Hypoxia is an important feature of tumor microenvironment due to insufficient oxygen supply in rapid-proliferation cancer cells [1]. It is well-known that hypoxia is correlated with tumor initiation, malignant progression, metastasis and resistance to chemotherapy and radiotherapy [2]. Imaging hypoxic areas is helpful to determine the treatment strategy and monitor the treatment response [3]. Therefore, it is important to develop detection methods which are able to track the dynamic changes of the tumor hypoxia in a non-invasive way.
Fluorescence imaging technology is such a non-invasive method and has been widely used in biological analysis. To date, many fluorescence probes have been reported for imaging tumor hypoxia. Among them, fluorescence probes being designed for responding to nitroreductase (NTR) are considered to be a promising strategy to track tumor hypoxia, because the NTR level is directly related to the degree of hypoxia in the solid turmor [4]. Nitroaromatic compounds have proven to be superior substrates for NTR which is upregulated in tumor cells. Many "turn-on" fluorescence probes have been reported to contain a nitroaromatic group as NTR recognition unit and a fluorophore as potential fluorescence reporting unit. The fluorescence signal would be switched from off to on when NTR induces a reduction reaction of nitro group into amino group.
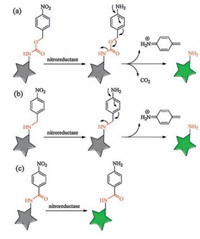
It is worthy to notice that there are several different ways to connect the two units of nitroaromatic group and fluorophore when designing such NTR probes. The commonly used linker could be a carbamate group [5-7]. The sensing mechanism in these probes is to turn on the fluorescence by the reduction of p-nitrobenzyl group to induce an 1, 6-elimination reaction and release a molecule of carbon dioxide (Scheme 1a). There is another widely used method to connect the two units in some reported NTR probes which have the p-nitrobenzyl group directly attached to the fluorophore by the linker of an ether group [8-11] or an ester group [12]. These probes possess the similar sensing mechanism as those with the first method to turn on the fluorescence by the reduction of p-nitrobenzyl group to induce an 1, 6-elimination reaction, but no release of carbon dioxide (Scheme 1b). Both the above-mentioned two methods would break the linker due to the 1, 6-elimination reaction when sensing NTR. There is the third method to construct the NTR probes in which the linker is not broken when sensing NTR (Scheme 1c) [13, 14]. The sensing mechanism include the reduction reaction of nitro group to amino group, but no 1, 6-elimination reaction. For example, Li et al. recently constructed ultrasensitive near-infrared turn-on fluorescence probes for NTR with unbroken linkers [4]. Importantly, they found that the linker is a key factor for sensing performance, and the probe with the linker of carbonyl group induces a rapid and significant enhancement of NTR activity after screening different unbroken linkers.
|
Download:
|
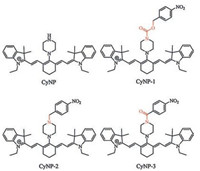
Therefore, it is interesting to investigate which kind of linkers, including broken or unbroken ones, would be the best one to induce the fastest and most enhancement of NTR activity for a given recognition unit and fluorescence reporting unit. Here, we present a linker-screening work on three new near-infrared fluorescence probes CyNP-1-3 for inducing the best NTR activity (Scheme 2). They are all derivatives of CyNP, an aminocyaine dye with a piperidine group (Scheme S1 in Supporting information). Although the three probes have the same fluorescence reporting unit and the same recognition unit, they have different linkers.
|
Download:
|
| Scheme 2. The structure of the aminocyanine dye CyNP and three NTR fluorescence probes CyNP-1, CyNP-2 and CyNP-3. | |
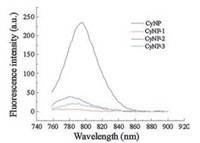
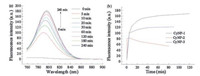
To check the potential of these three derivatives as a turn-on fluorescence probes, their emission spectra were recorded to compare with that of CyNP at the same experimental condition. As shown in Fig. 1, CyNP has emission centred at 790 nm when excited at 750 nm. But the fluorescence of all the three derivatives are quenched compared that of CyNP. Using 4-nitrophenyl group as a quencher is a common approach for designing chemosensors with a mechanism called d-PeT process [15]. This result gives them a chance to switch on fluorescence after sensing NTR because a good fluorescence probe should have a "dark" state before sensing. Among the three probes, CyNP-1 has the weakest fluorescence, which makes CyNP-1 to be the best candidate of NTR probes in these three derivatives. In fact, CyNP-1 did exhibit the best sensing performance with 40-fold enhancement (Fig. 2), while 3-fold for CyNP-2 (Fig. 2b and Fig. S1 in Supporting information) and 10-fold for CyNP-3 (Fig. 2b and Fig. S2 in Supporting information) at the same sensing condition.
|
Download:
|
| Fig. 1. The emission spectra of CyNP, CyNP-1, CyNP-2 and CyNP-3 ([dye] = 10 mmol/ L, in pH 7.4 Tris buffer, λex = 750 nm). | |
|
Download:
|
It is interesting to know how the difference of the linkers brings out the different sensing performance in the three probes. We carried HPLC to analyze the product of the sensing mixture of CyNP-1 (1 h reaction time, Fig. 3). As we expect, CyNP-1 was transformed into CyNP, although we can still observe the starting material CyNP-1. And the data of mass spectra (Fig. S3 in Supporting information) of the sensing mixture tell us the same story: CyNP-1 can smoothly transfer into CyNP companying that the linker is broken and carbon dioxide is release, which is consistent with the sensing mechanism shown in Scheme 1a. For CyNP-2, no expected HPLC data was obtained (not shown), and the mass spectra data tell us a different story. The nitro group in CyNP-2 was not reduced into amino group but into nitroso group (Fig. S4, Scheme S2 in Supporting information), and the linker was not broken. And the nitroso compound is known unstable and may become nonfluorescent with time. CyNP-3 was found to have a three-step reduction of nitrophenyl group into amino group (Fig. S5, Scheme S3 in Supporting information). And as we expected, its linker was not broken.

|
Download:
|
| Fig. 3. The HPLC spectra CyNP, CyNP-1 and the sensing mixture of CyNP-1 responding to NTR. | |
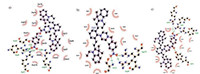
To explain the difference of sensing performance among the three probes, docking calculations were carried out for the probes with nitroreductase. As shown in Fig. S6 in Supporting information, CyNP-2 has higher docking energy (-4.59 ~ -9.74 kcal/mol) than those of CyNP-1 (-5.41 ~ -10.25 kcal/mol) and CyNP-3 (-6.94 ~ -10.07 kcal/mol). From the lowest energy conformation of the docking results shown in Fig. 4, the three probes have different hydrogen-bonding interaction with NTR. CyNP-1 has five hydrogen bonds with NTR, but three hydrogen bonds for CyNP-2, meanwhile CyNP-3 has four hydrogen bonds. So, the lower affinity of CyNP-2 may attribute to the less hydrogen bonds with NTR. Because the formation of hydrogen bonding is the key catalytic step of the reaction of NTR and its substrate, which explains why the nitrophenyl group in CyNP-2 cannot be completely reduced into amino group. CyNP-1 has the lowest docking energy by containing five hydrogen bonds with NTR and become the best probe. CyNP-3 has the second lower docking energy by containing four hydrogen bonds with NTR and can be used as a good NTR probe. But the fluorescence enhancement of CyNP-3 is weaker than that of CyNP-1.
|
Download:
|
| Fig. 4. Calculated binding model of CyNP-1 (a), CyNP-2 (b), CyNP-3 (c). The C, N, and O atoms o are shown in black, blue, and red, respectively. Red curves are indicated the hydrophobic areas and the amino acid residues of the NTR. Hydrogen bonds are indicated with green dotted lines. | |
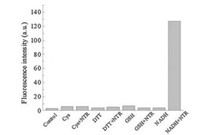
Based on the above screening results, we chose CyNP-1 as the best probe for further detection of nitroreductase. Selectivity is one of most important requirement for all kinds of detection methods. The sensing selectivity of CyNP-1 was investigated by using some biothiols as interfering species [10]. As shown in Fig. 5, CyNP-1 displays high selectivity for nitroreductase only in the presence of NADH.
|
Download:
|
| Fig. 5. Fluorescence response of CyNP-1 (15 μmol/L) to different reductive species (Cys (L-cysteine, 1 mmol/L), DTT (dithiothreitol, 1 mmol/L), GSH (glutathione, 1 mmol/L), NTR (10 μmol/L), NADH (0.5 mmol/L), reaction time 30 min, λex = 750 nm, λem = 795 nm). | |
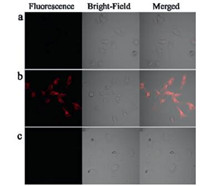
We futher check the capability of the probe CyNP-1 for monitoring the cellular hypoxia. Hela cells, which is known to produce nitroreductase under hypoxic conditions [10, 13], were incubated under either normoxic (20% O2, Fig. 6a) or different hypoxic (0.1% O2, Fig. 6b) conditions for 6 h, respectively, and then further incubated with CyNP-1 (15 μmol/L) for 40 min at 37 ℃. An obvious fluorescence signal can be observed at the channel of 655 nm ~755 nm when excitation at 635 nm. This result is consistent with the fluorescence response in solution (Fig. S7 in Supporting information). Dicoumarin is known to be an inhibitor of nitroreductase [11, 16, 17] and can be used to double check whether the probe CyNP-1 work with nitroreductase. As shown in Fig. 5c, the fluorescence signal can be not observed in the presence of dicoumarin, which means the hypoxic cells did produce nitroductase which can be detected by CyNP-1.
|
Download:
|
| Fig. 6. Confocal fluorescence image of Hela cells under normoxic (a) (20% O2) and hypoxic (b) (0.1% O2) conditions stained with CyNP-1 (15 μmol/L). And confocal fluorescence images of Hela cells (c) under hypoxic (0.1% O2) condition by incubating dicoumarin before staining CyNP-1. | |
In conclusion, we designed and synthesized three near-infrared fluorescence probes for imaging tumor hypoxia. The three probes contain same recognition unit and fluorescence reporting unit but different linkers. We screened out CyNP-1 as the best probe for detection of nitroreductase. And the probe CyNP-1 was employed for monitoring the cellular hypoxia in Hela cells. Importantly, the work provides a screening method by adjusting the linkers to develop other fluorescence probes based on enzyme-catalyzed reaction for detection other enzymes.
AcknowledgmentThis work was supported financially by the National Natural Science Foundation of China (Nos. 21421005, 21576038), the Fundamental Research Funds for the Central Universities of China (No. DUT16TD21), and Science Program of Dalian City (Nos. 2014J11JH133, 2015J12JH207).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.023.
| [1] |
H. Harada, M. Inoue, S. Itasaka, et al., Nat. Commun. 3(2012) 1-10. |
| [2] |
J.M. Brown, W.R. Wilson, Nat. Rev. Cancer 4(2004) 437-447. DOI:10.1038/nrc1367 |
| [3] |
X.C. Zheng, X. Wang, H. Mao, et al., Nat. Commun. 6(2015) 1-12. |
| [4] |
Y.H. Li, Y. Sun, J.C. Li, et al., J. Am. Chem. Soc. 137(2015) 6407-6416. DOI:10.1021/jacs.5b04097 |
| [5] |
J.G. Bae, L.E. McNamara, M.A. Nael, et al., Chem. Commun. 51(2015) 12787-12790. DOI:10.1039/C5CC03824C |
| [6] |
T. Guo, L. Cui, J.N. Shen, et al., Chem. Commun. 49(2013) 10820-10822. DOI:10.1039/c3cc45367g |
| [7] |
L. Cui, Y. Zhong, W.P. Zhu, et al., Org. Lett. 13(2011) 928-931. DOI:10.1021/ol102975t |
| [8] |
E. Nakata, Y. Yukimachi, H. Kariyazono, et al., Bioorg. Med. Chem. 17(2009) 6952-6958. DOI:10.1016/j.bmc.2009.08.037 |
| [9] |
Y.M. Shi, S.C. Zhang, X.R. Zhang, Analyst 138(2013) 1952-1955. DOI:10.1039/c3an36807f |
| [10] |
C. Xue, Y.J. Lei, S.C. Zhang, Y.W. Sha, Anal. Methods 7(2015) 10125-10128. DOI:10.1039/C5AY02312B |
| [11] |
Z. Li, X.Y. He, Z. Wang, et al., Biosens. Bioelectron. 63(2015) 112-116. DOI:10.1016/j.bios.2014.07.024 |
| [12] |
T.I. Kim, H. Kim, Y. Choi, Y. Kim, Sens. Actuators B:Chem. 249(2017) 229-234. DOI:10.1016/j.snb.2017.04.093 |
| [13] |
S.S. Wang, H. Liu, J. Mack, et al., Chem. Commun. 51(2015) 13389-13392. DOI:10.1039/C5CC05139H |
| [14] |
Q. Jiang, Z.Y. Zhang, J. Lu, et al., Bioorg. Med. Chem. 21(2013) 7735-7741. DOI:10.1016/j.bmc.2013.10.019 |
| [15] |
A. Roth, H. Li, C. Anorma, J. Chan, J. Am. Chem. Soc. 137(2015) 10890-10893. DOI:10.1021/jacs.5b05339 |
| [16] |
Z. Anusevicius, L. Miseviciene, J. Sarlauskas, et al., Arch Biochem. Biophys. 528(2012) 50-56. DOI:10.1016/j.abb.2012.08.014 |
| [17] |
R.L. Koder, A.F. Miller, Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1387(1998) 395-405. DOI:10.1016/S0167-4838(98)00151-4 |
 2017, Vol. 28
2017, Vol. 28 


