b University of Chinese Academy of Sciences, Beijing 100049, China
Bulk heterojunction (BHJ) organic solar cells (OSCs) have attracted more and more interest on immeasurable prospects of commercial application due to the unique characters of low costs, light weights, flexibility, translucence and simple preparation technology [1, 2]. Over the past decades, various strategies, such as material design [3], morphology control [4-6] and interfacial engineering [7, 8] have been adopted to improve the power conversion efficiencies (PCEs) of BHJ OSCs. Thereinto, the studies on donor materials promote the rapid development of BHJ OSCs in recent years [9]. With enormous efforts, the PCEs of single junction OSCs have reached to 13.1% [10] and 11.53% [11] for polymer and small molecule donor materials, respectively. Compared with polymers, small molecules possess remarkable superiorities, such as well-defined chemical structures, high purity, no batch-to-batch variation and adjustable molecular constructions [12-14]. These features ensure the reproducibility of the device efficiency and also provide more possibility of the molecular design. Despite the progress of small molecules lagged far behind that of polymers in the early stages, following the intense studies with employing donor-acceptor (D-A) structures, the device performance of numerous small molecules has been close to 10%, comparable to that of polymers [11, 15-18].
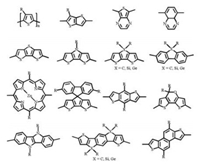
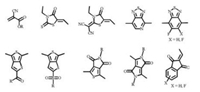
The energy conversion efficiency of solar cells is defined by the general formula: PCE = Voc × Jsc × FF/Pin, where the PCE depends on the three decisive parameters, open circuit voltage (Voc), short circuit current density (Jsc) and fill factor (FF); Pin is the given incident light power density [6, 19-21]. However, in the design of small molecular donor materials, these parameters are not independent of each other. For example, tuning the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels possibly lead to the change of band gaps or amounts of exciton generation [3]. As a consequence, comprehensive factors should be considered to legitimately design the versatile donor materials with high performance. The usage of D-A structural frameworks is an effective solution [22-24]. According to the molecular orbital theory, the frontier molecular orbitals of electron donating and electron-withdrawing units in D-A structural molecules will be recombined to form a new absorption band, named intramolecular charge transfer absorption band (ICT), with smaller band gap conducive to red shift of light absorption [25, 26]. In addition, the shortened band gap should be mainly resulted from the lowered LUMO energy levels, and consequently, the strategy of D-A molecular design would reduce the molecular band gaps but without sacrificing Voc. Furthermore, due to the existence of push and pull electronics effect in D-A systems, π electrons can delocalize to the whole molecule more effectively leading to better conjugated system and charge transfer [32]. D-A structures also enrich the design due to plentiful donor and acceptor units could be chosen from. For instance, oligothiophenes, benzodithiophene (BDT), dithienosilole (DTS), indacenodithiophene (IDT), naphtho[1, 2-b: 5, 6-b']dithiophene (NDT) etc. are widely used as electron-donating units [1, 4]. The commonly used electronwithdrawing units include benzodiathiazole (BT), diketopyrrolopyrrole (DPP), alkyl cyanoacetate, rhodanine, dicyanovinyl, 1, 3-indanedione and so on (Figs. 1 and 2) [3, 9].
|
Download:
|
| Fig. 1. Chemical structures of commonly used donor units. | |
|
Download:
|
| Fig. 2. Chemical structures of commonly used acceptor units. | |
Hence, many kinds of D-A constructions, D-A-D, A-D-A, A-π-D-π-A, A-A-D-A-A etc., were finely applied to enhance the performance of OSCs by tuning the energy levels, broadening absorptions or improving charge mobility [9]. Meanwhile, the intramolecular and intermolecular electron cloud densities based on different D-A constructions and also exhibit significant impacts on intermolecular coupling and subsequently film morphology control. Though there are various configurational D-A structures applied in OSCs, a summary of the relationship between material D-A constructions and device performance has been not reported. In this review, we summarize recent design of solution-processed small molecule donor materials through different D-A structural frameworks and emphasize their influences on optical properties, phase separation, charge transport and device performance. At the same time, our group has also done a lot of works in this area and achieved remarkable results, which will be introduced in the brief overview together.
2. D-A-D structural small moleculesD-A-D structural small molecules use an electron-withdrawing unit as central core and two electron-donating units as terminal groups in the axisymmetric framework. This structure widely applies DPP as acceptor core in small molecules [27-35]. DPP has two strong electron-withdrawing lactam structures and rigid plane leading to strong absorption and π-π stacking. Moreover, the properties of small molecules, such as molecular crystallinity, absorption and charge carrier mobility are strongly affected by the nature of the substituents on N, N-positions of DPP.
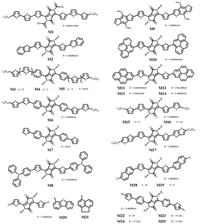
In 2008, DPP was applied to D-A-D structural small molecule solution-processed (OSCs) for the first time by Nguyen [27]. They synthesized the M1 (Fig. 3) consisting of a DPP core with t-butoxy carbonyl groups attached on the N, N-positions and terthiophene terminal arms. The material showed a broad optical absorption band in solution as well as film. Using PC61BM as electron acceptor, the highest PCE of 2.33% (Table 1) was created for small molecular BHJ OPVs at that time. Soon after, they utilized a fused benzofuran unit to instead of oligothiophene, aiming at increasing the Voc. Blended with PC71BM, the material (M2) (Fig. 3) exhibited a PCE of 4.4% and Voc of 0.96 V (Table 1) with good quality films after thermal annealing [28]. In order to understand the relationship between chemical structure and device performance of DPP-based molecules, Nguyen group gave a comprehensive study on a series of D-A-D structural phenyl substituted DPP-based small molecules (M3-M7) in 2014 (Fig. 3) [29]. Among them, M4 displayed the best PCE of 3.45% (Table 1). Whereas, M6 with bulky ethyl-hexyl groups produced the poorest PCE of 0.76% (Table 1) due to the scarce exciton separating and limited charge carrier mobility, both of which caused by unsatisfactory film morphology. When linear alkyl chains on terminal thiophenes were removed from M4 to form M7, the result was similar to M6, only a low PCE of 1.11% (Table 1) was obtained. M3 and M5 showed different conjugation length compared with M4, and they exhibited inferior device performance because of disordered molecular ordering and undesirable solubility for M3 and M5, respectively.
|
Download:
|
| Fig. 3. Chemical structures of small molecules based D-A-D framework. | |
|
|
Table 1 The electrochemical, optical properties and photovoltaic performances of small molecules based on D-A-D framework. |
In 2011, Fréchet et al. synthesized a series of small molecules (M8-M14) (Fig. 3) with different electron-rich end groups [30]. These end groups were expected to affect π-stacking by their varying degrees of planarity. Through device manufacture, DPP with C2-pyrene (M12) showed the best PCE of 4.1% with a high FF of 58% (Table 1) because of its completely planar moiety. When changing the site of pyrene substitution from C2 to C1, the device performance dropped to 0.7% (Table 1), elucidating that molecules consisting of fluorinated benzothiadiazole acceptor units as cores and oligothiophenes donor units of different length symmetry of end groups had a dramatic influence on device efficiency. Besides, modulating N-alkyl substituents on DPP core could ulteriorly optimize device performance. A number of other terminal groups have been studied in DPP-based small molecules, including selenophene, thieno[3, 2-b]thiophene, (fluoronaphthyl)thienyl, dibenzofuran, acenaphtene etc. [31-33]. Recently, Jassen group reported a work on introducing two hexyl side chains to different positions of the end thiophenes (M22-M25) (Fig. 3). Learning from the absorption and fluorescence spectra, they found that J-and Htype aggregates could be effectively tuned by modulating alkyl chains positions [34].
Except DPP as acceptor core for D-A-D structural small molecules, other electron-withdrawing groups are also explored. In 2014, our group designed and synthesized a series of small (1 to 6 thiophene units) as end arms [35]. Among these molecules, M26 and M27 (Fig. 4) showed better performance for solutionprocessed OSCs with optimized PCE of 3.81% and 3.21% (Table 1), respectively. The lower PCE of M27 is due to its lower Voc resulted from its higher HOMO energy level. Through comparative study, we found that the molecular length had significant impact on the properties of materials, especially the crystalline behaviors, phase separation and active layer morphologies.
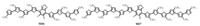
|
Download:
|
| Fig. 4. Chemical structures of small molecules our group reported based D-A-D framework. | |
The above studies indicate that DPP is commonly used in D-A-D structural small molecules as the central core due to its excellent photoelectric properties and simple synthetic pathways [36]. Some effective strategies, for instance, adopting diverse planar donors as end groups, choosing different alkyl side chains and changing their positions, could finely modulate the energy levels, optical properties, molecular solubility and packing [29, 30]. Beyond that, our group's studies also demonstrate that more electron-withdrawing units could be used to design D-A-D structural small molecules with better efficiencies.
3. A-D-A structural small moleculesJust on the contrary of D-A-D structure, A-D-A structural small molecules use an electron-donating group as core and end-capped with electron-withdrawing groups. In A-D-A small molecules, oligothiophenes [15, 37-43], BDT [44-46], porphyrin [47, 48] etc. are commonly applied as central groups and exhibit great device efficiencies. Among them, oligothiophene-based small molecules are studied most widely and systematically.
Oligothiophenes capitalized on polymer and small molecule OSCs shows excellent natures of highly p electrons delocalization and high hole mobility. Connecting with electron-withdrawing groups, the photoelectrical properties, band gaps and molecular packing of oligothiophene-based small molecules would be well adjusted to achieve high performance for OPVs. In 2006, Bäuerle et al. firstly synthesized an A-D-A small molecule M28 (Fig. 5) containing a quinquethiophene core and two dicyanovinyl terminal groups [37]. Compared to the thin films of unsubstituted quinquethiophene, the introduction of dicyanovinyl strong electron-withdrawing units led to the optical band gap decreasing from 2.5 eV to 1.77 eV. With PCBM as acceptor, the device through vacuum processing based on the material showed a relatively excellent PCE of 3.4% (Table 2) in that time. Later, a lot of research on A-D-A structural oligothiophene-based small molecules for solution-processed OSCs was reported by Chen group. Various electron-withdrawing groups were introduced to oligothiophene cores, including dicyanovinyl, 1, 3-indanedione, 2-(3-oxo-2, 3-dihydroinden-1-ylidene)malononitrile, rhodanine, cyanoacetate etc. [15, 38-41] In addition, based on 2-(1, 1-dicyanomethylene) rhodanine as the end groups, a series of small molecules (M37-M42) (Fig. 5) with different number thiophene units were synthesized to explore the delicate balance among various influencing factors [15]. Through deep investigation, they found that molecules with odd number thiophenes demonstrated much higher Jsc than those based on even number thiophenes, which should benefit from their well interpenetrating network with a domain size less than 20 nm. Among this series of molecules, M38 displayed the best device performance and its PCE was 10.08% with Voc of 0.94 V, Jsc of 15.88 mA/cm2 and FF of 69% (Table 2). The high PCE was attributed to its broader absorption and more effective molecular packing. More significantly, blended with three-dimensional perylene diimide, a small molecule acceptor, the all-small molecule OSCs exhibited excellent device performance with PCE achieved 6.1% (Table 2) [42]. The result indicated that this small molecule could be used for the study of all-small molecule OSCs. In 2016, Peng et al. introduced two fluorine atoms to central thiophenes of M43 to form a novel small molecule M44 (Fig. 5) [43]. Compared with M43, this molecule owned lower HOMO energy level and better molecular packing leading to higher Voc.

|
Download:
|
| Fig. 5. Chemical structures of small molecules based A-D-A framework. | |
|
|
Table 2 The electrochemical, optical properties and photovoltaic performances of small molecules based on A-D-A framework. |
BDT is also a commonly used donor unit for D-A structural small molecules. Employing DPP as electron-withdrawing terminal units, the A-D-A small molecules based on BDT show preferable device performance. Zhan et al. reported a 5-alkylthiophene-2-ylsubstituted BDT-based small molecule M45 (Fig. 5) for BHJ solar cells in 2013 [44]. Linking with DPP, the molecule showed strong absorption at the band of 500–750 nm with a narrow band gap of 1.67 eV and high hole mobility. Blended with PC61BM, the PCE of 5.79% (Table 2) was achieved after thermal annealing. This device efficiency was the top value among DPP-based small molecules at that time. Later, a series of related studies had also been published, such as alkyl side chains instead of thiophene-based side chains on BDT or replacing the end groups [45, 46].
Except above research, porphyrin is also used in DPP-based AD-A structural donor materials. In 2015, molecule M46 (Fig. 5) with a porphyrin ring tying to two DPP units by ethynylene bridges was designed and synthesized by Peng group [47]. The material attained a broad absorption coverage in the visible and nearinfrared from 400 nm to 900 nm, which led to a high Jsc of 16.76 mA/cm2 (Table 2). Ethynylene used to connect porphyrin and DPP resulted in lowing molecular HOMO level, enhancing intermolecular π-π stacking and facilitating intramolecular charge transfer. When pyridine additive and thermal annealing were applied to M46/PC61BM, the device performance got an effective promotion with PCE of 8.08% (Table 2) [47]. When changing the side chains on DPP with 2-ethylhexyl (M47) (Fig. 5), solar radiation from 300 nm to 900nm could be harvested to ameliorate the Jsc with an outstanding PCE of 9.06% (Table 2) [48]. Recently, in order to obtain a higher Voc with a wide absorption, Cao and coworkers reported a small molecule M49 (Fig. 5) by introducing benzothiophene groups to the meso positions of the porphyrin core. The molecule showed a PCE of 9.08% with Voc of 0.80 V, Jsc of 16.82 mA/cm2 and FF of 67.54% (Table 2) [49].
On account of the rich electrical properties of oxygen atom, 5H-dithieno[3, 2-b: 2', 3'-d]pyran (DTP) was applied as an electrondonating unit in low band gap polymers for high performance polymer solar cells [50]. In 2015, Jo et al. utilized DTP and DPP as donor unit and acceptor units to synthesize a low band gap A-D-A small molecule M50 (Fig. 5) [50]. Blended with PC71BM and P (NDI2OD-T2), a polymeric electron acceptor, for small molecule OSCs, it showed favorable PCEs of 6.88% and 4.8% (Table 2), respectively. This work elucidated that the M50 was a versatile donor for fullerene derivatives and non-fullerene acceptors.
In A-D-A structural small molecule materials, oligothiophenebased materials are systematically studied. By introducing different acceptor end groups, these materials possess good transport properties, easily tunable optical and electro chemical properties. The above studies also show that subtle adjustment would be a powerful way for meliorating molecular properties. In relative terms, other donor units in A-D-A structural molecules, such as BDT, porphyrin, DTP, mainly employ DPP as electronwithdrawing groups due to its strong electron-withdrawing property, which could lower band gaps and red-shift absorption.
4. A-π-D-π-A structural small moleculesBeing different with A-D-A structural small molecules, the A-π-D-π-A structural small molecules have two more π-bridges between donor core and acceptor arms. The π-bridge plays an important role in D-A structural small molecules, because it not only possesses a high charge transport property in the D-A backbone, but also enhances the conjugation degree of the molecule [51]. This kind of structure is mainly applied to BDTbased donor materials with high performance [16, 18, 52-59]. Other molecules, for instance, DTS [60, 61], porphyrin [62], spiro[cyclopenta[1, 2-b: 5, 4-b']dithiophene-4, 9'-fluorene] (STF) [63], NDT [64] and dihydroindolo-indole (DINI) [65] based donor materials also utilize this structure framework to design target compounds.
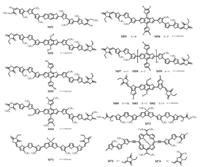
BDT unit owns symmetric and plain conjugated structure which could facilitate forming π-π stacking. In order to pursue the higher OPV performance, Chen et al. replaced the central thiophene unit in M31 with the more electron-rich and planar BDT unit to obtained the A-π-D-π-A small molecule M51 (Fig. 6) [52]. Following that, a series of similar structural BDT-based small molecules were rapidly developed. In these structural molecules, oligothiophenes were commonly employed as π-bridge, and it not only broadened the absorption but also promoted strong π-π stacking of conjugated skeletons [51]. M52 (Fig. 6), a splendid material with alkylthiol displacing alkyloxy on central BDT core was reported, showing the high PCE of 9.95% (Table 3) after combining thermal and solvent vapor annealing in 2014 [16]. In organic semiconductors, alkylthio side chains exhibited some unique optoelectronic properties and benefited for better molecular packing. Some similar works were also published in succession. In 2015, based on previous works [66, 67], Li et al. synthesized small molecule M53 (Fig. 6), which owning PCE of 9.2% (Table 3) without solvent additive or post-processing [53]. Interestingly, by changing the position of alkyl side chains on π-bridges, the small molecule M54 (Fig. 6) showed nematic liquid crystalline behavior and possessed excellent optoelectronic properties with FF of 77% (Table 3). Using M54 to fabricate thick-layer small molecule solution-processed OSCs still held high FF of ~70% and high PCE of ~8% (Table 3) [54]. In 2016, fluorine atoms were introduced to thienyl substituted BDT (TBDT) of M55 (Fig. 6) to obtain the small molecule M56 (Fig. 6) by Peng group [18]. Because of the effect of fluorine atoms, some unique qualities of M56 were achieved: lower lying HOMO energy level, stronger absorption and higher crystallinity. Mixing with PC71BM, high performance of 9.24% and 9.80% (Table 3) were obtained without and with a solvent annealing, respectively. About the same time, another interesting work about the influences of TBDT number was reported. Among these molecules (M57-M59) (Fig. 6), M58 gave the best efficiency of 8.56% (Table 3) with desired interconnected structure. In addition, in large-area OSCs (up to 77.8 cm2), the molecule also exhibited outstanding properties with a PCE of 7.45% [55]. Recently, three small molecules (M60-M62) (Fig. 6) based on different side chains on BDT moiety were synthesized. In these molecules, bithiophene was employed as π-bridge. Compared with another two molecules, M62 possessed stronger π-π stacking and a multi-length scaled phase separation being in favor of higher carrier mobilities. As a result, M62 obtained a highest PCE of 8.68% (Table 3) [56].
|
Download:
|
| Fig. 6. Chemical structures of small molecules based A-π-D-π-A framework. | |
|
|
Table 3 The electrochemical, optical properties and photovoltaic performances of small molecules based on A-π-D-π-A framework. |
Our group also pays attention on A-π-D-π-A structural BDTbased small molecules. In order to access to appropriate miscibility with fullerene derivatives, we designed and synthesized two small molecules (M63, M64) (Fig. 7) consisting of TBDT as core, trithiophene as π-bridge and oxo-alkylated nitrile as acceptor end groups substituted by octyl and pentyl chains in 2014 [57]. Through shortening alkyl chains, we found that it could induce tighter molecular stacking and better crystallinity when blended with PC71BM. Hence, it showed a PCE of 5.6% with Voc of 0.87 V, Jsc of 9.94 mA/cm2 and an excellent FF of 65% (Table 3). In 2015, we reported three TBDT-based small molecules (M65-M67) (Fig. 7) with three subtle changed terminal groups [58]. The different electron-withdrawing ability on acceptor units led to different molecular interactions, morphologies, HOMO levels and absorptions in films. The maximum PCEs of M65 and M66 were both up to 6.4% (Table 3). However, the optimized PCE of M67 is 3.0% because of its undesirable molecular packing and crystalline. In 2016, the donor material of highest performance based on inverted small molecule OSCs was synthesized by our group. This series of small molecules (M68-M70) (Fig. 7) contained TBDT as donor unit, 2-(thiophen-2-yl)thieno[3, 2-b]thiophene as π-bridges with stronger aromaticity and gradient decreased electron density and indenadione derivatives as terminal groups substituted by various fluorine atoms (0F, 1F and 2F, respectively) [59]. We found that introducing fluorine atoms to end acceptors could not only promote absorption red-shift, but also lead to an optimal active layer morphology with an enhanced domain purity, formation of hierarchical domain size and a directional vertical phase gradation. By employing PC71BM as acceptor material of inverted small molecule solution-processed OSCs, the three novel small molecules gave amazing cell performance of 8.30%, 10.4% and 11.3% (Table 3), respectively [59]. This remarkable work suggested that the introduction of fluorine atoms can control the active layer morphology effectively.
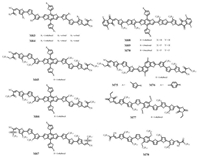
|
Download:
|
| Fig. 7. Chemical structures of small molecules our group reported based A-π-D-π-A framework. | |
Other donor units are also adopted as electron-donating cores in A-π-D-π-A structural small molecules with various electronwithdrawing end units. In 2011, Chen et al. published a work about DTS-based small molecule (M71) (Fig. 6) with terthienyl π-bridges and octyl cyanoacetate end arms [60]. It exhibited orderly arrangement in the thin film and gave a PCE of 5.84% (Table 3). When using 3-ethylrhodanine to replace octyl cyanoacetate as terminal groups and 2-ethylhexyl as side chains on DTS, M72 (Fig. 6) was synthesized. After thermal and solvent annealing, the PCE based on M72 was up to 8.02% (Table 3) due to better morphology in films [61]. The strategies of changing bridge atoms on DTS, converting alkyl chains and tuning π-bridge length were also applied to achieve desired device performance [69, 70]. To imitate the chlorophylls absorbing light and storing light energy, Zhu and co-workers developed two new porphyrin-based small molecules (M73, M74) (Fig. 6), based on A-π-D-π-A framework for OSCs [62]. Benefiting from applying terthiophene π-bridge and 2-octylundecyl side chains on porphyrin, a broaden coverage in the visible and near-infrared region and an optimized molecular packing were realized. The PCEs of M73 and M74 reached 7.66% and 8.21% (Table 3), respectively.
Some big fused-ring donor moieties, such as DINI, IDT and STF, possess larger rigid and co-planar structure leading to relatively high hole mobility. In 2016, our group reported two A-π-D-π-A type small molecules based on NDT with alkylthienyl and alkylphenyl as side chains, severally [64]. Compared with M75, M76 (Fig. 7) exhibited the advantage on fabricating thick layer BHJ OSCs. When the layer thickness was up to 300 nm, the PCE of M76-based OSCs still reached 7.20% with high FF of 70%. This is due to its weak electron-donating unit with alkylphenyl side chains led to better intermolecular π-π interactions and phase separation for thick film OSCs. In the same year, a research based on dialkoxyphenyldithiophene (PDT) donor unit was studied by us [68]. There are O…S weak non-covalent interaction between phenyl oxygen atom and adjacent thiophene sulfur atom in PDT which supporting in strong donating ability and decreasing diheral angles between PDT and π bridges to formed more planar molecular backbone. Hence, we designed and synthesized two small molecules (M77, M78) (Fig. 7) consisting of PDT core, bithiophene π-bridges and oxoalkylated nitrile or 3-ethylrhodanine end groups. These molecules showed greater red-shift compared to their BDT analogues, the red-shift of 30 nm was found. After blended with PC71BM, the PCE of 6.64% and 4.16% (Table 3) were for M77 and M78, respectively. Owing to a large phase separation, the PCE of M78 was lower [68].
There are multifarious small molecules apply A-π-D-π-A structure to design donor materials, which indicate that the framework is suitable for most of the donor and acceptor units benefiting from the addition of π-bridges. The most common electron-donating cores are big planar fused-ring, for instance, BDT, NDT, IDT, porphyrin, DTS and its derivatives, they show superior planarity, molecular crystalline and charge migration. Our group gives more attention on this type structure with studying different donor units, introducing diversiform acceptor groups and designing excellent π-bridge, and has made admirable promotion for small molecule BHJ OSCs.
5. D-A-D-A-D structural small moleculesOn the basis of A-D-A small molecules, incorporating one more electron-donating end group into both ends of the structure forms the D-A-D-A-D structural skeleton. This symmetrical structure further augments the molecular conjugation length, which could further effectively adjust light absorption and energy level.
D-A-D-A-D structure was given a lot of attention following the success of M79 (Fig. 8) reported by Heeger and co-workers [71]. In 2011, they synthesized this molecule with an amazing PCE of 6.7% (Table 4) for small molecule OSCs in that time. Two high electron affinity [1, 2, 5]thiadiazolo[3, 4-c]pyridine acceptor units were linked to a DTS core, and then end-capped by two hexylsubstituted bithiophenes. Because of the rational design, the molecule showed a broaden absorption from 600 nm to 800 nm, which resulting in an excellent Jsc of 14.4 mA/cm2 (Table 4). In order to avoid the loss of Voc, which caused by the acidic nature of PEDOT: PSS, Bazan et al. designed a versatile small molecule (M80) (Fig. 8) by applying 5-fluorobenzo[c] [1, 2, 5]thiadiazole [72]. Through adding an additive and thermal annealing, a PCE of 7.0% (Table 4) could be achieved. Subsequently, some investigations about the engineering of additions and solutions were carried out by employing M80 as donor material [78, 79]. A polymer additive polystyrene was found to improve device performance up to 8.2% [78]. In 2016, a series of small molecules (M80-M84) (Fig. 8) with different numbers of fluorine atom (from 0F to 4F) on benzo [c][1, 2, 5]thiadiazole were studied by Son and co-workers [73]. Depending on a comprehensive study, they found that small molecules with even-numbered fluorine atoms possessed more remarkable material properties compared to those with oddnumbered fluorine atoms. Therefore, M80 and M84 showed relatively higher PCE of 8.14% and 7.06% (Table 4). Further germanium atom has been used to replace the silicon atom [74]. Due to the larger germanium atom could form longer bond length of C-Ge, the small molecule (M85) (Fig. 8) based on 4, 4'-bis-(2-ethylhexyl)-dithieno[3, 2-b: 2', 3'-d]germole (DTG) exhibited the comparable performance in optical, thermal, and electrical properties. As donor for inverted BHJ OPVs, it showed a high PCE of 7.3% with Voc 0.731 V, Jsc 14.9 mA/cm2 and FF 56.9% (Table 4).

|
Download:
|
| Fig. 8. Chemical structures of small molecules based D-A-D-A-D framework. | |
|
|
Table 4 The electrochemical, optical properties and photovoltaic performances of small molecules based on D-A-D-A-D framework. |
Following the success of silaindacenodithiophene (SIDT) based polymers [80] and M80 [72], Bazan et al.designed a small molecule M86 (Fig. 8) with a relatively weaker donor unit SIDT as central core for lowering HOMO level [75]. As a result, the optimized device based on the small molecule showed a higher Voc (0.91 V). Moreover, the study also showed the liquid crystalline properties during the evolution of the film. In 2016, Beaujuge et al. reported three small molecules (M87-M89) (Fig. 8) based on TBDT as central unit with different sequence of benzo[1, 2-b: 4, 5-b']dithiophene-6, 7-difluoroquinoxaline and alkyl substituted thiophene along the π-conjugated backbone [76]. Among these molecules, the D-A-DA-D structural M87 possessed favorable molecular packing and aggregation patterns in thin films with optimized PCE of 6.6%. In contrast, M89 whose acceptor units were outermost from the central core showed the poor PCE of 2.0% due to significantly low charge transport characteristics. In the same year, our group designed and synthesized a D-A-D-A-D molecule (M90) (Fig. 9) with TBDT as donor units and 5, 6-difluorobenzo[c][1, 2, 5]thiadiazole as acceptor units [77]. For comparison, D-A structural polymer analog was also synthesized. Compared with polymer analog, M90 exhibited better molecular crystalline. Blended with PC71BM for OPVs, the high device performance of 6.02% for M90 could be achieved without any post processing, while polymer analog just showed a low PCE of 2.41%. After adding additives, the optimal PCEs of 8.1% (Table 4) were obtained for M90.

|
Download:
|
| Fig. 9. Chemical structure of small molecule our group reported based D-A-D-A-D framework. | |
The above studies reveal that the D-A-D-A-D structural small molecules is widely used to DTS-based donor materials and exhibits excellent photoelectrical properties. The DTS-based small molecules have strong absorption from 600 nm to 800 nm, but with nethermore Voc because of the higher HOMO energy level [71, 72]. In contrast, BDT-based molecules show the higher Voc due to the lower HOMO energy levels, but with lager band gaps [76, 77]. Furthermore, the success of introducing silicon atom to DTS arouses researchers to pay attention to analogous heterocycle, such as, SIDT and DTG. Our group proposes an unusual extraordinary approach to design D-A-D-A-D structural small molecule by tailoring polymers and could obtain comparable efficiency with their polymer counterpart, suggesting that there are still various efficient D-A-D-A-D small molecules worth to explore.
6. Other D-A structural small moleculesThe above several typical D-A constructions are mostly used in high performance small molecule donor materials for solutionprocessed OSCs. Beyond those, due to the diversity as well as the varying marshalling sequences of donor and acceptor units, there are some other structures are applied to design and synthesize the superior small molecules.
In 2016, Singh et al. synthesized a small molecule M91 (Fig. 10) based on A-A-D-A-A structural framework [81]. This molecule applied DTG as donor unit and 5-fluorobenzo[c][1, 2, 5]thiadiazole as electron-withdrawing groups. It displayed a long-range π-π stacking benefiting for light absorption. Under optimized conditions, the M91/PC71BM blend showed a PCE of 9.1% with a Voc of 0.79 V, a Jsc of 15.9 mA/cm2, and a FF of 73% (Table 5) [81]. A similar work also used A-A-D-A-A structure to design two small molecules based on DTS (M92, M93) (Fig. 10) by Wong et al. [82]. In order to understand the advantages of small molecules and polymers, series of medium-sized narrow band gap conjugated molecules (M94-M102) (Fig. 10) based on D-A-D-A-D-A-D and D-A-D-A-D-AD-A-D were designed and synthesized with DTS and a variety of electron-withdrawing groups in 2014 [83]. The detail study showed that the acceptor subunits in the medium-sized frameworks had more obvious effect on HOMO energy levels and crystallization behavior. Moreover, the fluorine atoms exhibited positive influence on improving performance of BHJs. Among these small molecules, M102 showed the highest PCE of 6.5% with Voc of 0.71 V, Jsc of 13.9 mA/cm2 and FF of 67% (Table 5).
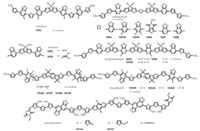
|
Download:
|
| Fig. 10. Chemical structures of small molecules based on novel D-A framework. | |
|
|
Table 5 The electrochemical, optical properties and photovoltaic performances of small molecules based on novel D-A framework. |
In 2016, Wang and co-workers reported three D-A-π-D-π-A-D structural small molecules (M103-M105) (Fig. 10) with furan, thiophene, or selenophene as π-bridges between donor core and acceptor units [84]. With different atom in π-bridge, these molecules showed discrepant film morphology. Through CH2Cl2 annealing, M104 showed the best device performance of 8.7% with an impressive FF of 75% (Table 5). In the same year, several IDTbased or FBT-based D-A structural repeating units small molecules (M106-M109) (Fig. 10) were also synthesized by Wang et al. [85]. They found that the molecules (M107 and M109) with a FBT as the central unit gave slightly higher PCEs than those (M106 and M108) with IDT as the central unit, and all the molecules exhibited higher device performance than their relative polymer.
In 2016, in order to enhance extinction coefficients of small molecule donor materials, our group designed and synthesized two 2-methylene-indene-1, 3-dione end-capped derivatives, M110 and M111 (Fig. 11), based on A-D-A-D-A and A-D-A-D-A-D-A conjugate skeleton, respectively [86]. Meanwhile, the parent molecules M112 and M113 (Fig. 11) were also prepared for comparative study. Compared with parent molecules, the results of UV-visible spectrum and density functional theory (DFT) calculation indicated that M110 and M111 owned superior molecular planarity and better LUMO orbital expanding, which promoted extinction coefficients enhancement as well as lowering band gaps without sacrificing Voc. Blended with PC71BM, M110 exhibited the highest PCE of 9.25% with Voc of 0.95 V, Jsc of 13.85 mA/cm2 and FF of 73% (Table 5). The excellent PCE of 8.91% (Table 5) was also obtained based on M111 as donor material for small molecule OPVs.

|
Download:
|
| Fig. 11. Chemical structures of small molecules our group reported based novel D-A framework. | |
Recently, Peng group reported an impressive work about two A-π-A-π-D-π-A-π-A structural small molecules (M114, M115) (Fig. 10) [11]. These two small molecules applied naphtho[1, 2-c: 5, 6-c']bis[1, 2, 5]thiadiazole as acceptor units in small molecule donor design for the first time. With large π-extended ring and strong electron affinity, they showed outstanding qualities of high planarity, wide absorptions and low-lying HOMO levels. Importantly, due to the introduction of sulfur atoms on BDT side chains, a very small energy loss of 0.57 eV was observed in the M115 devices with a record PCE of 11.53% (Table 5). And M114 also exhibited an amazing PCE of 10.02% (Table 5).
The above studies have showed interesting but relatively complicated D-A structural small molecules. Expanding the D-A frameworks is a potential molecular design strategy to achieve preferable properties as well as discover more donor or acceptor units for excellent small molecules.
7. Conclusion and outlookThis article reviews recent progress of small molecule donor materials for high performance BHJ OSCs. At present, the symmetrical D-A structural frameworks has been identified as a successful strategy to design small molecule donor materials with excellent properties. Befitting energy levels, proper absorption spectrums, orderly molecular packing and optimal active layer morphology could be achieved by protean marshalling sequences and diversity of donor and acceptor units. Besides, the introduction of π-bridges further improves the molecular conjugation availing for molecular packing and charge transport. Furthermore, the side chains modification also showed vital influences on solubility and molecular packing.
In previous investigation, A-D-A small molecules showed preferable device performance. Recent years, some donor molecules with multiple acceptor units also exhibited outstanding properties in the system of fullerenes. This is due to the introduction of multiple acceptor units could adjust light absorption effectively without sacrificing Voc. Hence, the D-A constructions provide lots of room and possibility for further improvement of PCE based on small molecules OSCs with fullerene acceptors. Furthermore, nowadays, D-A small molecular OSCs based on nonfullerene acceptors obtained excellent PCE (ca. 10%) [87-91]. This is another big chance for small molecules to further acquire breakthrough of PCE. In addition, long-life and stable small molecules should be designed at the same time for realizing commercial application in the future.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 21474022, 51603051), Youth Innovation Promotion Association CAS and Beijing Nova Program (No. Z171100001117062), and the Chinese Academy of Sciences.
| [1] |
A. Mishra, P. Bäuerle, Angew. Chem. Int. Ed. 51(2012) 2020-2067. DOI:10.1002/anie.201102326 |
| [2] |
H.W. Luo, Z.T. Liu, Chin. Chem. Lett. 27(2016) 1283-1292. DOI:10.1016/j.cclet.2016.07.003 |
| [3] |
W. Ni, X. Wan, M. Li, Y. Wang, Y. Chen, Chem. Commun. 51(2015) 4936-4950. DOI:10.1039/C4CC09758K |
| [4] |
Y. Huang, E.J. Kramer, A.J. Heeger, G.C. Bazan, Chem. Rev. 114(2014) 7006-7043. DOI:10.1021/cr400353v |
| [5] |
C.J. Brabec, M. Heeney, I. McCulloch, J. Nelson, Chem. Soc. Rev. 40(2011) 1185-1199. DOI:10.1039/C0CS00045K |
| [6] |
Y. Chen, X. Wan, G. Long, Acc. Chem. Res. 46(2013) 2645-2655. DOI:10.1021/ar400088c |
| [7] |
C.C. Chueh, C.Z. Li, A.K.Y. Jen, Energy Environ. Sci. 8(2015) 1160-1189. DOI:10.1039/C4EE03824J |
| [8] |
F. Wang, Z.A. Tan, Y. Li, Energy Environ. Sci. 8(2015) 1059-1091. DOI:10.1039/C4EE03802A |
| [9] |
S.D. Collins, N.A. Ran, M.C. Heiber, T.Q. Nguyen, Adv. Energy Mater. 7(2017) 1602242-1602287. DOI:10.1002/aenm.201602242 |
| [10] |
W. Zhao, S. Li, H. Yao, et al., J. Am. Chem. Soc. 139(2017) 7148-7151. DOI:10.1021/jacs.7b02677 |
| [11] |
J. Wan, X. Xu, G. Zhang, et al., Energy Environ. Sci. 10(2017) 1739-1745. DOI:10.1039/C7EE00805H |
| [12] |
B. Walker, C. Kim, T.Q. Nguyen, Chem. Mater. 23(2010) 470-482. |
| [13] |
J. Roncali, P. Leriche, P. Blanchard, Adv. Mater. 26(2014) 3821-3838. DOI:10.1002/adma.201305999 |
| [14] |
X.X. Shen, G.C. Han, Y.P. Yi, Chin. Chem. Lett. 27(2016) 1453-1463. DOI:10.1016/j.cclet.2016.05.030 |
| [15] |
B. Kan, M. Li, Q. Zhang, et al., J. Am. Chem. Soc. 137(2015) 3886-3893. DOI:10.1021/jacs.5b00305 |
| [16] |
B. Kan, Q. Zhang, M. Li, et al., J. Am. Chem. Soc. 136(2014) 15529-15532. DOI:10.1021/ja509703k |
| [17] |
M. Li, F. Liu, X. Wan, et al., Adv. Mater. 27(2015) 6296-6302. DOI:10.1002/adma.201502645 |
| [18] |
Z. Wang, X. Xu, Z. Li, et al., Adv. Electron. Mater. 2(2016) 1600061-1600067. DOI:10.1002/aelm.201600061 |
| [19] |
F. Gao, O. Inganas, Phys. Chem. Chem. Phys. 16(2014) 20291-20304. DOI:10.1039/C4CP01814A |
| [20] |
Z. He, C. Zhong, X. Huang, et al., Adv. Mater. 23(2011) 4636-4643. DOI:10.1002/adma.201103006 |
| [21] |
S. Sweetnam, K.R. Graham, G.O. Ngongang Ndjawa, et al., J. Am. Chem. Soc. 136(2014) 14078-14088. DOI:10.1021/ja505463r |
| [22] |
X.W. Zhu, K. Lu, H. Li, R.M. Zhou, Z.X. Wei, Chin. Chem. Lett. 27(2016) 1271-1276. DOI:10.1016/j.cclet.2016.06.015 |
| [23] |
Y. Lin, X. Zhan, Acc. Chem. Res. 49(2016) 175-183. DOI:10.1021/acs.accounts.5b00363 |
| [24] |
Y.S. Chen, X.J. Wan, G.K. Long, Acc. Chem. Res. 46(2013) 2645-2655. DOI:10.1021/ar400088c |
| [25] |
E.E. Havinga, W. Tenhoeve, H. Wynberg, Synthetic Met. 55(1993) 299-306. DOI:10.1016/0379-6779(93)90949-W |
| [26] |
E.E. Havinga, W. Tenhoeve, H. Wynberg, Polym. Bull. 29(1992) 119-126. DOI:10.1007/BF00558045 |
| [27] |
A.B. Tamayo, B. Walker, T.Q. Nguyen, J. Mater. Chem. C 112(2008) 11545-11551. |
| [28] |
B. Walker, A.B. Tamayo, X.D. Dang, et al., Adv. Funct. Mater. 19(2009) 3063-3069. DOI:10.1002/adfm.v19:19 |
| [29] |
J.D.A. Lin, J. Liu, C. Kim, et al., RSC Adv. 4(2014) 14101-14108. DOI:10.1039/C3RA45662E |
| [30] |
O.P. Lee, A.T. Yiu, P.M. Beaujuge, et al., Adv. Mater. 23(2011) 5359-5363. DOI:10.1002/adma.201103177 |
| [31] |
J. Huang, H. Jia, L. Li, et al., Phys. Chem. Chem. Phys. 14(2012) 14238-14242. DOI:10.1039/c2cp42050c |
| [32] |
E. Kozma, D. Kotowski, F. Galeotti, et al., Mater. Chem. Phys. 147(2014) 365-370. DOI:10.1016/j.matchemphys.2014.06.048 |
| [33] |
R. Zhou, Q.D. Li, X.C. Li, et al., Dyes Pigm. 101(2014) 51-57. DOI:10.1016/j.dyepig.2013.09.022 |
| [34] |
M. Más-Montoya, R.A.J. Janssen, Adv. Funct. Mater. 27(2017) 1605779-1605790. DOI:10.1002/adfm.v27.16 |
| [35] |
L. Yuan, Y. Zhao, K. Lu, et al., J. Mater. Chem. C 2(2014) 5842-5849. DOI:10.1039/C4TC00921E |
| [36] |
A. Tang, C. Zhan, J. Yao, E. Zhou, Adv. Mater. 29(2017) 1600013-1600052. DOI:10.1002/adma.v29.2 |
| [37] |
K. Schulze, C. Uhrich, R. Schüppel, et al., Adv. Mater. 18(2006) 2872-2875. DOI:10.1002/(ISSN)1521-4095 |
| [38] |
Y. Liu, X. Wan, B. Yin, et al., J. Mater. Chem. C 20(2010) 2464-2468. DOI:10.1039/b925048d |
| [39] |
Y. Liu, X. Wan, F. Wang, et al., Adv. Energy Mater. 1(2011) 771-775. DOI:10.1002/aenm.v1.5 |
| [40] |
Z. Li, G. He, X. Wan, et al., Adv. Energy Mater. 2(2012) 74-77. DOI:10.1002/aenm.201100572 |
| [41] |
G. He, Z. Li, X. Wan, et al., J. Mater. Chem. A 1(2013) 1801-1809. DOI:10.1039/C2TA00496H |
| [42] |
N. Liang, D. Meng, Z. Ma, et al., Adv. Energy Mater. 7(2017) 1601664-1601669. DOI:10.1002/aenm.201601664 |
| [43] |
Z. Wang, Z. Li, J. Liu, et al., ACS Appl. Mater. Interfaces 8(2016) 11639-11648. DOI:10.1021/acsami.6b01784 |
| [44] |
Y. Lin, L. Ma, Y. Li, et al., Adv. Energy Mater. 3(2013) 1166-1170. DOI:10.1002/aenm.v3.9 |
| [45] |
C.E. Song, Y.J. Kim, S.R. Suranagi, et al., ACS Appl. Mater. Interf. 8(2016) 12940-12950. DOI:10.1021/acsami.6b01576 |
| [46] |
J. Wang, K. Shi, Y. Suo, et al., J. Mater. Chem. C 4(2016) 3781-3791. DOI:10.1039/C5TC03589A |
| [47] |
K. Gao, L. Li, T. Lai, et al., J. Am. Chem. Soc. 137(2015) 7282-7285. DOI:10.1021/jacs.5b03740 |
| [48] |
K. Gao, J. Miao, L. Xiao, et al., Adv. Mater. 28(2016) 4727-4733. DOI:10.1002/adma.v28.23 |
| [49] |
T. Liang, L. Xiao, K. Gao, et al., ACS Appl. Mater. Interf. 9(2017) 7131-7138. DOI:10.1021/acsami.6b15241 |
| [50] |
J.W. Jung, T.P. Russell, W.H. Jo, Chem. Mater. 27(2015) 4865-4870. DOI:10.1021/acs.chemmater.5b01799 |
| [51] |
R. Wu, L. Yin, Y. Li, Sci. China Mater. 59(2016) 371-388. |
| [52] |
Y. Liu, X. Wan, F. Wang, et al., Adv. Mater. 23(2011) 5387-5391. DOI:10.1002/adma.201102790 |
| [53] |
C. Cui, X. Guo, J. Min, et al., Adv. Mater. 27(2015) 7469-7475. DOI:10.1002/adma.201503815 |
| [54] |
K. Sun, Z. Xiao, S. Lu, et al., Nat. Commun. 6(2015) 6013-6021. DOI:10.1038/ncomms7013 |
| [55] |
S. Badgujar, G.Y. Lee, T. Park, et al., Adv. Energy Mater. 6(2016) 1600228-1600236. DOI:10.1002/aenm.201600228 |
| [56] |
Z. Zhou, S. Xu, W. Liu, et al., J. Mater. Chem. A 5(2017) 3425-3433. DOI:10.1039/C6TA10559A |
| [57] |
D. Deng, Y. Zhang, L. Yuan, et al., Adv. Energy Mater. 4(2014) 1400538-1400544. DOI:10.1002/aenm.201400538 |
| [58] |
D. Deng, Y. Zhang, L. Zhu, et al., Phys. Chem. Chem. Phys. 17(2015) 8894-8900. DOI:10.1039/C5CP00042D |
| [59] |
D. Deng, Y. Zhang, J. Zhang, et al., Nat. Commun. 7(2016) 13740-13748. DOI:10.1038/ncomms13740 |
| [60] |
J. Zhou, X. Wan, Y. Liu, et al., Chem. Mater. 23(2011) 4666-4668. DOI:10.1021/cm202588h |
| [61] |
W. Ni, M. Li, F. Liu, et al., Chem. Mater. 27(2015) 6077-6084. DOI:10.1021/acs.chemmater.5b02616 |
| [62] |
L. Xiao, S. Chen, K. Gao, et al., ACS Appl. Mater. Interf. 8(2016) 30176-30183. DOI:10.1021/acsami.6b09790 |
| [63] |
W. Wang, P. Shen, X. Dong, et al., ACS Appl. Mater. Interf. 9(2017) 4614-4625. DOI:10.1021/acsami.6b14114 |
| [64] |
X. Zhu, B. Xia, K. Lu, et al., Chem. Mater. 28(2016) 943-950. DOI:10.1021/acs.chemmater.5b04668 |
| [65] |
J. Sim, H. Lee, K. Song, et al., J. Mater. Chem. C 4(2016) 3508-3516. DOI:10.1039/C6TC00323K |
| [66] |
C. Cui, W.Y. Wong, Y. Li, Energy Environ. Sci. 7(2014) 2276-2284. DOI:10.1039/C4EE00446A |
| [67] |
S. Shen, P. Jiang, C. He, et al., Chem. Mater. 25(2013) 2274-2281. DOI:10.1021/cm400782q |
| [68] |
J. Zhao, B. Xia, K. Lu, et al., RSC Adv. 6(2016) 60595-60601. DOI:10.1039/C6RA09417A |
| [69] |
J. Min, Y.N. Luponosov, N. Gasparini, et al., J. Mater. Chem. A 3(2015) 22695-22707. DOI:10.1039/C5TA06706E |
| [70] |
C.D. Wessendorf, A. Perez-Rodriguez, J. Hanisch, et al., J. Mater. Chem. A 4(2016) 2571-2580. DOI:10.1039/C5TA07713C |
| [71] |
Y. Sun, G.C. Welch, W.L. Leong, et al., Nat. Mater. 11(2011) 44-48. |
| [72] |
T.S. van der Poll, J.A. Love, T.Q. Nguyen, G.C. Bazan, Adv. Mater. 24(2012) 3646-3649. DOI:10.1002/adma.v24.27 |
| [73] |
J.H. Yun, S. Park, J.H. Heo, et al., Chem. Sci. 7(2016) 6649-6661. DOI:10.1039/C6SC02448C |
| [74] |
M. Moon, B. Walker, J. Lee, et al., Adv. Energy Mater. 5(2015) 1402044-1402054. DOI:10.1002/aenm.201402044 |
| [75] |
J.A. Love, I. Nagao, Y. Huang, et al., J. Am. Chem. Soc. 136(2014) 3597-3606. DOI:10.1021/ja412473p |
| [76] |
K. Wang, R.Z. Liang, J. Wolf, et al., Adv. Funct. Mater. 26(2016) 7103-7114. DOI:10.1002/adfm.v26.39 |
| [77] |
L. Yuan, Y. Zhao, J. Zhang, et al., Adv. Mater. 27(2015) 4229-4233. DOI:10.1002/adma.v27.28 |
| [78] |
Y. Huang, W. Wen, S. Mukherjee, et al., Adv. Mater. 26(2014) 4168-4172. DOI:10.1002/adma.v26.24 |
| [79] |
C. McDowell, M. Abdelsamie, K. Zhao, et al., Adv. Energy Mater. 5(2015) 1501121-1501129. DOI:10.1002/aenm.201501121 |
| [80] |
R.S. Ashraf, B.C. Schroeder, H.A. Bronstein, et al., Adv. Mater. 25(2013) 2029-2034. DOI:10.1002/adma.201300027 |
| [81] |
V. Gupta, L.F. Lai, R. Datt, et al., Chem. Commun. 52(2016) 8596-8599. DOI:10.1039/C6CC03998G |
| [82] |
L.Y. Lin, C.W. Lu, W.C. Huang, et al., Org. Lett. 13(2011) 4962-4965. DOI:10.1021/ol2021077 |
| [83] |
X. Liu, Y. Sun, B.B. Hsu, et al., J. Am. Chem. Soc. 136(2014) 5697-5708. DOI:10.1021/ja413144u |
| [84] |
J.L. Wang, F. Xiao, J. Yan, et al., Adv. Funct. Mater. 26(2016) 1803-1812. DOI:10.1002/adfm.v26.11 |
| [85] |
J.L. Wang, K.K. Liu, J. Yan, et al., J. Am. Chem. Soc. 138(2016) 7687-7697. DOI:10.1021/jacs.6b03495 |
| [86] |
L. Yuan, K. Lu, B. Xia, et al., Adv. Mater. 28(2016) 5980-5985. DOI:10.1002/adma.201600512 |
| [87] |
H. Bin, Y. Yang, Z.G. Zhang, et al., J. Am. Chem. Soc. 139(2017) 5085-5094. DOI:10.1021/jacs.6b12826 |
| [88] |
Z. Zheng, O.M. Awartani, B. Gautam, et al., Adv. Mater. 29(2017) 1604241-1604246. DOI:10.1002/adma.201604241 |
| [89] |
Y. Yang, Z.G. Zhang, H. Bin, et al., J. Am. Chem. Soc. 138(2016) 15011-15018. DOI:10.1021/jacs.6b09110 |
| [90] |
D. Liu, B. Yang, B. Jang, et al., Energy Environ. Sci. 10(2017) 546-551. DOI:10.1039/C6EE03489F |
| [91] |
F. Zhao, S. Dai, Y. Wu, et al., Adv. Mater. 29(2017) 1700144-1700150. DOI:10.1002/adma.201700144 |
 2017, Vol. 28
2017, Vol. 28