b College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650500, China;
c Department of Safety Evaluation, Technology Center of China Tobacco Yunnan Industrial Co., Ltd., Kunming 650106, China
Because of the high sensitivity, specificity, simplicity of implementation and fast response time, fluorescent probes for detecting biological small molecules show innate advantages over other detection methods developed [1]. Biological thiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play crucial roles in many physiological and pathological processes, and are closely related to many diseases [2]. Cysteine (Cys), well known as an essential amino acid, is involved in protein synthesis, detoxification, and metabolism [3]. Abnormal levels of Cys can lead to slowed growth rate, hair depigmentation, edema, lethargy, liver damage, muscle and fat loss, etc. [4]. The high level of Hcy in the blood is a well-known risk factor for cardiovascular [5] and Alzheimer's disease [6]. In recent years, many fluorescent probes have been developed to detect and sense these biologically important species such as Cys, Hcy and GSH. Although some probes exhibit high selectivity in distinguishing these biothiols from other amino acids and biological small molecules, most of them fail to distinguish Cys/Hcy/GSH from each other due to the similar structures and reactivity of these biothiols. In fact, distinguishing them has been a tough challenge for researchers and has received considerable attention.
Except the well-studied selective detection methods using the cyclization of Cys/Hcy with aldehydes [7] or acrylates [3], the recent strategy of differentiating Cys from Hcy/GSH was achieved by taking advantage of either the Cys-induced SNAr substitutionrearrangement reaction [8] or Michael addition with steric and electrostatic interactions [9]. Yang's group reported another strategy, which enabled GSH to be highly selectively distinguished from Cys/Hcy using a thiol-halogen SNAr substitution reaction between GSH and a chlorinated Bodipy [10].
In our previous thiol sensing work, metal complex-displace coordination mechanism was applied. According to this mechanism, the sensor first displays a selective colorimetric and fluorescence change towards Cu2+ and forms sensor-Cu2+ based complex. With the addition of thiols, the fluorophore is released with a more stable thiol-Cu2+ complex formation [11]. However, it was very hard to selectively discriminate Cys (or Hcy, GSH) among these three thiols. In order to further improve the selectivity and explore the sensing mechanism, a new coumarin derivative based on Cys-induced SNAr substitution-rearrangement reaction was designed.
Herein, we describe the synthesis and the photophysical properties of sensor 1 that has been designed for the sensitive and selective "turn-on" fluorescence detection of Cys over Hcy and GSH in aqueous solvents CH3CN–HEPES (0.02 mol/L, pH 7.2, 1: 9, v/v). As expected, upon addition of 35 equiv. of amino acids, only Cys led to a significant enhancement of up to 20-fold in fluorescence intensity, and a color change occurred from purple to light pink. Furthermore, sensor 1 exhibited highly sensitive and selective fluorescence imaging of Cys in Hi5 cells and C. elegans. The application of sensor 1 for detecting Cys for in vitro assays as well as for in vivo imaging studies broadens the design of new fluorescent sensors in the thiol detection field.
All reagents were of analytical grade or the best grade commercially available, and were put into use without further purification. Deionized water was used throughout. HEPES buffer solutions (0.02mol/L, pH 7.2) were prepared in deionized water. Analyte solutions of the amino acids and anions of His, Met, Tyr, Val, Ser, Ala, Arg, Gly, Gln, Glu, Thr, lys, Hcy, GSH and Cys were prepared by dissolving the salts in distilled water to final concentrations of 0.1mol/L.
Scheme 1 outlines the synthetic route to probe 1. Compound 3 was synthesized according to a reported procedure with an improved yield of 80% [12]. Compound 2 was synthesized with a high yield of 60% by modifying the literature method [13]. Compound 3 was then reacted with 2 for 12h in ethanol to obtain the target compound 1 with 55% yield. The detailed experimental procedures of the new compounds are described in Supporting information.

|
Download:
|
| Scheme 1. Synthetic route of compound 1. | |
In order to understand the interaction of 1 with Cys, the UV–vis absorption and fluorescence spectra of 1 were studied in CH3CN-HEPES buffer (0.02mol/L, pH 7.2, 1: 9, v/v) solutions. Tyr, His, Met, Tyr, Val, Ser, Ala, Arg, Gly, Gln, Glu, Thr, Lys, NAC (N-acetylcysteine), Hcy, GSH and Cys were used to measure the selectivityof the probe 1, and fluorescence spectra were recorded thirty minutes after the addition of 35 equiv. of each of those amino acid molecules. As shown in Fig. S1 (Supporting information), the maximum absorption wavelength of 1 is at about 575nm in the absence of the tested molecules. Upon addition of different amino acids, ruleless changes in the absorption ranged from 510nm to 620nm was observed in the presence of almost every tested molecule. However, only the addition of Cys led to an appearance of a new peak at 375nm, accompanying a dramatic color change from purple to light pink (Fig. 1, inset). In the titration experimentof Cys, the 200nm blue-shift in the case of 1-Cys indicated the absorption selectivity of 1 towards Cys among all the tested amino acids (Fig. 1).

|
Download:
|
| Fig. 1. Absorbance responses of 1 (10 μmol/L) to different concentrations of Cys in CH3CN–HEPES (0.02 mol/L, pH 7.2, 1: 9, v/v). Inset: Color change of 1 (left) and 1 in presence of Cys (right). | |
Meanwhile, in the fluorescence experiment, only Cys generated a significant "turn-on" fluorescence response at 456nm (Fig. 2) compared with the other studied molecules. The fluorescence of 1 was very weak, and a fluorescence enhancement of up to 20-fold was observed only in th epresent of Cys. Nosignificant fluorescence change was observed in the presence of other ions.

|
Download:
|
| Fig. 2. (a) Fluorescence responses of 1 (10 μmol/L) in CH3CN–HEPES (0.02mol/L, pH 7.2, 1: 9, v/v) with 35 equiv. of NAC, His, Met, Tyr, Val, Ser, Ala, Arg, Gly, Gln, Glu, Thr, lys, Hcy, GSH, Cys. Excitation at 380nm; (b) Fluorescence intensity of 1 (10 μmol/L) at 456nm after addition of 35 equiv. of selected ions (a: blank, b: Tyr, c: NAC, d: Arg, e: Gly, f: His, g: Val, h: Gln, i: Glu, j: lys, k: Ser, l: Met, m: Thr, n: Ala, o: GSH, p: Hcy, q: Cys.); (c) The color (top) and fluorescence (bottom) images of 1 solutions in the presence of NAC, GSH, Hcy, Cys and other above molecules. | |
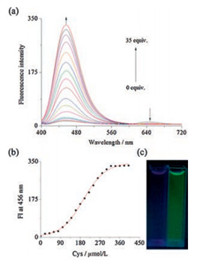
To get further insight into the binding of Cys with 1, the fluorescence spectra of 1 were recorded while titrating with different equiv. of Cys. As shown in Fig. 3, 1 exhibited a very weak fluorescence at 645nm. Upon addition of increasing amounts of Cys, the fluorescenceband was graduallyblue-shiftedand replaced by a new band at 456nm. An obvious 20-fold enhancement in intensity, which saturated with 35 equiv. of Cys within thirty minutes, was observed. As shown in Fig. 3c, the obvious fluorescence enhancement, which was induced by Cys, was obvious to the naked eye. These results suggest excellent selectivity of probe 1 towards Cys.
|
Download:
|
| Fig. 3. (a) Fluorescence emission titration spectra of 1 (10 μmol/L) in the presence of varying concentrations of Cys in CH3CN–HEPES (0.02 mol/L, pH 7.2, 1: 9, v/v); (b) The plot of the fluorescence intensity at 456 nm as a function of Cys concentration; (c) Fluorescent change of 1 in presence of Cys (right). | |
To explore the possibility of using 1 as a Cys selective fluorescent probe, the optical responses towards other thiol compounds at different pH and the interference experiment were carried out. As shown in Fig. S2 (Supporting information), probe 1 showed stable selective responses to Cys at pH ranged from 5 to 10. It gave the support for the further biological application of 1 at physiological pH. In the interference experiment, 1 (10 μmol/L) was mixed with 35 equiv. of Cys and various interfering molecules including His, Met, Tyr, Val, Ser, Ala, Arg, Gly, Gln, Glu, Thr, Lys, NAC, Hcy, and GSH. The interference experiment was monitored by the fluorescence emission changes. It showed that in the presence of above molecules, the emission spectra of 1 remained almost identical to that obtained in the presence of Cys alone. Therefore, 1 was proved to be a promising selective fluorescent detector for Cys in the presence of other interfering molecules. In the test paper experiment, 1 could also sense the existence of Cys by distinguishable color changes (Fig. 4).

|
Download:
|
| Fig. 4. (a) The color and (b) fluorescence images of probe 1 test papers in the presence of NAC, GSH, Hcy, Cys and other tested molecules. | |
To investigate the detection limit of 1 for Cys, 1 (2 μmol/L) was treated with various concentrations of Cys (0–70 μmol/L). The fluorescence intensity at 456 nm was plotted as a function of the Cys concentration. The fluorescence intensity of 1 was found to be linearly proportional to Cys concentrations of 0–70 μmol/L. A detection limit as low as 4.63 ×10-7 mol/L concentration of Cys was achieved by using 1, with a signal-to-noise ratio of 3 [14] (Fig. S4 in Supporting information).
Furthermore, the sensing mechanism of 1 towards Cys was explored (Fig. 5a). In probe 1, the introduction of chloro group enabled the thiol-halogen SNAr nucleophilic substitution. Thus, the chloro group was expected to be replaced by thiol group in Cys (or Hcy) to produce the intermediate product 2a (or 2b), and the following rearrangement gave the product 3a (or 3b). Guo et al. provided a reasonable interpretation for the sensing mechanism for Cys-induced SNAr substitution-rearrangement reaction [12]. Therefore, we believe that the reaction does not stop at this stage, and an intramolecular cyclization between the thiol group and the adjacent site in 3a–4a is the critical factor for this selective sensing. However, it is difficult to discriminate between Cys and Hcy because of the similar photophysical properties of 3a and 3b. The reaction of 1 with Cys ultimately yields the product 4a, but that of 1 with Hcy cannot go beyond the stage of 3b. In the case of GSH, even the initial thiol-halogen SNAr nucleophilic substitution can lead to an intermediate product 5 (Fig. S5 in Supporting information); however, it is difficult for it to undergo further intramolecular rearrangement due to the unstable 10-membered macrocyclic transition state of the corresponding amino-coumarin.

|
Download:
|
| Fig. 5. (a) The proposed binding mechanism of compound 1 with Cys and (b) 1H NMR spectra of compound 1 (0.03 mol/L) (b-1), with Cys (0.15 mol/L) (b-2) and Hcy (0.15 mol/L) (b-3) in DMSO-d6-D2O. | |
Further NMR spectroscopic analysis also provided evidence for the intramolecular cyclization reaction in 1-Cys (Fig. 5b). With the addition of varying concentrations of Cys and Hcy to 1 (30 mmol/L) in DMSO-d6-D2O, the coumarin protons Ha and Hb displayed the obvious upfield shifts, indicating the breaking of the conjugation between 7-diethylamine-coumarin moiety and indol cation by the intramolecular cyclization reaction. Mass-spectrometry analysis of a product obtained from the reaction of 1 with Cys in CH3CN-HEPES also supported the interpretation (Fig. S5). These results are in good agreement with our proposed reaction mechanism.
To test the capability of 1 for cell imaging, Hi5 were imaged using 1 as the imaging agent. As shown in Fig. 6, in the absence of 1, the cells were non-fluorescent. With the addition of Cys, the Hi5 cells exhibited green fluorescence in the cytoplasm, providing a clear fluorescence image. This indicates that 1 binds with Cys present in the cytoplasm of the Hi5 cells.

|
Download:
|
| Fig. 6. Bright field (top) and fluorescent imaging (bottom) for Cys in Hi5 cells. (a) Probe 1 (10 μmol/L) only; (b) Probe 1 (10 μmol/L) and Cys (170 μmol/L); (c) Probe 1 (10 μmol/L) and Cys (350 μmol/L) incubated for 4 h at 20 ℃. Scale bar: 5 μm. | |
In our previous studies, we used some probes to assess the distribution of Cys in C. elegans and tested the probes for their potential in biological applications [15]. In 2006, Shi and Ma et al reported a coumarin-based Cys sensor [16]. Compared with their work, our probe showed more obvious color changes and emission enhancement. To check the feasibility of probe 1 to be used in in vivo imaging, the present study also used C. elegans, as the biological model, to assess the distribution of Cys visually in the nematode. C. elegans larvae at the developmental stage 4 (L4) were used. Fig. 7 shows that the addition of Cys leads to a significant fluorescence emission from dark to green. This result also confirms the excellent cell permeability and the potential of 1 to visually trace the enrichment and distribution of Cys in biological specimens.

|
Download:
|
| Fig. 7. Fluorescence imaging using probe 1 (bottom) and bright field (top) for Cys in C. elegansin. (a) Probe 1 (10 μmol/L) only; (b) Probe 1 (10 μmol/L) and Cys (170 μmol/L); (c) Probe 1 (10 μmol/L) and Cys (350 μmol/L) incubated for 4 h at 20 ℃. Scale bar: 5 μm. | |
This study examined the binding properties of a new coumarinbased compound (1). The results show 1 as a new turn-on fluorescent probe for cysteine detection. The probe exhibited high selectivity towards Cys over other various thiol and amino acids at pH 7.2 in aqueous media CH3CN–HEPES (0.02 mol/L, pH 7.2, 1: 9, v/v). Remarkable enhancement of up to 20-fold in fluorescence intensity was observed only in the presence of cysteine. In addition, compound 1 was successfully applied to the fluorescence imaging of cysteine in Hi5 cells and in C. elegans, suggesting 1 as a cysteine selective stain that could be applied in in vivo bioimaging.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21462050 and 21672185), the Foundation of the Department of Science and Technology of Yunnan Province of China (Nos. 2013HB062, 2014HB008, 2016FB020, 2016FB023), and the Program for Excellent Youth Talents, Yunnan University (No. XT412003).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.051.
| [1] |
(a) Y. Zhou, J. F. Zhang, J. Y. Yoon, Chem. Rev. 114(2014) 5511-5571; (b) J. C. Xu, J. Pan, X. M. Jiang, et al., Biosens. Bioelectron. 77(2016) 725-732; (c) X. M. Li, R. R. Zhao, Y. L. Wei, et al., Chin. Chem. Lett. 27(2016) 813-816; (d) H. R. Qu, Z. Y. Zhang, N. Wang, et al., Chin. Chem. Lett. 26(2015) 1249-1254. |
| [2] |
(a) D. M. Townsend, K. D. Tew, H. Tapiero, Biomed. Pharmacother. 57(2003) 134-144; (b) Z. A. Wood, E. Schroder, J. R. Harris, L. B. Poole, Trends. Biochem. Sci. 28(2003) 32-40. |
| [3] |
X.F. Yang, Y.X. Guo, R.M. Strongin, Angew. Chem. Int. Ed. 50(2011) 10690-10693. DOI:10.1002/anie.201103759 |
| [4] |
S. Shahrokhian, Anal. Chem. 73(2001) 5972-5978. DOI:10.1021/ac010541m |
| [5] |
H. Refsum, P.M. Ueland, O. Nygord, S.E. Vollset, Annu. Rev. Med. 49(1998) 31-62. DOI:10.1146/annurev.med.49.1.31 |
| [6] |
S. Seshadri, A. Beiser, J. Selhub, et al., Engl. J. Med. 346(2002) 476-483. DOI:10.1056/NEJMoa011613 |
| [7] |
O. Rusin, N.N.S. Luce, R.A. Agbaria, et al., J. Am. Chem. Soc. 126(2004) 438-439. DOI:10.1021/ja036297t |
| [8] |
(a) L. M. Ma, J. H. Qian, H. Y. Tian, M. B. Lan, W. B. Zhang, Analyst 137(2012) 5046-5050; (b) L. Y. Niu, Y. S. Guan, Y. Z. Chen, et al., Chem. Commun. 49(2013) 1294-1296. |
| [9] |
(a) H. S. Jung, J. H. Han, T. Pradhan, et al., Biomaterials 33(2012) 945-953; (b) X. Zhou, X. J. Jin, G. Y. Sun, X. Wu, Chem. Eur. J. 19(2013) 7817-7824. |
| [10] |
L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., J. Am. Chem. Soc. 134(2012) 18928-18931. DOI:10.1021/ja309079f |
| [11] |
(a) Y. L. Duan, Y. G. Shi, J. H. Chen, et al., Tetrahedron Lett. 53(2012) 6544-6547; (b) Y. G. Shi, J. H. Yao, Y. L. Duan, et al., Bioorg. Med. Chem. Lett. 23(2013) 2538-2542. |
| [12] |
J. Liu, Y.Q. Sun, Y.Y. Huo, et al., J. Am. Chem. Soc. 136(2014) 574-577. DOI:10.1021/ja409578w |
| [13] |
F. Liu, J. Du, D. Song, M.Y. Xu, G.P. Sun, Chem. Commun. 52(2016) 4636-4639. DOI:10.1039/C5CC10658C |
| [14] |
H.A. Anila, R.G. Upendar, A. Firoj, et al., Chem. Commun. 51(2015) 15592-15595. DOI:10.1039/C5CC04876A |
| [15] |
R.R. Zhao, Q.L. Xu, Y. Yang, et al., Tetrahedron. Lett. 57(2016) 5022-5025. DOI:10.1016/j.tetlet.2016.09.081 |
| [16] |
X.Y. He, X.F. Wu, W. Shi, H.M. Ma, Chem. Commun. 52(2016) 9410-9413. DOI:10.1039/C6CC04628B |
 2017, Vol. 28
2017, Vol. 28 


