More than 10 million people are ruthlessly deprived of lives by kidney diseases all over the world every year [1, 2]. In recent years, the incidence has been increasing, especially among the young ones. Undoubtedly, kidney disease has been regarded as a kind of disease that seriously endangers human health [3, 4]. On clinicopathological analysis, urine of the patients is the most obvious sign for some diseases including kidney disease. As one of three conventional detections in medical laboratory, routine urine test is a preliminary one, and its result is an important clue to disclose the essence of pathological process and thus cannot be ignored [5, 6]. More importantly, pH value of urine can reflect the acid-base balance of human body and the function of the kidney, so it has been used as an auxiliary method for doctors to diagnose and identify kidney disease [7]. Without taking drugs, the pH value of human urine is 4.6–8.0, namely the pH is between weak acid and weak base [8, 9]. Once out of these values, the abnormal urine indicated a first feature of some diseases. For instance, alkaline urine appeared in the patients with cystitis, alkalosis or urinary tract infection (UTI) on clinical laboratory, and the patients with acidosis and chronic kidney diseases (nephritis and nephropthisis) usually occurred with acidic urines [10-12].
Lanthanide ions have unique electronic structures that make their complexes to possess excellent optical, electrical and magnetic properties [13, 14]. As ligands of lanthanide ions, multi-podal carboxylates have received great attention due to the flexibility, modifiable skeleton structures and functional groups [15, 16]. Among them, salicylic acid derivatives as "antenna" can transfer energy to lanthanide ions to get luminescence complexes [17]. Especially, their europium (Ⅲ) or terbium (Ⅲ) complexes have remarkable advantages, such as narrow emission band, better monochromaticity, long lifetime, high quantum yield, etc. [18, 19]. And the complexes have stable fluorescence because they cannot easily be influenced by other cations. Therefore, design and synthesis of europium (Ⅲ) or terbium (Ⅲ) complexes based on multi-podal carboxylate ligands as a fluorescence probe to detect guest species is still in high demand. In connection of our previous work [16, 20], herein we reported three new terbium complexes as pH probes with highly sensitive and "Off–On–Off" fluorescent response [21]. Due to the different push-pull electronic effect of their salicylic acid ligands, the fluorescence efficiencies of three terbium (Ⅲ) complexes varied significantly. LⅡ·Tb complex with the best fluorescence performance was thus successfully applied for pH detection in some patients' urines.
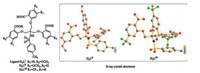
As shown in Fig. 1, three tripodal salicylic-acid ligands were easily synthesized (Scheme S1 in Supporting information) based on our previous work [15, 16, 20]. The structures of H3LⅠ, H3LⅡ and H3LⅢ were identified by 1H NMR, 13C NMR, IR and ESI-MS (Figs. S10-S22 in Supporting information). Moreover, the structures of H3LⅡ and H3LⅢ were directly analyzed by X-ray crystal structures (Fig. 1, and Table S1 in Supporting information). And their corresponding complexes LⅠ·Ln, LⅡ·Ln, LⅢ·Ln (Ln = Tb, Gd) were obtained in ethanol-water (1: 1, v/v) solution at room temperature. All complexes were identified by IR, EA and ESIMS (Figs. S23–S31 in Supporting information).
|
Download:
|
| Fig. 1. The structures of H3LⅠ, H3LⅡ, and H3LⅢ (all of hydrogen atoms and crystalline molecules of solvents are omitted for clarity in X-ray crystal structures). | |
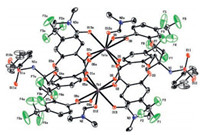
Compared with the free ligands, the νas (C=O) and νs (C=O) of LⅠ·Ln, LⅡ·Ln, LⅢ·Ln (Ln = Tb, Gd) in IR spectra (Fig. S12, S16, S21, and S23-S28 in Supporting information), have obvious red-shift, indicating coordination of Ln (Ⅲ) with the carbonyl oxygen atoms. And the elemental analysis suggested that all these complexes have the similar formula of [Ln2·(L)2·(H2O)4]. Meanwhile, the X-ray crystal structure of LⅢ·Tb was obtained in DMF. As shown in Fig. 2, two carboxylate ligands formed a cage-like dimer with two Tb ions to form a 2: 2 complex, which was in agreement with the elemental analysis. Each Tb was eight-coordinated, and two carboxyl oxygen atoms from two DMF molecules and six carboxyl oxygen atoms from two different ligands were the coordinated atoms, which was in agreement with the IR analysis. The coordinated modes of the ligand were cis-cis bidentate bridge and bidentate chelating. The ESI mass spectra also provided another evidence for the stoichiometry of these complexes in solution (Figs. S29–S31 in Supporting information).
|
Download:
|
| Fig. 2. X-ray crystal structure of LⅢ·Tb (all of hydrogen atoms and crystalline DMF molecules are omitted for clarity). | |
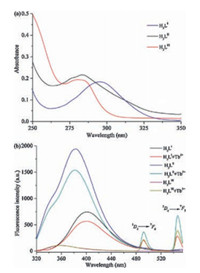
In order to confirm the antenna effect of ligands, molar absorption coefficients and the triplet energy levels of the ligands were obtained by UV–vis absorption spectra of ligands (Fig. 3a) and phosphorescence spectra of Gd (Ⅲ) complexes (Figs. S32-S34 in Supporting information). As shown in Fig. 3a (UV–vis spectra), the maximum absorption peaks of H3LⅠ, H3LⅡ and H3LⅢ were at 295 nm, 283 nm and 280 nm respectively, which were attributed to the 1π→π* transition. The molar absorption coefficient values of H3LⅠ, H3LⅡ and H3LⅢ were calculated as 3.69 ×103 L mol-1 cm-1, 4.32 ×103 L mol-1 cm-1, 3.98 × 103 L mol-1 cm-1 respectively, which indicated that all of the ligands have strong ability to absorb light in the UV region. The low temperature phosphorescence spectra of LⅠ·Gd, LⅡ·Gd and LⅢ·Gd were measured at 77 K in a methanol-ethanol mixture (1: 1, v/v) (Figs. S32–S34) [16], in which the triplet energy of H3LⅠ, H3LⅡ and H3LⅢ were calculated to be 21459 cm-1, 24633 cm-1, and 23474 cm-1, respectively. As a key factor, energy level matching between the triplet energy level of ligand and 5D4 (20, 500 cm-1) level of Tb (Ⅲ) could affect the luminescence properties of terbium complex. According to Latva's rule [22], if the energy gap (ΔE = 3ππ* -5D4) is within 2500– 4500 cm-1, ligands can effectively transfer the energy absorbed to Tb (Ⅲ) ions and show a higher quantum yield. If the ΔE is under 1500 cm-1, the energy return will occur, therefore leading to decrease of luminescence efficiency. For LⅠ·Tb, LⅡ·Tb and LⅢ·Tb, the energy gaps were calculated to be 929 cm-1, 4033 cm-1, and 2974 cm-1 respectively. These results showed that H3LⅡ and H3LⅢ were more effective sensitization to Tb (Ⅲ).
|
Download:
|
| Fig. 3. (a) UV–vis absorption spectra of H3LⅠ, H3LⅡ, and H3LⅢ (5.0 μmol/L) in Trisbuffer (1% DMSO, v/v, pH 6.5) solution. (b) Fluorescence spectra of H3LⅠ (λex = 295 nm), H3LⅡ (λex = 283 nm), H3LⅢ (λex = 280 nm), and H3L (5.0 μmol/ L) + Tb (Ⅲ) (5.0 μmol/L) in Tris-buffer (1% DMSO, v/v, pH 6.5) solution. | |
At room temperature, the fluorescence spectra of H3LⅠ, H3LⅡ, H3LⅢ and H3L + Tb (Ⅲ) in the Tris-buffer (1% DMSO, v/v, pH 6.5) solution were shown in Fig. 3b. H3LⅠ and H3LⅡ have strong emission peaks at about 400 nm and 381 nm, respectively. But H3LⅢ has a weak emission peak at about 358 nm. With the addition of Tb (Ⅲ) ions into the solution, the characteristic fluorescence emission peaks of Tb (Ⅲ) ions were appeared at 492 nm (5D4→7F6) and 547 nm (5D4→7F5). There results showed that the energy absorbed by three ligands could be transferred to Tb (Ⅲ) ion. But the fluorescence intensities were different: H3LⅡ + Tb > H3LⅢ + Tb > H3LⅠ + Tb, which in agreement with the discussion about the triplet energy levels of these ligands.
In general, the -OCH3 in H3LⅠ or H3LⅡ is the electron-donating group, and the -CF3 in H3LⅢ is the electron-withdrawing group. In order to study push-pull electronic effect on the fluorescence properties of ligands, the optimized structures and the density function theory (DFT) calculations were carried out with B3LYP/6-31G (d, p) basis set using the Gaussian 09 software (Fig. 4, and Figs. S35–S37 in Supporting information). For H3LⅠ, the contribution of electron density in HOMO was similar to that of LUMO, in which they all located on one arm of salicylic-acid moiety. But the electron of methoxyl group in H3LⅠ concentrated on the oxygen atom, which indicated the electron density on methoxyl group could not transfer to the salicylic-acid group efficiently. On the contrary, the electron density of HOMO in H3LⅡ distributed all over salicylic-acid ring including methoxyl group. However, the electron cloud on LUMO concentrated only on the salicylic-acid group, excluding -OCH3. Obviously, most of the electron clouds on this methoxyl group were efficiently transferred to the salicylicacid, which showed that -OCH3 in H3LⅡ was a good electrondonating group. As for HOMO in H3LⅢ, the electron cloud distributed on one arm of the salicylic-acid group, excluding -CF3. The electron density of carboxylic group in its LOMO decreased, while that of one of trifluoromethyl groups increased. These results indicated the -CF3 in H3LⅢ acted as an electronwithdrawing group. According to the triplet energy analysis and the computational analysis, the triplet energy level of H3LⅡ with an electron-donating -OCH3 could match well with a 5D4 energy level of Tb (Ⅲ). Thus, LⅡ·Tb exhibited good fluorescence properties. Due to the electron-withdrawing CF3 in H3LⅢ, the fluorescence intensity of LⅢ·Tb was weaker than that of LⅡ·Tb. Although there was also a -OCH3 in H3LⅠ, it was inefficient. Moreover, the energy return occurred in LⅠ·Tb based on Latva's rule, indicating the poor fluorescence properties of LⅠ·Tb as well.

|
Download:
|
| Fig. 4. HOMO-LUMO orbital plots of H3LⅠ, H3LⅡ and H3LⅢ. | |
As shown in Fig. 5 and Fig. S39 in Supporting information, two characteristic emission peaks of Tb (Ⅲ) complexes at 492 nm (5D4→7F6) and 547nm (5D4→7F5) can be clearly observed within the pH range of 5.0–8.0 for LⅠ·Tb, 4.6–8.2 for LⅡ·Tb and 3.6–9.0 for LⅢ·Tb. At lower pH value or higher pH value, these two characteristic peaks almost disappeared. All of the Tb (Ⅲ) complexes showed sensitive "Off–On–Off" fluorescence response in the whole pH range, so theycan be used as fluorescent probes to identify proton in aqueous solution.

|
Download:
|
| Fig. 5. Fluorescence response of (a) LⅠ·Tb, (b) LⅡ·Tb, and (c) LⅢ·Tb (5.0 μmol/L) to pH variation at 492 nm and 547 nm in Tris-buffer solution, respectively. (d) Fluorescence color changes of (top) LⅠ·Tb, (middle) LⅡ·Tb, and (bottom) LⅢ·Tb. From left to right: pH 2.0, 3.0, 4.0, 5.0, 6.0, 6.6, 7.0, 8.0, 9.0. | |
In the strongly acidic condition, coordination modes of Tb (Ⅲ) complexes changed as a result of protonation of one or more carboxyl groups of ligands. Based on the results of positive-ion ESIMS of LⅠ·Tb, LⅡ·Tb, LⅢ·Tb in aqueous solution at pH 2.0 (Figs. S39– S41 in Supporting information), the peaks of m/z 748.8 [H3LⅠ+Na]+, 748.2 [H3LⅡ+Na]+ and 862.8 [H3LⅢ+Na]+ were obtained which showed that all of the Tb (Ⅲ) complexes dissociated at low pH value. In addition, Tb (Ⅲ) ions were unstable and easy to be hydrolyzed in the strong alkaline condition. The positive-ion ESIMS of LⅠ·Tb, LⅡ·Tb and LⅢ·Tb in aqueous solution at pH 11.0 gave the evidence (Figs. S42-S44 in Supporting information). Moreover, the fluorescence quantum yields [23] of all of the complexes were obtained at different pH values as shown in Table 1, which were also in good agreementwith the "Off–On–Off" fluorescent changes. As shown in Fig. 5d, thesignificant fluorescence color changes from nonfluorescent (Off) to green (On) then to nonfluorescent (Off) were clearly observed by the naked-eye.
|
|
Table 1 The fluorescent quantum yields of LⅠ·Tb, LⅡ·Tb and LⅢ·Tb. |
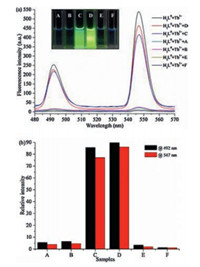
Due to the sensitive "Off–On–Off" fluorescence response for proton, these Tb (Ⅲ) complexes should have potential application in pH detection of urine. Considering the pH range (4.6–8.2) of the fluorescence response of LⅡ·Tb was consistent with the urine pH values of healthy person (4.6–8.0), it was applied to auxiliary diagnose and identify the kidney or urinary tract disease in clinical practice. Four fresh urine samples of patients with metabolic acidosis (A), chronic glomerulonephritis (B), metabolic acidosis (E), cystitis (F), and two fresh urine samples of healthy person (C, D) were collected from the Second Affiliated Hospital of Lanzhou University in order to routine urine pH detection, As shown in Fig. 6, when the addition of the samples C and D to the solution of LⅡ·Tb, the two fluorescence peaks at 492nm and 547nm were obviously observed. The colors of the solution were still green, which showed "Turn-On" fluorescence response under the ultraviolet lamp. Notably, the fluorescence peaks at 492nm and 547nm were quenched at once, when the four patient's urine samples were added to the solution of LⅡ·Tb. And the four patient's urine samples showed obvious nonfluorescence which can be directly observed by the naked eye. They showed "Turn-Off" fluorescence response. In other words, if a urine sample without any drugs and abnormal diets shows "Off" fluorescence response through this monitoring method, the individual may be suffered from some diseases, especially urinary system or kidney diseases. These results indicated that LⅡ·Tb can be used as a fluorescent probe for pH detection in clinical practice, therefore can auxiliary diagnose some diseases. The efficiency of the accurate diagnosis and treatment of diseases could be potentially improved.
|
Download:
|
| Fig. 6. (a) Fluorescence spectra of LⅡ·Tb in urine pH detection of samples A–F in Tris-buffer solution (1% DMSO, v/v). Inset: The fluorescence colors of samples A–F. (b) Relative fluorescence intensity of LⅡ·Tb in urine pH detection of samples at 492 nm and 547 nm. | |
Undoubtedly, various metal ions exist in human urine, and LⅡ·Tb may be interfered by them, especially Na+, K+ and Ca2+ ions. Thus, 17 common metal ions (Zn2+, Sr2+, Pb2+, Ni2+, Na+, Mn2+, Mg2+, Li+, K+, Hg2+, Fe3+, Fe2+, Cu2+, Co2+, Ca2+, Ba2+, Al3+) were used to measure the selectivity of LⅡ·Tb at different pH values. With the addition of these metal ions, the fluorescence intensities of LⅡ·Tb did notchange obviously, as shownin Fig. 7. Moreover, the quantity of the added metal ions in the solution was 10 times of the Tb (Ⅲ) complexes. These results showed that LⅡ·Tb as a sensitive pH fluorescent probe showed high selectivity under the existence of other metal ions.

|
Download:
|
| Fig. 7. The interference experiments of LⅡ·Tb with various metal ions in Tris-buffer solution (1% DMSO, v/v). (a) pH 4.6, (b) pH 6.6, and (c) pH 8.0. The gray and blue bars represent the fluorescence emission of LⅡ·Tb at 492nm and 547nm, respectively. The red and green bars represent the fluorescence emission of LⅡ·Tb with addition of 10.0 equiv. of metal ions at 492nm and 547nm, respectively. From 1 to 17: Al3+, Ba2+, Ca2+, Co2+, Cu2+, Fe2+, Fe3+, Hg2+, K+, Li+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, Sr2+, Zn2+. | |
Three new tripodal salicylic-acid ligands (H3LⅠ, H3LⅡ, H3LⅢ) were synthesized by changing different electron-pull or push groups. The fluorescence intensity of their Tb (Ⅲ) complexes at 492nm (5D4→7F6) and 547nm (5D4→7F5) showed "Off–On–Off" fluorescence response at various pH values in the Tris-buffer solution. Furthermore, LⅡ·Tb with high fluorescence intensity was chosen as a probe and successfully applied to the pH detection of routine urine in clinical practice. Without any drugs and abnormal diets, the patient's samples added with LⅡ·Tb had nonfluorescence. The method can be used in clinical diagnosis, especially for urinary system or kidney diseases.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21572091 and 21472075), and the Fundamental Research Funds for the Central Universities (No. lzujbky-2016-51) by Ministry of Education of China.
Appendix A. Supplementary dataSupplementary data associatedwith this article can be found, in the online version, at doi:10.1016/j.cclet.2017.08.037.
| [1] |
B.L. Kasiske, D.C. Wheeler, Nat. Rev. Nephrol. 5(2009) 650-657. DOI:10.1038/nrneph.2009.153 |
| [2] |
A. Vivante, F. Hildebrandt, Nat. Rev. Nephrol. 12(2016) 133-146. |
| [3] |
V. Krane, C. Wanner, Nat. Rev. Nephrol. 7(2011) 385-397. DOI:10.1038/nrneph.2011.62 |
| [4] |
S.R. Mulay, A. Linkermann, H.J. Anders, J. Am. Soc. Nephrol. 27(2015) 27-39. |
| [5] |
A.F. Hundley, A.B. Onderdonk, J.A. Greenberg, J. Reprod. Med. 48(2003) 853-857. |
| [6] |
H. Rayner, M. Thomas, D. Milford, Understanding Kidney Diseases[M]. Switzerland: Springer International Publishing, 2016.
|
| [7] |
D.M. Good, P. Zürbig, A. Argilés, et al., Mol. Cell. Proteom. 9(2010) 2424-2437. DOI:10.1074/mcp.M110.001917 |
| [8] |
G.H. Tesch, Nephrology 15(2010) 609-616. DOI:10.1111/j.1440-1797.2010.01361.x |
| [9] |
S. Kalantari, A. Jafari, R. Moradpoor, E. Ghasemi, E. Khalkhal, Int. J. Proteom. 4(2015) 782-798. |
| [10] |
S. Fujimoto, K. Hamai, Y. Sato, Y. Yamamoto, T. Eto, Nephrology 2(1996) 329-337. DOI:10.1111/nep.1996.2.issue-5 |
| [11] |
P.E. Drawz, J.R. Sedor, Nat. Rev. Nephrol. 7(2011) 458-468. DOI:10.1038/nrneph.2011.85 |
| [12] |
R.H. Mak, F. Schaefer, Nat. Rev. Nephrol. 10(2014) 428-429. |
| [13] |
X. Shen, B. Yan, RSC Adv. 34(2016) 28165-28170. |
| [14] |
C. Yin, F. Gao, F.J. Huo, P. Yang, Chem. Commun.(2004), 934-935. |
| [15] |
Y.W. Wang, Y.L. Zhang, W. Dou, et al., Dalton Trans. 39(2010) 9013-9021. DOI:10.1039/c001780a |
| [16] |
Y.L. Yang, Y.W. Wang, D.Z. Duan, et al., Org. Biomol. Chem. 11(2013) 6960-6966. DOI:10.1039/c3ob41319e |
| [17] |
A.J. Zhang, Y.W. Wang, W. Dou, et al., Dalton Trans. 40(2011) 2844-2851. DOI:10.1039/c0dt01514h |
| [18] |
J.C.G. Bünzli, Chem. Soc. Rev. 110(2010) 2729-2755. DOI:10.1021/cr900362e |
| [19] |
Z. Liu, W. He, Z. Guo, Chem. Soc. Rev. 42(2013) 1568-1600. DOI:10.1039/c2cs35363f |
| [20] |
Y.W. Wang, S.B. Liu, Y.L. Yang, et al., ACS Appl. Mater. Interfaces 7(2015) 4415-4422. DOI:10.1021/am5089346 |
| [21] |
C.C. Wang, S.Y. Yan, Y.Q. Chen, et al., Chin. Chem. Lett. 26(2015) 323-328. DOI:10.1016/j.cclet.2014.11.029 |
| [22] |
M. Latva, H. Takalo, V.M. Mukkala, et al., J. Lumin. 75(1997) 149-169. DOI:10.1016/S0022-2313(97)00113-0 |
| [23] |
C.A. Parker, W.T. Rees, Analyst 85(1960) 587-600. DOI:10.1039/an9608500587 |
 2017, Vol. 28
2017, Vol. 28