b Beijing Laboratory of Biomedical Materials, Beijing University of Chemical Technology Beijing 100029, China
Traditionally, drugs or cells are administrated intravenously into the human body [1, 2]. As a result, most of the drugs or functional cells will be lost during the long cycling process. To reach enough drug or cell concentration for therapy, high concentrated dose is normally inevitable [3, 4]. Furthermore, the quick decreased concentration of introduced drugs or cells after injection means the patients have to suffer from frequent injections to achieve satisfactory treatment effect. Above mentioned drawbacks like low delivery efficiency, toxicity from high concentrated drugs and frequent injections, remain a great challenge for medical science and clinical treatment. Thus, a new method of administration that can effectively deliver drug/cell to the target lesions to control release drugs or functional molecules produced by the implanted cells in situ is highly needed for academic research and practical applications.
Hydrogel, a soft matter with extremely high water-content, is constructed by crosslinked polymers. Due to its significant similarity in structure and property to human soft tissues, hydrogels have been broadly applied in bio-medical areas [5-8]. Self-healing hydrogel is a kind of hydrogel that can automatically restore its integrity and property/function after damage [9-11]. We believe the self-healing hydrogel is a new generation injectable carrier for targeting drug-delivery because when utilized as a carrier, the self-healing hydrogel can be injected and self-healed in vivo to deliver high concentrated drugs or cells into the desired position accurately, avoiding the unwanted burst release or delivery failure [12-15]. To date, varieties of self-healing hydrogels have been developed through physical or dynamic chemical linkages [16]. The physical binding contributes a large portion of self-healing hydrogels through hydrogen bond [17, 18], ionic bond [19, 20], metal coordination [21, 22], supramolecular interactions [18, 23] and hydrophobic interactions [17] in most cases. Those rather weak interactions cannot provide rapid self-healing effect and robust mechanical strength under low mass percentage. When applied in vivo, physical hydrogels might not be able to remain a gel state under the sophisticated human internal environment [24], let along to self-heal quickly. On the other hand, self-healing hydrogels constructed by dynamic chemical linkages have much stronger linkage in the hydrogel network, leading to the quicker self-healing process and higher mechanical strength compared with the physical self-healing hydrogels. Thus, dynamic chemical linkages such as boronic ester [25, 26], oxime [24, 26], disulfide [27, 28], acylhydrazone and imine[29-32], have been broadly utilized to prepare self-healing hydrogels for possible applications in broad areas.
However, the development of self-healing hydrogels for real applications, especially the clinical trial is still restricted by several bottle-necks: 1) Some self-healing hydrogels require expensive and complex gelators like synthetic peptides, metal ligands, etc.; 2) Some external stimuli (pH, temperature, UV radiation, etc.) are still necessary to realize the self-healing process; 3) Some self-healing hydrogels are even not biocompatible. Thus, to develop a selfhealing hydrogel that is not expensive, easy to prepare with excellent biocompatibility is the key for the future real application of that fancy materials. Here, in this mini review, we will summarize the recently appeared chitosan-based self-healing hydrogel, including the preparation of the hydrogel and some applications of that hydrogel for targeting drug delivery and 3D cell culture, and we will also make a brief introduction on the extension of that hydrogel to organic-inorganic composites and discuss about the future applications of those chitosan-based hydrogel/hybrid in bio-related fields.
Recently, our group developed a series of chitosan-based selfhealing hydrogels, using a benzalaldehyde terminated poly (ethylene glycol), DF-PEG, to crosslink chitosan or chitosan derivatives [33]. The hydrogel could be facilely prepared by simply mixing chitosan and DF-PEG solutions in benign conditions within 1 min. The aldehyde groups on DF-PEG could form dynamic Schiffbase (imine) with the amine group on chitosan, resulting in a selfhealing hydrogel constructed by a dynamic network. The reversible Schiff-base linkage keeps a balance between breakage and regeneration in the hydrogel environment, leading to the remarkable self-healing feature of the hydrogel. Compared with other dynamic bond, such as the borate or oxime, the imine bond has several advantages: 1) easily available substrates (aldehyde and amine compounds), 2) the dynamic balance of imine bond under neutral pH, which is suitable for the preparation and application of self-healing hydrogel in vivo. As a result, in 2011, a chitosan/DF-PEG hydrogel (CP hydrogel) have been facilely prepared by simply mixing chitosan solution with the DF-PEG aqueous solution. A hole has been punched in the middle of the hydrogel to observe its selfhealing process visually, and the hole was found to close up and disappeared in 2 h (Fig. 1), directly demonstrating the outstanding self-healing ability of that CP hydrogel. Thanks to the dynamic imine linkage, the hydrogel was found to be multi-responsive to some external stimuli like pH, amino acids, enzymes and vitamin B6 derivatives. Therefore, that CP hydrogel was applied for successful control-release of rhodamine B (a model of small molecular drugs) and lysozyme (a model of biomacromolecules) under different conditions, building a base for further biomedical usage of this type of hydrogel.

|
Download:
|
| Fig. 1. The preparation, self-healing process and the multi-responsive degradation of the CP hydrogel. Reprinted with permission [33]. Copyright 2011, American Chemical Society. | |
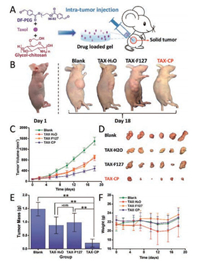
Subsequently, Yang et al. use glycol-chitosan, a chitosan derivative with better water solubility than the chitosan under neutral condition, to prepare a hydrogel react with DF-PEG (CP hydrogel), and studied the improved tumor chemotherapy effect using that CP hydrogel as a carrier to deliver Taxol (referred as CPT hydrogel) (Fig. 2) [34]. Unlike the widely used traditional injectable hydrogels (F127 hydrogel for example) which undergo the sol-gel phase transformation after injection, the CP hydrogel could be injected as a solid hydrogel, the broken hydrogel pieces caused by injection could self-heal after 30 min to regenerate a whole hydrogel with similar storage moduli as the native hydrogel, thus avoiding the uncontrolled gelation process and preventing the possible burst release. The communitive release of the drug from the self-healing CP hydrogel was thoroughly studied. Then, the prepared CPT hydrogel was applied in a in vivo test using tumorbearing nude mice as the model animal. The CPT hydrogel showed obvious therapeutic advantage compared with control groups (Taxol solution, Taxol@F127 hydrogel) during the 18-day in vivo evaluation with a marvelous tumor inhibition rate (TGI) of 70%. The followed histological studies confirmed the excellent anti-tumor result of the CPT hydrogel, suggesting the CP hydrogel could enhance the therapeutic efficiency of the laden drugs by concentrating drug at the desired position (tumor site) for effective control-release. Thus, the CP hydrogel could be regarded as a potential new carrier for drug delivery.
|
Download:
|
| Fig. 2. (A) Schematic of the intra-tumor injection experiment. (B) Pictures of nude mice from different groups. (C) The tumor volume of mice in four groups. (D) The picture of the tumors of the four groups on the 18th day. (E) The mass of the dissected tumors on the 18th day. (F) Weight changes of mice during the observation period. Reprinted with permission [34]. Copyright 2017, Royal Society of Chemistry. | |
As the development of the modern medical science, drug therapy is not an all-mighty way. Patients suffer from some diseases like leukemia could not be cured chemically, requiring rapid progress of alternative therapies. Cell therapy which has more than a hundred years history, recently drew great attention from researchers in biomedical and material science due to the deeper understanding of cytobiology. Unlike drug therapy that cure the dysfunctional cells or tissues, cell therapy simply and directly restores the functionality of the disordered organ/tissue by replacing the dysfunctional cells or tissues with healthy ones, thus is considered as the next generation therapeutic approach. However, restricted to the immune effect as well as the extremely low delivery efficiency to the disease site just like traditional drug therapy, cell therapy also meets with the bottle-neck. Using hydrogel as the cell carrier for cell therapy could not only shelter the implanted cells from the immune attack, but also anchor high concentrated therapeutic cells at the designed site to dramatically improve the utilization of implanted cells compared with currently intravenous administration [35-37].
To investigate that hypothesis, in 2012, we applied the CP hydrogel for 3D cell culture to evaluate whether the CP hydrogel is a suitable candidate for cell delivery [38]. The implanting process could be simply carried out by mixing the glycol-chitosan solution, DF-PEG solution and the cell suspension at room temperature to get the cell-contained hydrogel in 5 min. The benign gelation process ensures the excellent viability of the embedded cells. And the gelation time (about 5 min) allows researchers to implant cells with even distribution within the hydrogel. The embedded cells showed high viability (~97%) through the 3-day observation evaluated by dyeing and confocal imaging (Fig. 3). As the hydrogel could be injected through a syringe and self-healed, the cell-laden hydrogel was also injected and studied. Confocal image showed the viability of injected cells kept at ~87% in the hydrogel network after 72 h, confirming the CP hydrogel a biocompatible carrier for cell therapy.

|
Download:
|
| Fig. 3. Confocal images of the embedded cells in the CP hydrogel after 24 h (A, A') and 72 h (B, B'). Reprinted with permission [38]. Copyright 2012, Royal Society of Chemistry. | |
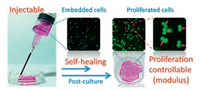
Nowadays, 3D cell culture in hydrogels have been widely accepted for better simulate and study the cell behaviors in vivo. But cell proliferation especially in 3D environment has yet been widely studied although it is a key factor for cell therapy. In 2017, by tuning the mass of DF-PEG, we prepared a series of self-healing CP hydrogel with different mechanical strength [39]. All those hydrogels could be injected with syringe and self-heal as a whole piece of gel with almost completely recovered modulus. To evaluate the relationship between the hydrogel mechanical strength and the proliferation of the embedded cells, cells were implanted into a series of hydrogels. Through a 7-day culture, higher proliferation rate of L929 cells was found in stiffer hydrogels in the testing range, implying that the proliferation rate could be regulated by manipulating the hydrogel matrix stiffness. Those cell-contained hydrogels were injected through needles, and same assessment was carried out to evaluate the cell damage caused by extrusion through the needle. We found that the injected cells in the self-healed CP hydrogels could proliferate in a considerable rate following the same relationship between the hydrogel stiffness and cell proliferation, confirming that CP hydrogel is a potential matrix for 3D cell culture and cell therapy (Fig. 4). Furthermore, this CP hydrogel was successfully applied for neural stem cell culture and neural repair in zebrafish [40].
|
Download:
|
| Fig. 4. The 3D cell proliferation within the CP hydrogel after injection. Reprinted with permission [39]. Copyright 2017, Elsevier Ltd. | |
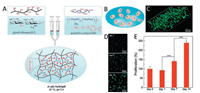
Similarly, on the basis of the dynamic Schiff base, Cao et al. prepared a multi-benzaldehyde functionalized PEG analogue to crosslink glycol-chitosan (Fig. 5) [41]. Due to the dynamic imine bond that build up the network, the hydrogel also has self-healing capability after broken. The inner structure as well as the degradation profile of the hydrogel have been thoroughly studied. Subsequently, that hydrogel was applied for 2D and 3D culture of chondrocyte cell. Chondrocyte cells showed excellent viability in the hydrogel during the 14-day observation and the cell proliferation was also observed by kit-8 assay, confirming that hydrogel is a promising artificial extracellular matrix for cartilage tissue engineering.
|
Download:
|
| Fig. 5. (A) The preparation of the hydrogel. (B) The schematic illustration of the cell-embedded hydrogel. (C) The confocal image of the loaded cells. (D) The morphology of the cells on different time points. (E) The proliferation of the cells. Reprinted with permission [40]. Copyright 2015, Royal Society of Chemistry. | |
Besides, taken the CP hydrogel as a basic material, other attempts have been conducted to develop new functional materials. In 2012, we introduced Fe3O4 magnetic nanoparticles to the CP hydrogel to facilely prepare a novel magnetic self-healing hydrogel (Fig. 6) [42]. Driven by a magnet, that magnetic selfhealing hydrogel could cross through a channel with a glass barrier in the middle. It is noticeable that the network of that hydrogel could quickly re-build due to the dynamic Schiff base linkage. Thus, the magnetic self-healing hydrogel could pass the narrow channel as an integrity after cleavage by the glass barrier. The organicinorganic combination endows the hydrogel new function and great potential for drug delivery, separation, image enhancement and remote-controlled actuators, etc.

|
Download:
|
| Fig. 6. (A) The preparation of the magnetic CP hydrogel. (B) The magnetic hydrogel passing a channel driven by a magnet. Reprinted with permission [41]. Copyright 2012, Royal Society of Chemistry. | |
In 2013, by introducing silica into the CP hydrogel, we prepared a biohybrid hydrogel (CPS hydrogel) with hierarchically porous structure through an in situ silica sol-gel process within the CP hydrogel (Fig. 7) [43]. Thanks to its hierarchical structure, the hybrid hydrogel has excellent capacity for quick cell adsorption. In the red blood cell (RBC) adsorption experiment, the synergism of the hierarchical structure and the positive charge of the chitosan gave the CPS hydrogel extraordinary cell concentration ability. The crosslinked hydrogel network could "lock" the adsorbed blood cells in like a "cage", indicating it a potential hemostatic material or a candidate for plasma separation in clinical trials.

|
Download:
|
| Fig. 7. (A) The preparation of the inorganic/organic hybrid hierarchical CPS hydrogel. (B) SEM images of RBCs adsorbed on a chitosan-silica hybrid (a), chitosanPEG xerogel (b) chitosan monoliths (c), porous silica (d), and hierarchical CPS biohybrid (e, e'). Reprinted with permission [42]. Copyright 2013, Wiley-VCH. | |
A biocompatible self-healing CP hydrogel has been developed using chitosan as the main gelator. By simple mixing with drug (Taxol for example), this CP hydrogel has been applied as a high efficient drug carrier for anti-tumor treatment. Meanwhile, the CP hydrogel has also been applied as a 3D cell scaffold, in which cell viability, proliferation before/after injection were thoroughly studied, suggesting this CP hydrogel could be used as an ideal carrier for drug/cell delivery and lead to much better therapeutic effect compared with traditional approaches. Furthermore, we integrated inorganic nanoparticles (NPs) to the CP hydrogel to prepare a series of organic-inorganic composites, suggesting that CP hydrogel an excellent basic platform to develop new functional materials [44].
As a biocompatible, multi-responsible, versatile platform to combine other functional materials, this CP hydrogel might have broad applications in medical and clinical fields including arthroscopic treatment, wound treatment, tumor treatment, etc., and might also be utilized as a promising basis to develop related conductive, luminous, robotic soft devices, etc.
AcknowledgmentThis research was supported by the National Natural Science Foundation of China (No. 21534006).
| [1] |
D.E. Owens, N.A. Peppas, Int. J. Pharm. 307(2006) 93-102. DOI:10.1016/j.ijpharm.2005.10.010 |
| [2] |
M. Roberts, M. Bentley, J. Harris, Adv. Drug Del. Rev. 64(2012) 116-127. DOI:10.1016/j.addr.2012.09.025 |
| [3] |
R. Langer, Nature 392(1998) 5-10. |
| [4] |
R. Langer, Science 249(1990) 1527-1533. DOI:10.1126/science.2218494 |
| [5] |
P. Gupta, K. Vermani, S. Garg, Drug Discov. Today 7(2002) 569-579. DOI:10.1016/S1359-6446(02)02255-9 |
| [6] |
J.L. Drury, D.J. Mooney, Biomaterials 24(2003) 4337-4351. DOI:10.1016/S0142-9612(03)00340-5 |
| [7] |
N. Peppas, P. Bures, W. Leobandung, H. Ichikawa, Eur. J. Pharm. Biopharm. 50(2000) 27-46. DOI:10.1016/S0939-6411(00)00090-4 |
| [8] |
N.A. Peppas, J.Z. Hilt, A. Khademhosseini, R. Langer, Adv. Mater. 18(2006) 1345-1360. DOI:10.1002/(ISSN)1521-4095 |
| [9] |
D.L. Taylor, Adv. Mater. 28(2016) 9060-9093. DOI:10.1002/adma.201601613 |
| [10] |
V. Yesilyurt, M.J. Webber, E.A. Appel, et al., Adv. Mater. 28(2016) 86-91. DOI:10.1002/adma.201502902 |
| [11] |
I. Jeon, J. Cui, W.R. Illeperuma, J. Aizenberg, J.J. Vlassak, Adv. Mater. 28(2016) 4678-4683. DOI:10.1002/adma.v28.23 |
| [12] |
T.R. Hoare, D.S. Kohane, Polymer 49(2008) 1993-2007. DOI:10.1016/j.polymer.2008.01.027 |
| [13] |
R.K. Jain, Sci. Am. 271(1994) 58-65. |
| [14] |
Y. Qiu, K. Park, Adv. Drug Deliv. Rev. 53(2001) 321-339. DOI:10.1016/S0169-409X(01)00203-4 |
| [15] |
L. Yu, J. Ding, Chem. Soc. Rev. 37(2008) 1473-1481. DOI:10.1039/b713009k |
| [16] |
Z. Wei, J.H. Yang, J. Zhou, et al., Chem. Soc. Rev. 43(2014) 8114-8131. DOI:10.1039/C4CS00219A |
| [17] |
B. Xing, C.-W. Yu, K.-H. Chow, et al., J. Am. Chem. Soc. 124(2002) 14846-14847. DOI:10.1021/ja028539f |
| [18] |
W. Lu, X. Le, J. Zhang, Y. Huang, T. Chen, Chem. Soc. Rev. 46(2017) 1284-1294. DOI:10.1039/C6CS00754F |
| [19] |
J. Yang, M. Ma, X. Zhang, F. Xu, Macromolecules 49(2016) 4340-4348. DOI:10.1021/acs.macromol.6b00874 |
| [20] |
T. Bai, S. Liu, F. Sun, et al., Biomaterials 35(2014) 3926-3933. DOI:10.1016/j.biomaterials.2014.01.077 |
| [21] |
F. Wang, J. Zhang, X. Ding, et al., Angew. Chem. Int. Ed. 122(2010) 1108-1112. DOI:10.1002/ange.200906389 |
| [22] |
S.Y. Zheng, H. Ding, J. Qian, et al., Macromolecules 49(2016) 9637-9646. DOI:10.1021/acs.macromol.6b02150 |
| [23] |
D. Limón, C. Jiménez-Newman, A.C. Calpena, et al., Chem. Commun. 53(2017) 4509-4512. DOI:10.1039/C6CC09392B |
| [24] |
S. Ghosh, J.D. Cabral, L.R. Hanton, S.C. Moratti, Acta Biomater. 29(2016) 206-214. |
| [25] |
R. Guo, Q. Su, J. Zhang, et al., Biomacromolecules 18(2017) 1356-1364. DOI:10.1021/acs.biomac.7b00089 |
| [26] |
J. Collins, M. Nadgorny, Z. Xiao, L.A. Connal, Macromol. Rapid Commun. 38(2017). |
| [27] |
S. De Koker, J. Cui, N. Vanparijs, et al., Angew. Chem. Int. Ed. 55(2016) 1334-1339. DOI:10.1002/anie.201508626 |
| [28] |
J. Lee, M.N. Silberstein, A.A. Abdeen, S.Y. Kim, K.A. Kilian, Mater. Horiz. 3(2016) 447-451. DOI:10.1039/C6MH00091F |
| [29] |
N. Sreenivasachary, J.-M. Lehn, Proc. Natl. Acad. Sci. U. S. A. 102(2005) 5938-5943. DOI:10.1073/pnas.0501663102 |
| [30] |
D.E. Whitaker, C.S. Mahon, D.A. Fulton, Angew. Chem. Int. Ed. 125(2013) 990-993. DOI:10.1002/ange.201207953 |
| [31] |
Y. Yang, J. Zhang, Z. Liu, et al., Adv. Mater. 28(2016) 2724-2730. DOI:10.1002/adma.201505336 |
| [32] |
W. Huang, Y. Wang, Y. Chen, et al., Adv. Healthc. Mater. 5(2016) 2813-2822. DOI:10.1002/adhm.v5.21 |
| [33] |
Y. Zhang, L. Tao, S. Li, Y. Wei, Biomacromolecules 12(2011) 2894-2901. DOI:10.1021/bm200423f |
| [34] |
L. Yang, Y. Li, Y. Gou, et al., Polym. Chem. 8(2017) 5071-5076. DOI:10.1039/C7PY00112F |
| [35] |
Q. Feng, K. Wei, S. Lin, et al., Biomaterials 101(2016) 217-228. DOI:10.1016/j.biomaterials.2016.05.043 |
| [36] |
C.T. Wong Po Foo, J.S. Lee, W. Mulyasasmita, A. Parisi-Amon, S.C. Heilshorn, Proc. Natl. Acad. Sci. U. S. A. 106(2009) 22067-22072. DOI:10.1073/pnas.0904851106 |
| [37] |
J.A. Rowley, G. Madlambayan, D.J. Mooney, Biomaterials 20(1999) 45-53. DOI:10.1016/S0142-9612(98)00107-0 |
| [38] |
B. Yang, Y. Zhang, X. Zhang, et al., Polym. Chem. 3(2012) 3235-3238. DOI:10.1039/c2py20627g |
| [39] |
Y. Li, Y. Zhang, F. Shi, et al., Colloid Surf. B 149(2017) 168-173. DOI:10.1016/j.colsurfb.2016.10.021 |
| [40] |
T.C. Tseng, L. Tao, F.Y. Hsieh, et al., Adv. Mater. 27(2015) 3518-3524. DOI:10.1002/adma.v27.23 |
| [41] |
L. Cao, B. Cao, C. Lu, et al., J. Mater. Chem. B 3(2015) 1268-1280. DOI:10.1039/C4TB01705F |
| [42] |
Y. Zhang, B. Yang, X. Zhang, et al., Chem. Commun. 48(2012) 9305-9307. DOI:10.1039/c2cc34745h |
| [43] |
C. Fu, S. Wang, L. Feng, et al., Adv. Healthc. Mater. 2(2013) 302-305. DOI:10.1002/adhm.v2.2 |
| [44] |
G. Férey, Chem. Soc. Rev. 37(2008) 191-214. DOI:10.1039/B618320B |
 2017, Vol. 28
2017, Vol. 28 


