b CAS Key Laboratory of Bio-inspired Materials and Interfacial Science, CAS Center for Excellence in Nanoscience, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Responsive polymers have a tremendous impact on advanced materials, as they can endow static materials with dynamic properties, including wettability switch, mechanical actuation, and mass transport [1]. Those polymers undergo irreversible or reversible changes by external stimuli (e.g., thermal, pH, photo, and electrochemistry) and further induce dynamical alteration of macro-scale physicochemical properties of responsive polymer materials. For example, thermal-responsive polymers, like poly(N-isopropylacrylamide) (PNiPAAm), reversibly change from hydrophilic to hydrophobic when the external temperature increases to lower critical solution temperature (LCST) [2]. Poly(acrylic acid) (PAA), a pH-responsive polymer, can undergo pH-sensitive phase separation where the materials contribute to ability of high sorption and release [3]. Photo-responsive azobenzene-polymers can lead to efficient self-assemble in liquid state and mass transport in solid state [4]. Electrochemical-responsive polymers, like polypyrrole, can accommodate a variety of anions or cations and the release them under electric fields [5]. By the virtue of controllable properties of responsive polymer materials, they have been used in widespread fields from mechanical activation to nanomedicine, which have promoted modern life [6].
With advantages of noninvasion and spatiotemporal control [7], photo-responsive polymer materials can be widely used for photoelectric films [8], photo-actuators [9, 10], detector [11-13], optical devices [14, 15], self-healing hydrogel [16], biomedicine [17-19], and information storage [20, 21]. For example, photoactivated film fabricated by azobenzene-polymer (cross-linked AAZO-poly(diallyldimethylammonium chloride)) can realize deformation and recovery as light-driven actuator, which is due to the photo-isomerization of azobenzene-group between trans-and cis-azobenzene controlled by external light [22]. Recently, the mentioned outstanding applications above of photo-responsive polymer materials have been summarized by some excellent papers [23, 24]. Besides, the photo-responsive polymer materials are also promising candidates for biological applications like controllable drug delivery [25-27], and cell manipulation [28], arising from their noninvasive and spatiotemporal strategy during property change. For example, photoresponsive particles can serve as vehicle for controllable drug delivery via particle disintegration [29]. Photo-responsive surfaces and hydrogels [30], have been used for cell adhesion and responsive detachment. Up till now, the photo-responsive polymers usually are constructed by introducing photo-responsive moieties to bulk polymers by copolymerization or modification as side chain as summarized in Fig. 1 [31-34]. For example, photoresponsive monomer like 4-methacryloyloxyazobenzene (MOAB) can be copolymerized with other monomer like methylmethacrylate (MMA) by a free radical polymerization to construct photoresponsive polymers (PMMAMOAB) in Fig. 1A [32]. On the other hand, photo-responsive polymers (poly(benzospiropyran hexyl methacrylate)) can be synthesized by modification of photoresponsive moieties (spiropyran) to bulk polymers with reactive sites like azide group by "click" chemistry as Fig. 1B shows. The spiropyran groups in the side chain can then provide photoresponsiveness for the linear, star-like and graft copolymers [33]. Therefore, the photo-responsive moieties is the key to understand the photo-responsive polymer materials and their biological applications. In this review, we will briefly summarize the photo-responsive moieties and discuss the photo-responsive polymer materials from zero-dimensional micelles, two-dimensional surfaces to three-dimensional hydrogels for various biological fields including drug delivery and cell manipulation.
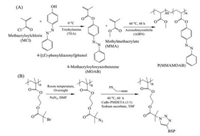
|
Download:
|
| Fig. 1. Construction of photo-responsive polymers by introducing photoresponsive moieties to bulk polymers by (A) copolymerization or (B) modification as side chain. Reproduced with permission [32, 33]. Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA. Copyright 2014, Royal Society of Chemistry. | |
2. Photo-responsive polymers
The photo-responsive polymers always consist of photoresponsive moieties and bulk polymer [32-37]. In this review, we mainly focus on the discussion of photo-responsive moieties, which can be divided into irreversible and reversible moieties. For irreversible moieties, like o-nitrobenzyl and coumarinyl, can undergo photo-induced bond cleavage and thus this transition is one-off [7]. Whereas the reversible moieties, such as azobenzene and spiropyran, can give rise to photo-responsive cyclical isomerization between isomers when treated by specific light [38].
2.1. Irreversible photo-responsive moietiesThe widely applied irreversible moieties (Fig. 2) include onitrobenzyl (ONB), coumarinyl, pyrenylmethyl, and 2-diazo-1, 2-naphtoquinone moieties have been used for the fabrication of photo-responsive polymers [7, 39, 40]. Polymers bearing them as side groups have been served as one-off photo-responsive materials, like zero-dimensional micelles and two-dimensional surfaces [41]. The cleavage of the chemical bond always switches photo-responsive polymers from hydrophobic to hydrophilic, leading to the destruction of micelles or physicochemical change of surfaces [42]. The mechanism of bond cleavage may be different, for instance, pyrenylmethyl moieties need the presence of protonic solvents during the UV induced photolysis reaction while the ONB moieties do not. Besides, the ONB moieties can respond to nearinfrared (NIR) light in the way of two photons absorption. However, the efficiency of NIR-induced bond cleavage is much lower than that of UV-induced condition [7]. In this view of point, coumarinyl moieties are more favor for the construction of photo-responsive polymers for biological applications because they can respond to UV and NIR light more efficiently. After bond cleavage reaction of those irreversible moieties, hydrophilic carboxyl groups always expose on the surface of photo-responsive polymer materials, switching the surface wettability from hydrophobic to hydrophilic. In summary, the irreversible photo-responsive moieties can be used for one-off wettability transition of photo-responsive polymer materials.
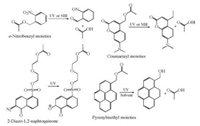
|
Download:
|
| Fig. 2. Photo-induced transition of irreversible moieties in photo-responsive polymers. | |
2.2. Reversible photo-responsive moieties
In comparison to the irreversible moieties with one-way photoinduced bond cleavage, the reversible photo-responsive moieties can present cyclic transformation between isomers including azobenzene, spiropyran, spirooxazines, and diarylethene (Fig. 3). There are generally two kinds of isomerization mechanism in reversible moieties: 1) cis-trans isomerization without bond cleavage (e.g., azobenzene) and 2) dissociation process with bond cleavage (e.g., spiropyran) [43]. For example, azobenzene can transform between cis and trans isomers due to the rearrangement of the nucleus and thus the electric dipole moment change is almost 3 D [44]. Whereas spiropyran can isomerize from hydrophobic neutral spiropyran (SP) isomer under visible light to hydrophilic zwitterionic merocyanine (MC) isomer under UV light. The reversible isomerization can result in 8-15 D of electric dipole moment change which is because of the reversible bond cleavage when treated by UV light [43].

|
Download:
|
| Fig. 3. Photo-induced isomerization of reversible moieties in photo-responsive polymers. | |
The reversible isomerization of those moieties can realize the physicochemical change of photo-responsive polymer materials which can be further applied for biomedicine such as drug delivery and cell manipulation. For azobenzene, the amphiphilic copolymers with trans-azobenzene on the hydrophobic blocks can assemble to micelles in solution because of the hydrophobic-hydrophilic balance of block copolymers. While the hydrophobic-hydrophilic balance is destructed after UV light because the trans-azobenzene moieties transform to more polar cis-azobenzene, resulting in the micelle disassembly [26]. Whereas the hydrophobic/hydrophilic shift may be not enough to induce complete micelle disassembly. To improve the photo-responsive change, the specific recognition of trans-azobenzene and cyclodextrin (CD) can serve as photo-responsive network with reversible sol-gel transition which is important for controllable drug delivery [45]. In addition to azobenzene moieties, spiropyran as an emerging candidate exhibits two isomers with vastly different physicochemical properties, such as wettability, charge and polarity, which guarantee the widespread utility of spiropyran-polymers. For example, smart surfaces modified by poly(SP) can reversibly switch from hydrophobic to hydrophilic. By combining the micro/ nanostructured substrate, the water contact angle (CA) of those smart surfaces is 138.8° ± 1.3° at visible light, and can decrease to 42.7° ± 1.7° when treated by UV light (365 nm) for 5 min [46]. The reversible and fast switch is required to fabricate photo-responsive materials for practical applications.
3. Biological applications of photo-responsive polymersThe photo stimuli with properties of noninvasion and spatiotemporal control make themselves promising candidates in biological applications such as drug delivery, cell manipulation, and tissue engineering. In this part, we will briefly classify the applications of photo-responsive polymer materials from zerodimensional micelles, two-dimensional surfaces to three-dimensional hydrogels.
3.1. Zero-dimensional micellesIt is a mature strategy to construct zero-dimensional micelles by the self-assembly of amphiphilic block copolymers. Photoresponsive zero-dimensional micelles have recently received tremendous attention due to its controllable delivery with high spatiotemporal resolution in vivo which is important for biomedicine [47]. In solution, the hydrophilic blocks are able to form shell and the hydrophobic blocks bearing photo-responsive moieties assemble to be core. During the self-assemble process, containers (e.g., drugs, proteins, and DNA) can be encapsulated into the micellar core. Usually, photo-responsive moieties on the hydrophobic blocks undergo photo-cleavage or photo-isomerization after photo irradiation causing the destruction of micelles. Consequently, the preloaded containers can be released when and where the administrator wants. Based on the photochemical reaction, we briefly divide photo-responsive moieties for micelle assembly into two categories: 1) photo-cleavable moieties, like ONB and coumarinyl; 2) photo-isomerized moieties, like azobenzene and spiropyran. In the following part, photo-responsive polymers for micelles and their applications in drug delivery will be discussed in those two aspects.
For the photo-cleavable moieties, they cleave chemical bond at specific site triggered by photo irradiation. The controllable micelles disruption of photo-responsive polymers is realized by the bond cleavage induced wettability switch or copolymer degradation. For example, the amphiphilic copolymer composing photo-responsive 2-diazo-1, 2-naphtoquinone groups can selfassemble into micelles [48]. When treated by UV light, the hydrophobic 2-diazo-1, 2-naphtoquinone groups (DNQ) transform to hydrophilic 3-indenecarboxylic acid (IC) via a Wolff rearrangement as shown in Fig. 4. This change of wettability can result in the micelle disruption and the following release of preloaded drug (Coumarin 102). Another approach for controllable micelles disruption is realized by the degradation of copolymers containing photo-cleavable moieties. For instance, amphiphilic polymers (PNiPAAm-ONB-PXCL) with classic photo-cleavable ONB can self-assemble into micelles with DOX encapsulated in the hydrophobic core [49]. The UV irradiation triggered the bond cleavage of ONB moieties and subsequent degradation of the amphiphilic polymers. During in vitro culture of Hela cells with DOX-loaded micelles, the DOX-loaded micelles greatly inhibited the proliferation of cells once upon treated by UV light for only 15 s. Although the UV responsive polymers demonstrated their ability of controllable delivery of active molecules in vitro, it is still challenging to realize in vivo because the low tissue penetration of UV light. Thus the NIR responsive moieties may be more suitable for practical biomedicine. Coumarinyl moieties can respond to NIR light and undergo bond cleavage which have the advantage for drug delivery. Recently, a nanovehicle for NIR induced drug delivery in Fig. 5 was fabricated by grafting photo-responsive polymers with coumarinyl moieties to hollow mesoporous spherical particles [17]. Under NIR laser (800 nm), the polymers could be easily hydrolyzed and the DOX in the particles can be released.

|
Download:
|
| Fig. 4. Amphiphilic photo-responsive polymers can assemble into micelles with loaded drug. When treated by UV light, the photo-responsive moieties turn from hydrophobic to hydrophilic where the micelles dissemble and result in drug release. Reproduced with permission [48]. Copyright 2011, Wiley-VCH Verlag GmbH & Co. KGaA. | |

|
Download:
|
| Fig. 5. NIR responsive micelles based on coumarinyl-polymers for drug delivery. Under NIR light, the coumarinyl moieties occur bond cleavage which induces the drug delivery. Reproduced with permission [17]. Copyright 2013, Royal Society of Chemistry. | |
Besides the photo-cleavable moieties, the photo-isomerized moieties are more adoptable to construct photo-responsive micelles due to their reversible switch of configuration. Based on the photo-isomerization of trans-and cis-azobenezene, the micelles can assemble and disassemble following the isomerization of azobenzene [50-55]. For example, amphiphile PMPC-azo (poly (ethylene glycol)-b-poly(5-methyl-5-propargylxycarbonyl-1, 3-dioxane-2-one)-azobenzene) can assemble to micelles in solution with hydrophobic core surrounding by hydrophilic shell. Upon UV irradiation, the azobenzene moieties transform from trans-isomer (dipole ~0 D) to cis-isomer (dipole ~4.4 D). The change of hydrophobic and hydrophilic balance of azobenzene resulted in micelle disruption as shown in Fig. 6A [26]. Another approach relying on the photo-isomerization of azobenzene is to utilize the host-guest recognition between azobenzene and CD. For instance, polymers with azobenzene as end group (adamantine-polystyrene-azobenzene (Ad-PS-Azo)), and poly(ethylene glycol) (PEG) modified by β-CD can form triblock copolymers via hostguest interaction and further assemble into micelles. Photoinduced transition from trans-azobenzene to cis-azobenzene vanish the recognition which result in micelle disruption as shown in Fig. 6B [52]. As another typical photo-isomerized molecule, spiropyran containing polymers are also used for photo-responsive micelles. The hydrophobic spiropyran can transform to hydrophilic merocyanine where the micelles disrupt [56]. That relatively greater change in physicochemical properties of polymers is more efficient for micelle disruption and the practical biomedicine. For example, photo-responsive polymers bearing SP as side groups were coated on mesoporous silica as upconversion nanoparticles (UCNPs) in it. That nanovehicle can load drugs in the core and release them when needed. Upon NIR light (980 nm), the UCNPs emit luminescence in the UV region, which can induce isomerization of SP to MC. The hydrophilic MC breaks the hydrophilic/hydrophobic balance which is followed by the micelles disruption. The in vitro and in vivo experiments confirmed that the preloaded DOX can be released efficiently and indicate a potential application in biomedicine [57].
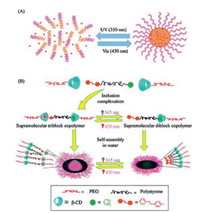
|
Download:
|
| Fig. 6. Two approaches of azobenzene-polymers of micelle disruption. (A) UV induced change between hydrophobic and hydrophilic can result in micelle disruption. (B) Vanishment of host-guest interaction between azobenzene and CD can result in micelle disruption. Reproduced with permission [26, 52]. Copyright 2014, Royal Society of Chemistry. | |
Either photo-cleavable or photo-isomerized polymers above demonstrate their ability to form zero-dimensional micelles and delivery of active molecules. By the aid of UCNPs, not only the potential side-effect of UV light on cells and tissues can be avoided, but also the NIR light with deeper penetration will shed a light on the practical application in vivo. Therefore, the photo-responsive micelles may solve the problems of biomedicine.
3.2. Two-dimensional surfacesPhoto-responsive polymers can also be used to fabricate twodimensional surfaces with dynamic photo-responsiveness via chemical modification. Under specific photo irradiation, chemical reaction like irreversible bond cleavage or reversible isomerization of photo-responsive moieties can induce physicochemical change of the surfaces. In this way, the photo-responsive two-dimensional surfaces have been adopted to harness cell adhesion and detachment which is vital for cell diagnostic, tissue engineering and cell medicine. Based on the transition between cell adhesion and detachment, photo-responsive two-dimensional surfaces can be classified into three kinds: 1) surfaces from cell anti-adhesion to cell adhesion; 2) surfaces from cell adhesion to cell detachment; 3) surfaces switch between cell adhesion and cell detachment.
Photo-responsive polymers bearing photo-cleavable moieties are always grafted from the substrates to construct twodimensional surfaces from cell anti-adhesion to cell adhesion. The bioadhesive linkers like arginine-glycine-aspartic acid (RGD) which is well known to promote integrin-mediated cell adhesion [58, 59] can be protected conveniently by ONB and its derivatives on the carboxylic acid side chain. After bond cleavage, the protected bioadhesive linkers like RGD on the surfaces can be exposed for enhancing cell adhesion. For example, poly-L-lysine (PLL) with nitrobenzyl protected RGD peptide as bioadhesive linker was modified to the commercial culture dish [60]. Before UV irradiation, the bioadhesive linkers were concealed by the nitrobenzyl cages and the cells cannot adhere onto the dish. After UV irradiation, the nitrobenzyl underwent bond cleavage which resulted in exposure of RGD peptide and cell adhesion as shown in Fig. 7. In this way, the spatiotemporal cell adhesion controlled by UV light can be achieved, which is required by tissue engineering and cell biology [61]. For example, surfaces that switch from cell anti-adhesion to cell adhesion can realize cell pattern by masked photo irradiation. This method provides a way to investigate cell migration and coculture of different cells [62].
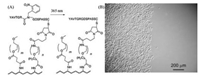
|
Download:
|
| Fig. 7. Photo-responsive surfaces from cell anti-adhesion to cell adhesion. (A) photo-responsive exposure of nitrobenzyl protected RGD for enhancing cell adhesion under UV treatment. (B) Spatial control of cell adhesion on the UV treated surface (left). Reproduced with permission [60]. Copyright 2008, Wiley-VCH Verlag GmbH & Co. KGaA. | |
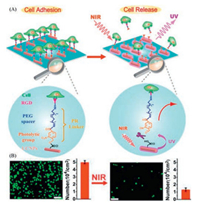
Compared to controllable transition from cell anti-adhesion to cell adhesion, the photo-controllable cell detachment is more promising for cell diagnostic and tissue engineering. The irreversible surfaces for cell adhesion and detachment can be modified by the photo-responsive polymers bearing photo-cleavable moieties. For instance, PEG with 4-(hydroxymethyl)-3-nitrobenzoic acid (ONA) as anchor to UCNPs and RGD on the other side of PEG was used for photo-responsive surface [63]. The RGD can enhance cell adhesion on the surfaces and the UCNPs can make the surface respond to NIR light rather than UV light to avoid the side-effect. When treated by NIR irradiation, the ONA moieties underwent bond cleavage and the RGD moieties with cells detached from the surface as shown in Fig. 8. When the RGD is replaced by specific antibody, the method can be adopted for specific cell capture and release. For example, anti-CD4Ab was linked to PEG with nitrobenzyl moieties to capture CD4-expressing T-cells (Molt-3 cells) where the cells can be released after UV treatment with high viability [64]. The method for specific cell capture and release can give a clue on cell separation and analysis after release.
|
Download:
|
| Fig. 8. ONA-based polymers modified UCNPs surface can realize controllable cell detachment. (A) Schematic introduction of NIR responsive cell detachment and (B) fluorescent images before and after NIR treatment indicate the controllable cell adhesion and detachment. Reproduced with permission [63]. Copyright 2014, American Chemical Society. | |
As well known, the photo-responsive polymers with photoisomerized moieties like azobenzene, spiropyran and their derivatives have provided a reversible way to control the transition between cell adhesion and detachment. As introduced in Section 2.2, the host-guest recognition between azobenzene and CD can be adopted for reversible cell adhesion and detachment [65]. For example, Azo-PEG-Azo polymers was used to fabricate patterned surfaces with photo-responsive cell adhesion and detachment. In this strategy, the β-CD was modified to cell surface to give the recognition ability with azobenzene to the cells. When the Azo-PEG-Azo surface presented trans-azobenzene state, the cells can adhere to the surface. Whereas when photo-induced transition of trans-azobenzene to cis-azobenzene happened, the cells detached from the surfaces due to the vanishment of host-guest recognition [66]. Although the azobenzene polymers provide a reversible way for controllable cell adhesion and detachment, the modification of β-CD to cells is complex. So the strategy based on photo-isomerized molecules induced physicochemical properties change of surfaces has been developed. Spiropyran as a popular photo-responsive moiety has two isomers named SP and MC which present relatively vast difference, such as wettability, that can be cooperated into polymers for reversibly control cell adhesion and detachment. In a recent report, poly(nitrobenzospiropyran-co-methyl methacrylate) (poly(NSP-co-MMA)) based surfaces can detach cells due to wettability switch induced by UV light [67]. The water CA of poly (NSP-co-MMA)-coated surfaces decreased under UV irradiation and cells detached from the hydrophilic surfaces as shown in Figs. 9A and B. Sumaru and coworker also used poly(SP-co-NiPAAm) to control the photo-responsive cell adhesion and detachment in Figs. 9C and D [68]. In that study, UV irradiation facilitated cell adhesion probably resulting from specific interaction of MC and cells. After treated by visible light, the MC isomer transformed to SP isomer again, this reversible isomerization guaranteed the reversible cell adhesion and detachment. The reversible photo-isomerization also provides a stepwise way to control cell pattern which is vital for tissue engineering. Polynitrospyropyranemethylmethaclyate (pNSpMMA)-based surface which holds PEG as inhibitor of cells can enable the stepwise cell patterning and co-culture of CHO-K1 and MDCK cells [69]. When treated by UV light, the surface is hydrophilic and the PEG release from the surface. The area without PEG can turn to hydrophobic immediately after irradiation to allow cell adhesion. Repeated UV irradiation with different masks can create new spots for cell adhesion and the co-culture of different cells. Therefore, the reversible photo-responsive surface for cell adhesion is urgently required in cell-related studies and biomedical applications.

|
Download:
|
| Fig. 9. Spiropyran-polymers for photo-responsive cell detachment. (A) and (B) illustrate the wettabilitychange induced cell detachment. Under UV irradiation, the surfaces change to hydrophilic and the cell detached as microscope images shows. (C) and (D) illustrate that MC increase cell adhesion maybe result from the specific interaction between cells and MC. The microscope image in (D) demonstrates the cells can adhere on UV treated surface. Reproduced with permission [67, 68]. Copyright 2004 [67], 2005 [68], American Chemical Society. | |
3.3. Three-dimensional hydrogels
Photo-responsive polymers can also be promising candidates to fabricate three-dimensional hydrogels for biological applications, such as drug delivery [70], cell encapsulation and release [71], and cell fate manipulation [72]. The hydrogels usually occur degradation due to the photo-responsive cleavage [73] or isomerization [16, 45, 74]. For example, a multi-responsive hydrogel system co-assembled from phenylalanine derivative gelator (LPF2) and azobenzene derivative (PPI) is constructed for controllable cell encapsulation and release [71]. The UV light induced transition from trans-azobenzene (E-PPI) to cis-azobenzene (Z-PPI) vanished hydrogen bonding interactions of the hydrogel which further resulted hydrogel collapse in Figs. 10A and B. And the NIH 3T3 cells entrapped in the hydrogel which had proliferated well, can be released to the bulk solution as shown in Fig. 10C. Besides the direct release of cells, the degradation of hydrogel can tune the morphology and fate of stem cell [72]. The hydrogel based on PEG with nitrobenzyl moieties can undergo photo-cleavage which changes the stiffness of hydrogel at some degree modulated by light intensity and wavelength. This flexible strategy for tuning cell fate could provide a real-time manipulation which may apply for tissue engineering. Besides, photo-responsive hydrogels have also been used for drug delivery temporal-spatially. For example, hydrogel constructed by amphiphilic triblock copolymers consist of poly(N-isopropylacrylamide)-b-poly(4-acryloylmorpholine)-b-poly(2-((((2-nitrobenzyl)oxy)carbonyl) amino)ethyl methacrylate) (PNIPAM-b-PNAM-b-PNBOC) can be engineered as new carrier to delivery both hydrophilic gemcitabine (GCT) and hydrophobic doxorubicin (DOX) [75]. This method will inspired people to apply photo-responsive hydrogel in clinical applications on-demand. Recently, the more promising bioapplications of hydrogels which are not limited to photo-responsive polymers are their spatiotemporal controllability for tissue engineering [76]. For example, hostguest hydrogel that consists of adamantane-hyaluronic acid and β-cyclodextrin-hyaluronic acid can be printed into filament and complex self-supporting structures by the aid of UV light at high resolutions [77]. Biological structures like granular structure [78], human femur structure and human right coronary arterial tree [79] have been demonstrated using hydrogels. In the aspect of organmimicking, the photo-responsive hydrogels may open a gate of dynamic and high resolution strategy for bioapplications.

|
Download:
|
| Fig. 10. Photo-responsive three-dimensional hydrogel for controllable cell encapsulation and release. (A) Schematic illustration of photo-responsive hydrogel collapse resulting from the vanishment of hydrogen bonding interactions. (B) Hydrogel can transform to solution with 30 min by UV light irradiation. (C) Cell number before UV light can increase as elongating culture time from one day to seven days which suggests the biocompatibility. Reproduced with permission [71]. Copyright 2015, American Chemical Society. | |
4. Conclusion
Photo-responsive polymer materials from zero-dimensional micelles, two-dimensional surfaces to three-dimensional hydrogels have been designed, synthesized and applied for various biological fields. Many remarkable works of photo-responsive polymers have been reported with advantages of noninvasion and spatiotemporal control. In this review, we briefly summarized the remarkable progress of photo-responsive polymers with irreversible or reversible moieties and their further biological applications in drug delivery and cell manipulation. However, there are still many challenges which need to be addressed. Firstly, the future challenges lie in extending the types of photo-responsive moieties to provide excellent photo-responsiveness with facile synthetic and modified methods. Secondly, the biocompatibility of photoresponsive materials should be improved by introducing appropriate photophysical tools like UCNPs or photo-thermal materials. Finally, the photo-responsive polymers should be expanded to widely biological and medical applications such as artificial muscle, iris and vessel. Although these challenges are still tough to overcome, the photo-responsive polymer materials are promising candidates in sweeping fields.
AcknowledgmentsThis research is supported by the National Natural Science Foundation of China (Nos. 21425314, 21501184, 20141061), Beijing Municipal Science & Technology Commission (No. Z161100000116037), the Top-Notch Young Talents Program of China, and Youth Innovation Promotion Association, CAS (No. 2017036).
| [1] |
F.D. Jochum, P. Theato, Chem. Soc. Rev. 42(2013) 7468-7483. DOI:10.1039/C2CS35191A |
| [2] |
T.L. Sun, G.J. Wang, L. Feng, et al., Angew. Chem. Int. Ed. 43(2004) 357-360. DOI:10.1002/(ISSN)1521-3773 |
| [3] |
H. Yu, X. Qiu, S.P. Nunes, K.V. Peinemann, Nat. Commun. 5(2014) 4110-4119. |
| [4] |
L. Rocha, C.M. Paiu ş, A. Luca-Raicu, et al., J. Photochem. Photobiol. A 291(2014) 16-25. DOI:10.1016/j.jphotochem.2014.06.018 |
| [5] |
S. Jeon, J.M. Moon, E.S. Lee, Y.H. Kim, Y. Cho, Angew. Chem. Int. Ed. 53(2014) 4597-4602. DOI:10.1002/anie.201309998 |
| [6] |
M.A.C. Stuart, W.T.S. Huck, J. Genzer, et al., Nat. Mater. 9(2010) 101-113. DOI:10.1038/nmat2614 |
| [7] |
O. Bertrand, J.F. Gohy, Polym. Chem. 8(2017) 52-73. DOI:10.1039/C6PY01082B |
| [8] |
S.W. Li, Y.Y. Feng, P. Long, C.Q. Qin, W. Feng, J. Mater. Chem. C 5(2017) 5068-5075. DOI:10.1039/C7TC00142H |
| [9] |
Y. Nabetani, H. Takamura, A. Uchikoshi, et al., Nanoscale 8(2016) 12289-12293. DOI:10.1039/C6NR02177H |
| [10] |
Y. Zhang, C. Peng, B. Cui, et al., Adv. Mater. 28(2016) 8538-8545. DOI:10.1002/adma.201602411 |
| [11] |
Q. Tang, Y.T. Nie, C.B. Gong, et al., J. Mater. Chem. 22(2012) 19812-19820. DOI:10.1039/c2jm34522f |
| [12] |
Y.Z. Yang, Q. Tang, C.B. Gong, et al., New J. Chem. 38(2014) 1780-1788. DOI:10.1039/C3NJ01598J |
| [13] |
M.E. Lee, A.M. Armani, ACS Sensors 1(2016) 1251-1255. DOI:10.1021/acssensors.6b00491 |
| [14] |
D.Y. Kim, D.G. Kang, M.H. Lee, et al., Chem. Commun. 52(2016) 12821-12824. DOI:10.1039/C6CC06901K |
| [15] |
L. De Sio, S. Serak, N. Tabiryan, T. Bunning, J. Mater. Chem. C 2(2014) 3532-3535. DOI:10.1039/c4tc00229f |
| [16] |
E. Borre, S. Bellemin-Laponnaz, M. Mauro, J. Chem. Eur. 22(2016) 18718-18721. DOI:10.1002/chem.v22.52 |
| [17] |
W.D. Ji, N.J. Li, D.Y. Chen, et al., J. Mater. Chem. B 1(2013) 5942-5949. DOI:10.1039/c3tb21206h |
| [18] |
P. Anilkumar, E. Gravel, I. Theodorou, et al., Adv. Funct. Mater. 24(2014) 5246-5252. DOI:10.1002/adfm.v24.33 |
| [19] |
K. Sumaru, K. Kikuchi, T. Takagi, et al., Biotechnol. Bioeng. 110(2013) 348-352. DOI:10.1002/bit.24617 |
| [20] |
P. Weis, D. Wang, S. Wu, Macromolecules 49(2016) 6368-6373. DOI:10.1021/acs.macromol.6b01367 |
| [21] |
X. Yang, L. Zhou, L. Lv, X. Zhao, L. Hao, Colloid Polym. Sci. 294(2016) 1623-1632. DOI:10.1007/s00396-016-3915-6 |
| [22] |
C.Q. Qin, Y.Y. Feng, W. Luo, et al., J. Mater. Chem. A 3(2015) 16453-16460. DOI:10.1039/C5TA01543J |
| [23] |
W. Feng, W. Luo, Y.Y. Feng, Nanoscale 4(2012) 6118-6134. DOI:10.1039/c2nr31505j |
| [24] |
V. Marturano, P. Cerruti, M. Giamberini, B. Tylkowski, V. Ambrogi, Polymers 9(2017) 8-26. |
| [25] |
J.J. Yu, Z.Q. Cao, Q. Zhang, et al., Chem. Commun. 52(2016) 12056-12059. DOI:10.1039/C6CC06458B |
| [26] |
D. Hu, Y.F. Li, Y.L. Niu, et al., RSC Adv. 4(2014) 47929-47936. DOI:10.1039/C4RA07345B |
| [27] |
J. Yang, W.D. He, C. He, et al., J. Polym. Sci. Part A:Polym. Chem. 51(2013) 3791-3799. DOI:10.1002/pola.v51.18 |
| [28] |
G. Koçer, J. Ter Schiphorst, M. Hendrikx, et al., Adv. Mater. 29(2017) 1606407-1606414. DOI:10.1002/adma.v29.27 |
| [29] |
L.L. Yu, N. Ren, K. Yang, M. Zhang, L. Su, J. Appl. Polym. Sci. 133(2016) 44003-44011. |
| [30] |
M.A. Cole, N.H. Voelcker, H. Thissen, H.J. Griesser, Biomaterials 30(2009) 1827-1850. DOI:10.1016/j.biomaterials.2008.12.026 |
| [31] |
W. Chen, Y. Ma, J.M. Pan, et al., Polymers 7(2015) 1689-1715. DOI:10.3390/polym7091478 |
| [32] |
P. Christogianni, M. Moniruzzaman, G. Kister, Macromol. Symp. 354(2015) 55-61. DOI:10.1002/masy.201400107 |
| [33] |
C. Ventura, P. Thornton, S. Giordani, A. Heise, Polym. Chem. 5(2014) 6318-6324. DOI:10.1039/C4PY00778F |
| [34] |
Z.Z. Tong, J.Q. Xue, R.Y. Wang, et al., RSC Adv. 5(2015) 4030-4040. DOI:10.1039/C4RA12844C |
| [35] |
J. Yang, Z.T. Li, Y.J. Zhou, G.C. Yu, Polym. Chem. 5(2014) 6645-6650. DOI:10.1039/C4PY01042F |
| [36] |
T. Suzuki, T. Moriya, R. Endo, N. Iwasaki, Polym. Chem. 8(2017) 761-768. DOI:10.1039/C6PY02036D |
| [37] |
A. Singh, O. Kuksenok, A.C. Balazs, Soft Matter 12(2016) 9170-9184. DOI:10.1039/C6SM02006B |
| [38] |
R. Klajn, Chem. Soc. Rev. 43(2014) 148-184. DOI:10.1039/C3CS60181A |
| [39] |
J. Jiang, X. Tong, D. Morris, Y. Zhao, Macromolecules 39(2006) 4633-4640. DOI:10.1021/ma060142z |
| [40] |
J. Babin, M. Pelletier, M. Lepage, et al., Angew. Chem. Int. Ed. 48(2009) 3329-3332. DOI:10.1002/anie.v48:18 |
| [41] |
J. Jiang, X. Tong, Y. Zhao, J. Am. Chem. Soc. 127(2005) 8290-8291. DOI:10.1021/ja0521019 |
| [42] |
J.M. Schumers, C.A. Fustin, J.F. Gohy, Macromol. Rapid Commun. 31(2010) 1588-1607. DOI:10.1002/marc.201000108 |
| [43] |
W. Szymanski, J.M. Beierle, H.A.V. Kistemaker, W.A. Velema, B.L. Feringa, Chem. Rev. 113(2013) 6114-6178. DOI:10.1021/cr300179f |
| [44] |
K.G. Yager, C.J. Barrett, J. Photochem. Photobiol. A 182(2006) 250-261. DOI:10.1016/j.jphotochem.2006.04.021 |
| [45] |
M.W. Wang, X.J. Zhang, L. Li, et al., Macromol. Mater. Eng. 301(2016) 191-198. DOI:10.1002/mame.v301.2 |
| [46] |
D. Wang, P.W. Jiao, J.M. Wang, et al., J. Appl. Polym. Sci. 125(2012) 870-875. DOI:10.1002/app.v125.2 |
| [47] |
J.F. Gohy, Y. Zhao, Chem. Soc. Rev. 42(2013) 7117-7129. DOI:10.1039/c3cs35469e |
| [48] |
C.J. Chen, G.Y. Liu, Y.T. Shi, et al., Macromol. Rapid Commun. 32(2011) 1077-1081. DOI:10.1002/marc.v32.14 |
| [49] |
R.S. Lee, S.W. Wang, Y.C. Li, J.Y. Fang, RSC Adv. 5(2015) 497-512. DOI:10.1039/C4RA13702G |
| [50] |
L. Shao, B. Hua, J. Yang, G.C. Yu, Chem. Commun. 52(2016) 6573-6576. DOI:10.1039/C6CC02434C |
| [51] |
S.L. Lin, Y.Y. Wang, C.H. Cai, et al., Nanotechnology 24(2013) 085602-085611. DOI:10.1088/0957-4484/24/8/085602 |
| [52] |
L.C. Liu, L.L. Rui, Y. Gao, W.A. Zhang, Polym. Chem. 5(2014) 5453-5460. DOI:10.1039/C4PY00645C |
| [53] |
Y. Yang, L. Yue, H.L. Li, et al., Small 8(2012) 3105-3110. DOI:10.1002/smll.v8.20 |
| [54] |
X. Tong, G. Wang, A. Soldera, Y. Zhao, J. Phys. Chem. B 109(2005) 20281-20287. DOI:10.1021/jp0524274 |
| [55] |
G. Wang, X. Tong, Y. Zhao, Macromolecules 37(2004) 8911-8917. DOI:10.1021/ma048416a |
| [56] |
S. Chen, Y. Gao, Z. Cao, et al., Macromolecules 49(2016) 7490-7496. DOI:10.1021/acs.macromol.6b01760 |
| [57] |
Q.J. Xing, N.J. Li, Y. Jiao, et al., RSC Adv. 5(2015) 5269-5276. DOI:10.1039/C4RA12678E |
| [58] |
J.D. Humphries, A. Byron, M.J. Humphries, J. Cell Sci. 119(2006) 3901-3903. DOI:10.1242/jcs.03098 |
| [59] |
U. Hersel, C. Dahmen, H. Kessler, Biomaterials 24(2003) 4385-4415. DOI:10.1016/S0142-9612(03)00343-0 |
| [60] |
Y. Ohmuro-Matsuyama, Y. Tatsu, Angew. Chem. Int. Ed. 47(2008) 7527-7529. DOI:10.1002/anie.v47:39 |
| [61] |
J. Nakanishi, J. Chem. Asian 9(2014) 406-417. DOI:10.1002/asia.201301325 |
| [62] |
S.F.M. van Dongen, P. Maiuri, E. Marie, C. Tribet, M. Piel, Adv. Mater. 25(2013) 1687-1691. DOI:10.1002/adma.v25.12 |
| [63] |
W. Li, J. Wang, J. Ren, X. Qu, J. Am. Chem. Soc. 136(2014) 2248-2251. DOI:10.1021/ja412364m |
| [64] |
D.S. Shin, J.H. Seo, J.L. Sutcliffe, A. Revzin, Chem. Commun. 47(2011) 11942-11944. DOI:10.1039/c1cc15046d |
| [65] |
P. Shi, E. Ju, J. Wang, et al., Mater. Today 20(2017) 16-21. DOI:10.1016/j.mattod.2016.12.006 |
| [66] |
P. Shi, E. Ju, Z. Yan, et al., Nat. Commun. 7(2016) 13088-13096. DOI:10.1038/ncomms13088 |
| [67] |
A. Higuchi, A. Hamamura, Y. Shindo, et al., Biomacromolecules 5(2004) 1770-1774. DOI:10.1021/bm049737x |
| [68] |
J.I. Edahiro, K. Sumaru, Y. Tada, et al., Biomacromolecules 6(2005) 970-974. DOI:10.1021/bm0493382 |
| [69] |
K. Kikuchi, K. Sumaru, J.I. Edahiro, et al., Biotechnol. Bioeng. 103(2009) 552-561. DOI:10.1002/bit.v103:3 |
| [70] |
L.A. Wells, M.A. Brook, H. Sheardown, Macromol. Biosci. 11(2011) 988-998. DOI:10.1002/mabi.v11.7 |
| [71] |
G.F. Liu, W. Ji, W.L. Wang, C.L. Feng, ACS Appl. Mater. Interfaces 7(2015) 301-307. DOI:10.1021/am506202s |
| [72] |
A.M. Kloxin, A.M. Kasko, C.N. Salinas, K.S. Anseth, Science 324(2009) 59-63. DOI:10.1126/science.1169494 |
| [73] |
S. Tamesue, S. Abe, T. Mitsumata, N. Tsubokawa, T. Yamauchi, J. Polym. Sci. Part A:Polym. Chem. 54(2016) 1317-1322. DOI:10.1002/pola.v54.10 |
| [74] |
S.Y. Dong, L.Y. Gao, J.Y. Li, D.H. Xu, Q.Z. Zhou, Polym.Chem. 4(2013) 3968-3973. DOI:10.1039/c3py00494e |
| [75] |
C. Wang, G. Zhang, G. Liu, J. Hu, S. Liu, J. Control. Release 259(2017) 149-159. DOI:10.1016/j.jconrel.2016.11.007 |
| [76] |
J. Leijten, J. Seo, K. Yue, et al., Mater. Sci. Eng. R 119(2017) 1-35. DOI:10.1016/j.mser.2017.07.001 |
| [77] |
C.B. Highley, C.B. Rodell, J.A. Burdick, Adv. Mater. 27(2015) 5075-5079. DOI:10.1002/adma.201501234 |
| [78] |
T. Bhattacharjee, S.M. Zehnder, K.G. Rowe, et al., Sci. Adv. 1(2015) e1500655. DOI:10.1126/sciadv.1500655 |
| [79] |
T.J. Hinton, Q. Jallerat, R.N. Palchesko, et al., Sci. Adv. 1(2015) e1500758. DOI:10.1126/sciadv.1500758 |
 2017, Vol. 28
2017, Vol. 28 


