [23] |
J. Liu, S.S. Chen, D.P. Qian, et al., Nat. Energy 1(2016) 16089. DOI:10.1038/nenergy.2016.89 |
| [24] |
D. Meng, H.T. Fu, C.Y. Xiao, et al., J. Am. Chem. Soc. 138(2016) 10184-10190. DOI:10.1021/jacs.6b04368 |
| [25] |
Y.W. Duan, X.P. Xu, H. Yan, et al., Adv. Mater. 29(2017) 1605115. DOI:10.1002/adma.201605115 |
| [26] |
J.J. Dittmer, R. Lazzaroni, Ph. Leclère, et al., Sol. Energy Mater. Sol. Cells 61(2000) 53-61. DOI:10.1016/S0927-0248(99)00096-3 |
| [27] |
L. Schmidt-Mende, A. Fechtenkötter, K. Müllen, et al., Science 293(2001) 1119-1122. DOI:10.1126/science.293.5532.1119 |
| [28] |
X.Y. Guo, L.J. Bu, Y. Zhao, et al., Thin Solid Films 517(2009) 4654-4657. DOI:10.1016/j.tsf.2009.02.082 |
| [29] |
A. Sharenko, C.M. Proctor, T.S. vanderPoll, et al., Adv. Mater 25(2013) 4403-4406. DOI:10.1002/adma.v25.32 |
| [30] |
A. Sharenko, D. Gehrig, F. Laquai, T.Q. Nguyen, Chem. Mater. 26(2014) 4109-4118. DOI:10.1021/cm5010483 |
| [31] |
Y.X. Chen, X. Zhang, C.L. Zhan, J.N. Yao, Phys Status Solidi A 212(2015) 1961-1968. DOI:10.1002/pssa.201532102 |
| [32] |
R. Singh, E. Aluicio-Sarduy, Z. Kan, et al., J. Mater. Chem. A 2(2014) 14348-14353. DOI:10.1039/C4TA02851A |
| [33] |
X. Zhang, Z.H. Lu, L. Ye, et al., Adv. Mater. 25(2013) 5791-5797. DOI:10.1002/adma.v25.40 |
| [34] |
X.L. Zhang, B. Jiang, X. Zhang, et al., J. Phys. Chem. C 118(2014) 24212-24220. DOI:10.1021/jp5093674 |
| [35] |
P.E. Hartnett, A. Timalsina, H.S.S.R. Matte, et al., J. Am. Chem. Soc. 136(2014) 16345-16356. DOI:10.1021/ja508814z |
| [36] |
Y.H. Cai, L.J. Huo, X.B. Sun, et al., Adv. Energy Mater. 5(2015) 1500032. DOI:10.1002/aenm.201500032 |
| [37] |
X.C. Li, H.B. Wang, J. Li, et al., J. Mater. Chem. C 5(2017) 2781-2785. DOI:10.1039/C7TC00263G |
| [38] |
X. Liu, G.P. Luo, X.Y. Cai, et al., RSC Adv. 5(2015) 83155-83163. DOI:10.1039/C5RA13188J |
| [39] |
G.D. Sharma, P. Balraju, J.A. Mikroyannidis, M.M. Stylianakis, Sol. Energy Mater. Sol. Cells 93(2009) 2025-2028. DOI:10.1016/j.solmat.2009.08.003 |
| [40] |
G.D. Sharma, P. Suresh, J.A. Mikroyannidis, M.M. Stylianakis, J. Mater. Chem. 20(2010) 561-567. DOI:10.1039/B918527E |
| [41] |
J.A. Mikroyannidisa, P. Sureshb, G.D. Sharma, Synth. Met. 160(2010) 932-938. DOI:10.1016/j.synthmet.2010.02.003 |
| [42] |
D. Kotowski, S. Luzzati, G. Scavia, et al., Dyes Pigm. 120(2015) 57-64. DOI:10.1016/j.dyepig.2015.04.006 |
| [43] |
Z.H. Lu, X. Zhang, C.L. Zhan, et al., Phys. Chem. Chem. Phys. 15(2013) 11375-11385. DOI:10.1039/c3cp51475g |
| [44] |
X. Zhang, J.N. Yao, C.L. Zhan, Chem. Commun. 51(2015) 1058-1061. DOI:10.1039/C4CC08457H |
| [45] |
X. Zhang, C.L. Zhan, J.N. Yao, Chem. Mater. 27(2015) 166-173. DOI:10.1021/cm504140c |
| [46] |
J.Y. Wang, Y.H. Yao, S.X. Dai, et al., J. Mater. Chem. A 3(2015) 13000-13010. DOI:10.1039/C5TA02589C |
| [47] |
J.B. Zhao, Y.K. Li, J.Q. Zhang, et al., J. Mater. Chem. A 3(2015) 20108-20112. DOI:10.1039/C5TA05339K |
| [48] |
W.T. Hadmojo, S.Y. Nam, T.J. Shin, et al., J. Mater. Chem. A 4(2016) 12308-12318. DOI:10.1039/C6TA04344E |
| [49] |
Q.F. Yan, Y. Zhou, Y.Q. Zheng, et al., Chem. Sci. 4(2013) 4389-4394. DOI:10.1039/c3sc51841h |
| [50] |
J.B. Zhao, Y.K. Li, H.R. Lin, et al., Energy Environ. Sci. 8(2015) 520-525. DOI:10.1039/C4EE02990A |
| [51] |
S.S. Chen, G.Y. Zhang, J. Liu, et al., Adv. Mater. 29(2017) 1604231. DOI:10.1002/adma.201604231 |
| [52] |
G.E. Park, H.J. Kim, S. Choi, et al., Chem. Commun. 52(2016) 8873-8876. DOI:10.1039/C6CC04229E |
| [53] |
P.E. Hartnett, H.S.S.R. Matte, N.D. Eastham, et al., Chem. Sci. 7(2016) 3543-3555. DOI:10.1039/C5SC04956C |
| [54] |
H.L. Zhong, C.H. Wu, C.Z. Li, et al., Adv. Mater. 28(2016) 951-958. DOI:10.1002/adma.v28.5 |
| [55] |
S.X. Li, W.Q. Liu, C.Z. Li, et al., J. Mater. Chem. A 4(2016) 14983-14987. DOI:10.1039/C6TA07368A |
| [56] |
D.L. Zhao, Q.H. Wu, Z.X. Cai, et al., Chem. Mater. 28(2016) 1139-1146. DOI:10.1021/acs.chemmater.5b04570 |
| [57] |
S.M. McAfee, S. Dayneko, P. Josse, et al., Chem. Mater. 29(2017) 1309-1314. DOI:10.1021/acs.chemmater.6b04862 |
| [58] |
S.M. McAfee, S.V. Dayneko, A.D. Hendsbee, et al., J. Mater. Chem. A 5(2017) 11623-11633. DOI:10.1039/C7TA00318H |
| [59] |
Q.Q. Shi, S.Y. Zhang, J.X. Zhang, et al., J. Am. Chem. Soc. 138(2016) 3946-3949. DOI:10.1021/jacs.5b12259 |
| [60] |
W. Jiang, L. Ye, X.G. Li, et al., Chem. Commun. 50(2014) 1024-1026. DOI:10.1039/C3CC47204C |
| [61] |
L. Ye, W. Jiang, W.C. Zhao, et al., Small 10(2014) 4658-4663. DOI:10.1002/smll.v10.22 |
| [62] |
Y. Zang, C.Z. Li, C.C. Chueh, et al., Adv. Mater. 26(2014) 5708-5714. DOI:10.1002/adma.201401992 |
| [63] |
C.H. Wu, C.C. Chueh, Y.Y. Xi, et al., Adv. Funct. Mater. 25(2015) 5326-5332. DOI:10.1002/adfm.201501971 |
| [64] |
J.S. Yu, Y.Y. Xi, C.C. Chueh, et al., Adv. Mater. Interfaces 3(2016) 1600476. DOI:10.1002/admi.201600476 |
| [65] |
Y.P. Yu, F. Yang, Y.J. Ji, et al., J. Mater. Chem. C 4(2016) 4134-4137. |
| [66] |
D. Sun, D. Meng, Y.H. Cai, et al., J. Am. Chem. Soc. 137(2015) 11156-11162. DOI:10.1021/jacs.5b06414 |
| [67] |
D. Meng, D. Sun, C.M. Zhong, et al., J. Am. Chem. Soc. 138(2016) 375-380. DOI:10.1021/jacs.5b11149 |
| [68] |
T. Liu, D. Meng, Y.H. Cai, et al., Adv. Sci. 3(2016) 1600117. DOI:10.1002/advs.201600117 |
| [69] |
A.D. Hendsbee, J.P. Sun, W.K. Law, et al., Chem. Mater. 28(2016) 7098-7109. DOI:10.1021/acs.chemmater.6b03292 |
| [70] |
Y. Zhong, M.T. Trinh, R.S. Chen, et al., J. Am. Chem. Soc. 136(2014) 15215-15221. DOI:10.1021/ja5092613 |
| [71] |
Y. Zhong, M.T. Trinh, R.S. Chen, et al., Nat. Commun. 6(2015) 8242. DOI:10.1038/ncomms9242 |
| [72] |
T.J. Sisto, Y. Zhong, B.Y. Zhang, et al., J. Am. Chem. Soc. 139(2017) 5648-5651. DOI:10.1021/jacs.6b13093 |
| [73] |
C. Zhang, T. Liu, W.X. Zeng, et al., Mater. Chem. Front. 1(2017) 749-756. DOI:10.1039/C6QM00194G |
| [74] |
S. Rajaram, R. Shivanna, S.K. Kandappa, K.S. Narayan, J. Phys. Chem. Lett. 23(2012) 2405-2408. |
| [75] |
R. Shivanna, S. Shoaee, S. Dimitrov, et al., Energy Environ. Sci. 7(2014) 435-441. DOI:10.1039/C3EE42484G |
| [76] |
L. Ye, K. Sun, W. Jiang, et al., ACS Appl. Mater. Interfaces 7(2015) 9274-9280. DOI:10.1021/acsami.5b02012 |
| [77] |
N.N. Liang, K. Sun, Z. Zheng, et al., Adv. Energy Mater. 6(2016) 1600060. DOI:10.1002/aenm.201600060 |
| [78] |
Z.H. Luo, W.T. Xiong, T. Liu, et al., Org. Electron. 41(2017) 166-172. DOI:10.1016/j.orgel.2016.10.044 |
| [79] |
Q.C. Wu, L. Li, J.F. Hai, et al., Dyes Pigm. 132(2016) 41-47. DOI:10.1016/j.dyepig.2016.04.040 |
| [80] |
N.N. Liang, D. Meng, Z.T. Ma, et al., Adv. Energy Mater. 7(2016) 1601664. |
| [81] |
H.T. Fu, D. Meng, X.Y. Meng, et al., J. Mater. Chem. A 5(2017) 3475-3482. DOI:10.1039/C6TA09049D |
| [82] |
X. Li, W.Q. Liu, C.Z. Li, et al., J. Mater. Chem. A 4(2016) 10659-10665. DOI:10.1039/C6TA04232E |
| [83] |
B. Wang, W.Q. Liu, H.B. Li, et al., J. Mater. Chem. A 5(2017) 9396-9401. DOI:10.1039/C7TA02582C |
| [84] |
Q.H. Wu, D.L. Zhao, A.M. Schneider, et al., J. Am. Chem. Soc. 138(2016) 7248-7251. DOI:10.1021/jacs.6b03562 |
| [85] |
Q.H. Wu, D.L. Zhao, J.H. Yang, et al., Chem. Mater. 29(2017) 1127-1133. DOI:10.1021/acs.chemmater.6b04287 |
| [86] |
M.H. Yi, J.D. Yi, J.K. Wang, et al., Dyes Pigm. 139(2017) 498-508. DOI:10.1016/j.dyepig.2016.12.057 |
| [87] |
Y.H. Liu, J.Y.L. Lai, S.S. Chen, et al., J. Mater. Chem. A 3(2015) 13632-13636. DOI:10.1039/C5TA03093E |
| [88] |
W.Q. Chen, X. Yang, G.K. Long, et al., J. Mater. Chem. C 3(2015) 4698-4705. DOI:10.1039/C5TC00865D |
| [89] |
W. Fan, N.N. Liang, D. Meng, et al., Chem. Commun. 52(2016) 11500-11503. DOI:10.1039/C6CC05810H |
| [90] |
Y.H. Liu, C. Mu, K. Jiang, et al., Adv. Mater. 27(2015) 1015-1020. DOI:10.1002/adma.201404152 |
| [91] |
H.R. Lin, S.S. Chen, H.W. Hu, et al., Adv. Mater. 28(2016) 8546-8551. DOI:10.1002/adma.v28.38 |
| [92] |
J.D. Yi, Y.L. Wang, Q. Luo, et al., Chem. Commun. 52(2016) 1649-1652. DOI:10.1039/C5CC08484A |
| [93] |
J. Lee, R. Singh, D.H. Sin, et al., Adv. Mater. 28(2016) 69-76. DOI:10.1002/adma.201504010 |
| [94] |
X. Liu, T. Liu, C.H. Duan, et al., J. Mater. Chem. A 5(2017) 1713-1723. DOI:10.1039/C6TA08739F |
| [95] |
A.D. Zhang, C. Li, F. Yang, et al., Angew. Chem. Int. Ed. 56(2017) 2694-2698. DOI:10.1002/anie.201612090 |
 2017, Vol. 28
2017, Vol. 28