Indium is a soft, ductile metal following Ga in Group ⅢA, and is rare in Earth's crust [1]. The breakthrough discovery of indium was its use in the form of indium tin oxide (ITO), which was a unique material used in touchscreen devices, smartphones and liquidcrystal-display televisions, and so on [2]. In3+ ion can interfere in the metabolism of Fe3+ in the cells [3]. And according to animal experiments studies, In3+ ion was found to be toxic to the liver and kidneys [4]. Furthermore, In3+ ion has shown high affinity for biological membranes than Cd2+ and Hg2+ [5]. Thus recognition and monitoring In3+ ion in biological or environmental samples has created the need and academic interests.
For indium detection, the main methods include atomic absorption spectrometry [6], atomic emission spectrometry [7], and ion-selective electrodes [8], all of which were expensive and complex. Due to the high selectivity, sensitivity and relatively simple handling, fluorescent probes are widely used to monitoring various cations [9]. To our knowledge, to date, there were only three reports about fluorescent probe for In3+ ion [10-12] compared to many Al3+ probes [13]. Nevertheless, the two probes detected In3+ ion only in CH3CN [10, 11], which would limit their practical application. And the other one for In3+ ion detection was interfered by many metal ions [12]. Therefore, the development of highly selective and sensitive fluorescent probe for In3+ ion in aqueous solution is still in high demand.
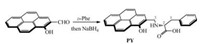
In connection with continuing study on dual-analyte chemosensors [14-21], we herein report a highly selective and sensitive fluorescence turn-on PY for the detection of In3+ ion in water and Al3+ ion in methanol, respectively. This chemosensor is based on the pyrene fluorophore with an α-amino acid moiety, and constitutes a new bifunctional (In3+/Al3+) probe by the convenient solvent-tuning fashion. Moreover, this probe can be used in the bioimaging of In3+ ion for the first time. As shown in Scheme 1, the probe PY was synthesized from 1-hydroxypyrene-2-carbaldehyde [22] and D-phenylalanine. The structure was confirmed by 1H, 13C NMR and ESI mass spectrometry (Figs. S1–S3 in Supporting information).
|
Download:
|
| Scheme 1. Synthesis of PY. | |
First, the fluorescence spectral properties of PY were studied in CH3OH solution at room temperature because the selectivity is not so good in ethanol. As illustrated in Fig. 1a, PY exhibited a strong, single emission band at 430 nm when excited at 364 nm, which emission was attributed to pyrene-pyrene static excimer [23]. Upon the addition of different cations (Li+, Na+, H+, Ag+, Mg2+, Sr2+, Ba2+, Pb2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Fe3+, Cr3+, Al3+, Ga3+, and In3+) to the solution, the fluorescence responses of PY were different. Almost no obvious changes of the fluorescence of PY were observed when Li+, Na+, Mg2+, Sr2+, Ba2+, Mn2+, Zn2+, and Cd2+ ions were added to the solution, respectively. Due to the chelation enhanced fluorescent quenching (CHEQ) [24], Pb2+, Fe2+, Ni2+, Cu2+, Hg2+, Ag+, and Fe3+ quenched the fluorescence of PY severely. But the addition of Cr3+, Co2+, Al3+, Ga3+, In3+, or H+ changed the fluorescence signals of PY remarkably. The characteristic monomer emission bands at approximately 400 nm and 420 nm of pyrene moieties appeared, and a new peak from the dynamic excimer of pyrene moieties at round 450 nm was also observed. Compared to the fluorescence intensity of monomer peak, Al3+ ion caused the significant enhancement of the two monomer peaks. These results showed that PY was highly selective for Al3+ in CH3OH solution. Then the fluorescence titrations of Al3+ were carried out (Fig. 1b). With the addition of Al3+ to the solution of PY, the monomer emissions at 400 nm and 420 nm increased significantly and the excimer emission at 430 nm decreased in some extent. Moreover the total fluorescence intensity at 400 nm of PY was enhanced 16.7-fold when 40.0 equiv. of Al3+ was present. And the fluorescent quantum yield (Φ) of PY (10.0 μmol/L) increased from 60.9% to 63.5% in the presence of Al3+ (40.0 equiv.). The detection limits were calculated to be 4.13 μmol/L and 8.08 μmol/L at 400 nm and 430 nm, respectively [25] (Figs. S4 and S5 in Supporting information). The Job's plot supported the formation of a 1:1 stoichiometry complexation between PY and Al3+ (Fig. S6 in Supporting information). Based on the stoichiometry, the association constant K of the complex was then calculated to be about 5.0 ×104 L/mol by using the emission changes at both 400 nm and 430 nm with Benesi–Hildbrand plots (Figs. S7 and S8 in Supporting information). In addition, the peak at m/z 618.0226 for [PY + Al3+ + 2ClO4--2H+]- in the negative-ion ESI mass spectrum provides additional evidence for the formation of a 1:1 complex of PY·Al3+ (Fig. S9 in Supporting information). Further, the short response time showed that PY has high sensitivity for Al3+ (Fig. S10 in Supporting information). And the competition experiments displayed all of other cations had no obvious interference with the detection of Al3+ ion (Figs. S11 and S12 in Supporting information). These results indicated that PY could act as a turn-on fluorescent probe for Al3+ in methanol.

|
Download:
|
| Fig. 1. (a) Fluorescent spectra of PY (10.0 μmol/L) with different cations (20.0 equiv.) in CH3OH (0.05% DMSO, v/v) (λex = 364 nm). (b) Fluorescent titrations spectra of PY (10.0 μmol/L) with Al3+ (0–40.0 equiv.). Inset: Fluorescence intensity at F400/F430 changes upon the addition of Al3+. | |
Next, the fluorescence spectral properties of PY were investigated in H2O (0.05% DMSO, v/v) at pH 4.8 (Fig. 2a). The fluorescence intensity of free PY was quenched significantly by water. And the fluorescence spectrum of free PY was different from that in methanol. Three emission bands at about 396 nm, 416 nm and 446 nm were observed, which also attributed to the monomer and excimer emission bands of pyrene. After the addition of different cations to the solution, most of them quenched the fluorescence of PY in different extent. Only In3+ caused a significant fluorescence enhancement of PY with a new emission band at 410 nm. The high selectivity of In3+ over Al3+ and Ga3+ was probably attributed to a pH effect in water, since Group ⅢA ions have strong hydration ability. Therefore, the pH effect was studied in detail (Fig. S13 in Supporting information). Within the range of pH 4.0 and 5.5, probe PY showed high selective recognition of In3+ over Al3+ and Ga3+ by turn-on fluorescence mode. The addition of In3+ ion to the solution resulted a 2.9-fold fluorescence enhancement at 410 nm (Fig. 2b), which denoted chelation-enhanced fluorescence (CHEF) [26]. The fluorescent quantum yield (Φ) of PY (10.0 μmol/L) increased from 46.1% to 48.8% in the presence of In3+ (40.0 equiv.). And the intensity decrease in the two bands at about 396 nm and 446 nm, which is resulting from breaking the monomer-excimer equilibrium of PY in water solution after the formation of PY·In3+ complex. The Job's plot (Fig. S14 in Supporting information), Benesi–Hildbrand plot (Fig. S15 in Supporting information), and the ESI–MS (Fig. S16 in supporting information) also indicated 1:1 binding model between PY and In3+ in NaOAc/HOAc (pH 4.8) buffer solution. By using the emission changes at 410 nm, the association constant K of PY·In3+ was calculated to be 7.1 ×104 L/mol (Fig. S15 in Supporting information). A peak at m/z 777.7 assigned to [PY + In3+ + 2ClO4- + H2O-2H+]- was observed in ESI–MS (Fig. S16 in Supporting information). The corresponding detection limit was found to be 3.42 μmol/L (Fig. S17 in Supporting information), which is low enough for detection of In3+. PY still showed the short response time for detection of In3+ (Fig. S18 in Supporting information). And all competitive cations had no obvious interference with the detection of In3+ ion (Fig. S19 in Supporting information). These results clearly showed that PY can function as a specific turn-on fluorescent probe for In3+ in NaOAc/HOAc (pH 4.8) buffer solution.

|
Download:
|
| Fig. 2. (a) Fluorescent spectra of PY (10.0 μmol/L) with different cations (20.0 equiv.) in H2O (0.05% DMSO, v/v) (pH 4.8) (λex = 364 nm). (b) Fluorescent titrations spectra of PY (10.0 μmol/L) with In3+ (0 to 40.0 equiv.) in NaOAc/HOAc buffer solution (pH 4.8) (0.05% DMSO, v/v). Inset: Plot of the fluorescence intensity of PY as a function of [In3+] at 410 nm. | |
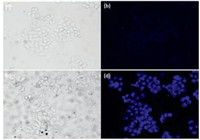
Finally, the fluorescence imaging of PY for sensing In3+ ions in HeLa cells was carried out. When HeLa cells were incubated with PY (10.0 μmol/L) for 30 min at 37 ℃, the weak fluorescence of cells was observed (Fig. 3b). As shown in Fig. 3d, at 37 ℃ the treated HeLa cells were incubated with In3+ (200.0 μmol/L) in culture medium for 30 min, the strong blue fluorescence was obtained. These results indicated that PY is cell membrane permeable and could be used for detecting In3+ within living cells.
|
Download:
|
| Fig. 3. Images of HeLa cells: (a) bright field image of Hela cells incubated with PY (10.0 μmol/L); (b) Fluorescence image of (a); (c) bright field image of HeLa cells incubated with PY (10.0 μmol/L) for 30 min, and then further incubation with In3+ (200.0 μmol/L) for 30 min at 37 ℃; (d) fluorescence image of (c). | |
In summary, a turn-on probe PY based on the integration of pyrene moiety and amino acid group was obtained. Through solvent-tuning, PY shows high selectivity and sensitivity for In3+ in NaOAc/HOAc (pH 4.8) buffer solution and for Al3+ in methanol by the quick formation of corresponding complex. The above orthogonal recognition of Al3+ and In3+ is resulted from subtle equilibrium shift of pyrene monomer-excimer in CH3OH and H2O, respectively.Moreover, probe PY shows good cell permeability and was successfully applied in bioimaging.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21572091 and 21472075), and the Fundamental Research Funds for the Central Universities (No. lzujbky-2016-51) by MoE of China.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.019.
| [1] |
A.J. Downs, Chemistry of Aluminium, Gallium, Indium and Thallium, Blackie Academic and Professional[M]. Chapman & Hall, 1993.
|
| [2] |
C. Renouf, Nat. Chem. 4(2012) 862. DOI:10.1038/nchem.1460 |
| [3] |
A.A. Moshtaghie, M.A. Ghaffari, Iran Biomed. J. 7(2003) 73-77. |
| [4] |
R.E. Chapin, M.W. Harris, E. Sidney Hunter Ⅲ, et al., Fundam. Appl. Toxicol. 27(1995) 140-148. DOI:10.1006/faat.1995.1117 |
| [5] |
Y. Suzuki, H. Matsushita, Ind. Health 7(1969) 143-154. DOI:10.2486/indhealth.7.143 |
| [6] |
N. Lewen, S. Mathew, M. Schenkenberger, T. Raglione, J. Pharm. Biomed. Anal. 35(2004) 739-752. DOI:10.1016/j.jpba.2004.02.023 |
| [7] |
O. Acar, A.R. Türker, Z. Kiliç, Spectrochim. Acta Part B 55(2000) 1635-1641. DOI:10.1016/S0584-8547(00)00258-5 |
| [8] |
V.K. Gupta, A.J. Hamdan, M.K. Pal, Talanta 82(2010) 44-50. DOI:10.1016/j.talanta.2010.03.055 |
| [9] |
X. Qian, Z. Xu, Chem. Soc. Rev. 44(2015) 4487-4493. DOI:10.1039/C4CS00292J |
| [10] |
S.K. Kim, S.H. Kim, H.J. Kim, et al., Inorg. Chem. 44(2005) 7866-7875. DOI:10.1021/ic050702v |
| [11] |
Y.C. Wu, H.J. Li, H.Z. Yang, Org. Biomol. Chem. 8(2010) 3394-3397. DOI:10.1039/c0ob00002g |
| [12] |
Q. Wei, B. Du, H. Zhang, Y. Li, Z. Li, J. Anal. Chem. 60(2005) 868-873. DOI:10.1007/s10809-005-0198-3 |
| [13] |
Y. Zhang, Y. Fang, N.Z. Xu, et al., Chin. Chem. Lett. 27(2016) 1673-1678. DOI:10.1016/j.cclet.2016.04.011 |
| [14] |
M. Dong, Y.W. Wang, Y. Peng, Org. Lett. 12(2010) 5310-5313. DOI:10.1021/ol1024585 |
| [15] |
Y.M. Dong, Y. Peng, M. Dong, Y.W. Wang, J. Org. Chem. 76(2011) 6962-6966. DOI:10.1021/jo201269e |
| [16] |
M. Dong, Y. Peng, Y.M. Dong, N. Tang, Y.W. Wang, Org. Lett. 14(2012) 130-133. DOI:10.1021/ol202926e |
| [17] |
Y. Peng, Y.M. Dong, M. Dong, Y.W. Wang, J. Org. Chem. 77(2012) 9072-9080. DOI:10.1021/jo301548v |
| [18] |
X. Sun, Y.W. Wang, Y. Peng, Org. Lett. 14(2012) 3420-3423. DOI:10.1021/ol301390g |
| [19] |
Y.L. Yang, Y.W. Wang, Y. Peng, Sci. China Chem. 57(2014) 289-295. DOI:10.1007/s11426-013-4999-1 |
| [20] |
Y.W. Wang, S.B. Liu, W.J. Ling, Y. Peng, Chem. Commun. 52(2016) 827-830. DOI:10.1039/C5CC07886E |
| [21] |
Z.H. Fu, L.B. Yan, X. Zhang, et al., Org. Biomol. Chem. 15(2017) 4115-4121. DOI:10.1039/C7OB00525C |
| [22] |
Y. Zhou, J.Y. Jung, H.R. Jeon, et al., Org. Lett. 13(2011) 2742-2745. DOI:10.1021/ol200846a |
| [23] |
S. Karuppannan, J.C. Chambron, Chem. Asian J. 6(2011) 964-984. DOI:10.1002/asia.v6.4 |
| [24] |
J.H. Chang, Y.M. Choi, Y.K. Shin, Bull. Korean Chem. Soc. 22(2001) 527-530. |
| [25] |
W. Lin, L. Yuan, Z. Cao, Y. Feng, L. Long, Chem. Eur. J. 15(2009) 5096-5103. DOI:10.1002/chem.v15:20 |
| [26] |
J.S. Kim, K.H. Noh, S.H. Lee, et al., J. Org. Chem. 68(2003) 597-600. DOI:10.1021/jo020538i |
 2017, Vol. 28
2017, Vol. 28 


