b Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China
Radicals typically refer to organic species with unpaired valence electron (/s). They are often very reactive, and their endogeneity was not widely acknowledged until a dedicated superoxidedetoxifying enzyme, superoxide dismutase (SOD) [1] was discovered. Over the years, numerous biological radicals based on carbon, nitrogen, oxygen, sulfur atoms etc. have been confirmed or proposed to be produced in vivo [2, 3]. Various biological mechanisms have been evolved to avoid damaging of the cell components [4]. For example, glutathione, ascorbic acid, uric acid and vitamin E are abundant in the hydrophilic and hydrophobic compartments as radical scavengers [5-9]. In fact, these highly caustic radicals have been harnessed by the immune system as a mechanism of host defense [10, 11]. Nevertheless, when not under strict control, undesired damages to the host cells by the radical species may occur and have been found to be associated to the development of various degenerative diseases, such as coronary heart disease, autoimmune diseases, Alzheimer's diseases, and cancers [3]. For this reason, understanding of the physiology and pathology of various radical species in various diseases has attracted tremendous research interests. Fluorescent probes, which yield a fluorescent signal upon recognition of the analyte-of-interest, are sensitive and suitable for use in complex biological milieu, and are highly desired by the biomedical community, over traditional methods based on electron spin resonance and mass-spectrometer [12].
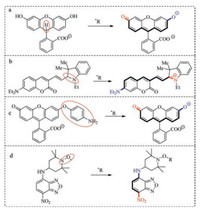
Development of sensitive and selective probes for radicals has attracted significant attention [13-19]. Reduced xanthene dyes and cyanine dyes have been routinely used in detection of highly reactive radicals (Fig. 1a, b) [12, 19]. Other receptors such as hydroquinone or p-aminophenol can be oxidized by radicals too (Fig. 1c) [20, 21]. Nitroxide which can efficiently quench the fluorescence of fluorophore was also used as radical receptor (Fig. 1d) [16]. Beside, directly hydroxylation or nitration of aryl was also used to detect oxidative radicals [12].
|
Download:
|
| Fig. 1. Selected radical fluorescent probes. | |
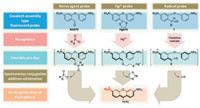
Detection sensitivity of a fluorescent probe is profoundly influenced by the background fluorescence from the probe. We are interested in developing novel scaffold, which could always yield a turn-on signal from a zero background. And the spectroscopic wavelength of the resulting fluorochrome is longer than that of the probe. Probes via "covalent assembly" approach can yield such a highly sensitive signal [22]. The basic rational of the covalentassembly type probes is that two independent fragments are joined together to furnish the electronic push-pull backbone of a fluorescent dye, via a cascade reaction triggered by the analyte-ofinterest. We have previously showcased that that such a principle could be used to develop probes for heavy transition metal ions [23], methylmercury [24], peroxynitrite [21], nerve agents [22], nitric oxide [25] and nitrite [26]. It is noteworthy that a rhodamine dye was employed in the design of probes for nerve agent (NA570) and Hg2+ (Hg570) respectively. Their detection mechanisms are analogous, i.e., in the presence of analyte-of-interest, both probes are activated and converted into an unstable chromogenic intermediate, which could go through an intramolecular electrophilic aromatic substitution, followed by an elimination to yield the final xanthene dye. Such a detection mechanism may be judiciously harnessed for detection of oxidative species, such as radical species. We envisage that compounds with the generic structure of 1 are potential "covalent-assembly" type chromogenic and fluorogenic probes for detection of oxidative radicals, with OR570 as a representative example (Fig. 2).
|
Download:
|
| Fig. 2. Detection mechanism of various covalent-assembly type fluorescent probes. | |
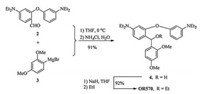
OR570 was conveniently synthesized by ethylated the intermediate 4 in a 92% yield, which was formed by reacting the Grignard reagent 3 with compound 2 in a 91% yield. Compound 2 was synthesized by a simple condensation of 2-bromo-4-diethylaminobenzaldehyde and 3-diethylamino-phenol in a 75% yield (Scheme 1).
|
Download:
|
| Scheme 1. Synthesis of OR570. | |
(4-(Diethylamino)-2-(3-(diethylamino)phenoxy)phenyl)2, 4-dimethoxyphenyl)methanol (4): 2.2 mL Grignard reagent 3 (1 mol/ L, 1.5 eqiv., prepared from 1-bromo-2, 4-dimethoxybenzene) was added to the 30 mL anhydrous THF solution of compound 2 (500 mg, 1.47 mmol, 1 eqiv.) at ice-bath. Kept reacting for 10 minutes before quenched with saturated ammonium chloride solution followed by column chromatography with a mixture of petroleum ether and EtOAc (100:5, v/v) as eluent to afford 4 (640 mg, colorless solid) in a 91% yield. 1H NMR (400 MHz, CDCl3): δ 7.23 (d, 1H, J = 8.20 Hz), 7.14 (d, 1H, J = 8.20 Hz), 7.05 (t, 1H, J = 8.16 Hz), 6.45–6.40 (m, 3H), 6.35 (dd, 1H, J = 8.00 Hz, 2.32 Hz), 6.33 (d, 1H, J = 7.82 Hz), 6.24 (d, 1H, J = 2.60 Hz), 6.23 (s, 1H), 6.18 (dd, 1H, J = 7.52 Hz, 2.40 Hz), 3.78 (d, 1H, J = 1.24 Hz), 3.78 (s, 3H), 3.73 (s, 3H), 3.30 (q, 4H, J = 7.04 Hz), 3.24 (q, 4H, J = 7.04 Hz), 1.13 (t, 6H, J = 7.04 Hz), 1.07 (t, 6H, J = 7.04 Hz); 13C NMR (100 MHz, CDCl3): δ 159.9, 159.1, 157.7, 155.0, 149.2, 148.3, 129.8, 128.8, 128.4, 124.5, 121.5, 107.5, 106.3, 104.6, 103.9, 103.2, 101.8, 98.5, 66.3, 55.3, 55.3, 44.4, 44.3, 12.5, 12.5. EI-HRMS (m/z): Calcd. for C29H39N2O4: 479.2904 [M + H]+; Found: 479.2913.
3-(3-(Diethylamino)phenoxy)-4-((2, 4-dimethoxyphenyl)(ethoxy)methyl)-N, N-diethylaniline (OR570): Compound 4 (300 mg, 0.63 mmol, 1 eqiv.) and NaH (30 mg, 1.25 mmol, 2 eqiv.) were mixed in 20 mL anhydrous acetonitrile at room temperature then iodoethane (195 mg, 1.25 mmol, 2 eqiv.) was added to the solution. Kept reacting for 2 hours before extracted with CH2Cl2 then followed by column chromatography with a mixture of petroleum ether and EtOAc (100:1, v/v) as eluent to afford OR570 (640 mg, colorless solid) in a 92% yield. 1H NMR (400 MHz, CDCl3): δ 7.35 (d, 1H, J = 8.20 Hz), 7.13 (d, 1H, J = 8.20 Hz), 7.04 (t, 1H, J = 8.16 Hz), 6.46 (dd, 1H, J = 8.00 Hz, 2.32 Hz), 6.41 (dd, 1H, J = 8.12 Hz, 2.32 Hz), 6.35 (d, 1H, J = 2.44 Hz), 6.32 (d, 1H, J = 8.68 Hz), 6.31 (d, 1H, J = 2.44 Hz), 6.25 (d, 1H, J = 2.44 Hz, 2.40 Hz), 6.16 (dd, 1H, J = 8.00 Hz, 2.44 Hz), 3.78 (s, 3H), 3.60 (s, 3H), 3.46 (d, 2H, J = 7.04 Hz), 3.29 (q, 4H, J = 7.04 Hz), 3.23 (q, 4H, J = 7.04 Hz), 1.13 (t, 9H, J = 7.04 Hz), 1.07 (t, 6H, J=7.04Hz); 13C NMR (100MHz, CDCl3): δ 159.9, 159.7, 158.0, 155.0, 149.1, 148.2, 129.5, 129.0, 128.3, 123.5, 121.0, 107.8, 105.7, 104.4, 103.9, 103.9, 101.5, 98.3, 70.9, 64.4, 55.3, 55.2, 44.3, 15.4, 12.5, 12.5. EI-HRMS (m/z): Calcd. for C31H43N2O4: 507.3217 [M+H]+; Found: 507.3219.
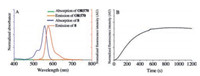
The absorption and emission spectra of the probe OR570 and the intended detection product (8) was recorded in CHCl3. The OR570 solution shows no absorption beyond 400nm, is colorless, and displays no background fluorescence with excitation at 530nm, while 8 exhibits a strong absorption with a maximum at 557nm (ε=93, 000Lmol-1cm-1) and emission at 570nm (ϕ=0.37). When observing with our naked eyes, the solution of the probe OR570 is colorless, while that of 8 is intensely magenta.
The viability of this scaffold as radical probe was validated by reactions between OR570 and NO2 in chloroform. A solution of NO2 in CHCl3 was prepared by bubbling NO2 into CHCl3. Upon addition of NO2 into a solution of OR570 (10 μmol/L), the solution turned magenta instantaneously and the kinetics of rhodamine formation was monitored with a fluorimeter, with excitation and the emission wavelength set to 557nm and 570nm respectively. The fluorescence signal rose steadily at the beginning 10min then leveled off, indicating this reaction could be finished within 10min (Fig. 3).
|
Download:
|
| Fig. 3. (A) Absorption(black)and emission(red)spectraof OR570and compound 8. (B) The kinetic curve of the formation of OR570 (10 μmol/L in CHCl3) upon addition of NO2 (a solution in CHCl3), with the excitation and emission wavelengths set to 557nm and 573nm. | |
A tentative mechanism has been proposed to rationalize the conversion of OR570 into 8 in the presence of oxidative radicals (Scheme 2). Highlyelectron efficient methine in OR570 is expected to be a good hydrogen atom donor, since the resulting secondary benzylic radical (5) is well delocalized and therefore stable. The presence of ethoxy group also helps to stabilize the radical. The single electron of 5 may be simply transferred to any other radical scavenger to yield 6 directly, which is unstable and will transform to the final fluorescent product 8. Or compound 5 may combine with molecular oxygen to generate peroxyl radical species (7). Since superoxide radicalanion is a goodleaving group with a pKa of 4.8 [3], conjugate elimination of 7 can also occur to yield the unstable intermediate 6, which collapses to 8 or signaling.
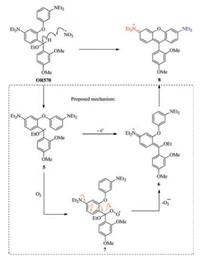
|
Download:
|
| Scheme 2. A tentative detection mechanism of OR570 toward highly oxidative radicals. | |
In conclusion, we have developed a scaffold (OR570) which has potential for detection of oxidative radicals. When used as a standalone "covalent-assembly" type fluorescent probe, merits of OR570 include simple structure, highly sensitive colorimetric and fluorometric turn-on signal fromzerobackground. OR570 may also be coupled with another fluorescent dye to develop twochannel fluorescent probes with more sophisticated signaling mechanism. Although OR570 was tested in organic solvent chloroform, its reactivity towards oxidative radicals was unambiguously exemplified. We believe that OR570 has the potential to detect oxidative radical in biological models.
AcknowledgementsThe work is supported by the Fundamental Research Funds for the Central Universities (Nos. 222201718004, WY1514053 and WY1516017) and the National Natural Science Foundation of China (Nos. 21372080, 21572061, and 21236002).
| [1] |
I. Fridovich, Ann. Rev. Biochem. 44(1975) 147-159. DOI:10.1146/annurev.bi.44.070175.001051 |
| [2] |
D.R. Blake, R.E. Allen, J. Lunec, Br. Med. Bull. 43(1987) 371-385. DOI:10.1093/oxfordjournals.bmb.a072188 |
| [3] |
P. Pacher, J.S. Beckman, L. Liaudet, Physiol. Rev. 87(2007) 315-424. DOI:10.1152/physrev.00029.2006 |
| [4] |
L.A. Pham-Huy, H. He, C. Pham-Huy, Int. J. Biomed. Sci. 4(2008) 89-96. |
| [5] |
B.N. Ames, R. Cathcart, E. Schwiers, P. Hochstein, Proc. Natl. Acad. Sci. U. S. A. 78(1981) 6858-6862. DOI:10.1073/pnas.78.11.6858 |
| [6] |
C. Michiels, M. Raes, O. Toussaint, J. Remacle, Free Radic. Biol. Med. 17(1994) 235-248. DOI:10.1016/0891-5849(94)90079-5 |
| [7] |
N. Smirnoff, Curr. Opin. Plant Biol. 3(2000) 229-235. DOI:10.1016/S1369-5266(00)00069-8 |
| [8] |
A. Chan, Can. J. Physiol. Pharm. 71(1993) 725-731. DOI:10.1139/y93-109 |
| [9] |
C.H. Foyer, G. Noctor, Plant Physiol. 155(2011) 2-18. DOI:10.1104/pp.110.167569 |
| [10] |
C. Bogdan, M. Rollinghoff, A. Diefenbach, Curr. Opin. Immunol. 12(2000) 64-76. DOI:10.1016/S0952-7915(99)00052-7 |
| [11] |
F.C. Fang, Nat. Rev. Microb. 2(2004) 820-832. DOI:10.1038/nrmicro1004 |
| [12] |
P. Wardman, Free Radic. Biol. Med. 43(2007) 995-1022. DOI:10.1016/j.freeradbiomed.2007.06.026 |
| [13] |
K. Yamada, F. Mito, Y. Matsuoka, et al., Nat. Chem. Biol. 12(2016) 608-613. DOI:10.1038/nchembio.2105 |
| [14] |
A.O.M. Barzegar, A. Zavras, C.H. Schiesser, et al., Org. Biomol. Chem. 14(2016) 2272-2281. DOI:10.1039/C5OB02441B |
| [15] |
R. Huber, T. Fiebig, H.A. Wagenknecht, Chem. Commun. 15(2003) 1878-1879. |
| [16] |
N.B. Yapici, S. Jockusch, A. Moscatelli, et al., Org. Lett. 14(2012) 50-53. DOI:10.1021/ol202816m |
| [17] |
L. Meng, Y. Wu, T. Yi, Chem. Commun. 50(2014) 4843-4845. DOI:10.1039/C4CC00975D |
| [18] |
F. Si, Y. Liu, K. Yan, et al., Chem. Commun. 51(2015) 7931-7934. DOI:10.1039/C5CC01075F |
| [19] |
K. Kundu, S.F. Knight, N. Willett, et al., Angew. Chem. Int. Ed. 48(2009) 299-303. DOI:10.1002/anie.v48:2 |
| [20] |
K. Setsukinai, Y. Urano, K. Kakinuma, et al., J. Biol. Chem. 278(2003) 3170-3175. DOI:10.1074/jbc.M209264200 |
| [21] |
Q. Zhang, Z. Zhu, Y. Zheng, et al., J. Am. Chem. Soc. 134(2012) 18479-18482. DOI:10.1021/ja305046u |
| [22] |
Z. Lei, Y. Yang, J. Am. Chem. Soc. 136(2014) 6594-6597. DOI:10.1021/ja502945q |
| [23] |
L. Song, Z. Lei, B. Zhang, et al., Anal. Methods 6(2014) 7597-7600. DOI:10.1039/C4AY01729C |
| [24] |
Z. Zhang, B. Zhang, X. Qian, et al., Anal. Chem. 86(2014) 11919-11924. DOI:10.1021/ac503900w |
| [25] |
Y. Yang, S.K. Seidlits, M.M. Adames, et al., J. Am. Chem. Soc. 132(2010) 13114-13116. DOI:10.1021/ja1040013 |
| [26] |
Y. Shen, Q. Zhang, X. Qian, et al., Anal. Chem. 87(2015) 1274-1280. DOI:10.1021/ac5039779 |
 2017, Vol. 28
2017, Vol. 28 


