Ascorbic acid (AA) plays vital physiological and biochemical functions in human bodies. It is associated with the regulation of gene expression and mRNA transcription, as well as attenuation of the process for ageing and cancer [1, 2]. In addition, AA serves as an essential cofactor in many biological processes to participate in the functions of both the nervous and endocrine systems [3]. However, human are unable to synthesize AA due to their deficiency in Lgulono-γ-lactone oxidase, which catalyze the terminal step in Lascorbic acid biosynthesis [4]. Therefore, AA can be only obtained from dietary sources and distribute to all other tissues for keeping the physiological balance. In this regard, it is quite important to detect AA in biological samples, which can offer better guidance to the human's rational diet.
Up to now, numerous analytical methods for monitoring AA in vitro or in vivo have been reported, such as UV spectrophotometry [5], electrochemical analysis [6, 7], and fluorescence spectroscopy [8-11].Amongthem, fluorescent sensors are ideal candidates dueto their intrinsic advantages, such as real-time imaging, high sensitivity and selectivity, and good reproducibility. However, prolonged light or heating will cause the loss and decomposition of AA. In addition, there are a large number of pigments including chlorophyll and some fluorescent protein in the biological samples, which produce a certain biorelated autofluorescence under short-wavelength UV-visible radiation as interference. NIR materials with high selectivity, rapidity and less photo-damage are also decent methods for detecting AA. Recently, some novel turn-on sensors based on the specific oxidation-reduction reaction between the CoOOH nanoflakes and AA have gained increasing interests for determining AA with some outstanding advantages [12, 13]. Based on this, different nanomaterials, such as quantum dots [14-16], carbon nanomaterials [17], have been employed as the designed fluorophores for AA sensing. For instance, Yan's group employed CdTe QDs as fluorophores for selective detection of AA after adding a certain amount of KMnO4 as quencher [18]. Lin et al. hybridized as-prepared tris(hydroxymethyl)-aminomethane-derived carbon dots (CDs) with CoOOH nanoflakes to form CDsCoOOH for simple yet facial monitoring of AA in brain microdialysate [19]. In addition, the Chang group reported a two-photon excited probes which were prepared by electrostatic force of silica nanoparticles with two-photon dye adsorbing cobalt oxyhydroxide (CoOOH) nanoflakes to image AA in living cells and tissues [20]. Our group designed a nanoprobe based on CoOOH nanoflake-modified persistent luminescence nanoparticle for determining and screening of AA in living cells and in vivo, which allowed monitoring of AA without external excitation [21]. Although these probes achieved impressive results, these probes probably have certain inadequacies with biotoxicity, the complicated preparation process and the poor water-solubility. Therefore, developing novel biocompatible luminescent materials for AA determination has become a highly challenging question.
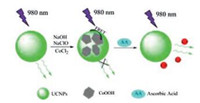
Herein, we have developed a novel nanoprobe for detecting AA in biological samples using the CoOOH-modified lanthanidedoped upconversion nanoparticles (UCNPs). UCNPs as luminescent nanoprobes for detection can improve signal-to-noise ratio and preclude possible false positive signals [22-24]. Beyond that, the photodamage to biological samples is barely minimal and the depth of light penetration is significantly remarkable. Owing to the broad absorption band of CoOOH nanoflakes well overlapping with the two emission wavelengths of UCNPs, the upconversion luminescence (UCL) can be efficiently quenched by the CoOOH nanoflakes via the generation of Förster resonance energy transfer (FRET). In the presence of AA, CoOOH was reduced to Co2+ and the luminescence of UCNPs could be restored immediately with the relative amounts of AA. Moreover, the signal-to-noise ratio can be greatly enhanced when applied 980 nm light for excitation, by which both reducing the biological background and increasing the sensitivity of the detection.
The design and synthesis of CoOOH modified-upconversion nanoparticles was illustrated in Scheme 1. Firstly, 50 μL OX-UCNPs (10 mg/mL), 100 μL NaOH aqueous solutions (0.8 mol/L) and 100 μL NaClO aqueous solution (0.2 mol/L) were mixed in a 2 mL microcentrifuge tube. Various amounts of CoCl2 (10 mmol/L, 0 ~ 100 μL) were then added into the tube and sonicated. Subsequently, CoOOH-modified UCNPs were collected by centrifugation and washed for three times with deionized water. Finally, the products were re-dispersed in 1 mL deionized water. The experimental detail has been deposited in Supporting information.
|
Download:
|
| Scheme 1. Schematic illustration of the design for AA detection using CoOOHmodified UCNPs. | |
In the following experiments, the CoOOH-modified UCNPs (addition of 0.9 mmol/L CoCl2) were chosen as an example.
At first, the detection of AA in handmade orange juice or pepper extract was studied. 1.0 mL of fresh-prepared CoOOH-modified UCNPs (0.5 mg/mL) was added to a 2 mL microcentrifuge tube, and then different concentrations (0, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 μmol/L) of AA solution were added respectively. The photoluminescence intensities at 541 nm of the above solutions were measured immediately with the excitation of a continuous wave (CW) 980 nm laser.
Then, 10 μL handmade orange juice or pepper extract was added into the 1 mL nanoprobe solution respectively and the luminescence intensity at 541 nm was recorded before and after the addition of handmade orange juice with the excitation of a CW 980 nm laser. AA content of the handmade juice or pepper extract was calculated by the follow formula:
AA content (mg/100 mL) = C × V × M = 100 × 176.13 × C
Here, "C" is the AA molar concentration (μmol/L) which was obtained though the linear correlation between the fluorescence intensity and AA concentrations; "V" is the volume as 1 mL; "M" is AA's molar mass as 176.13 g/mol.
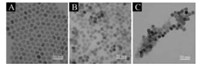
To accomplish the detection of AA in biological sample, nanoprobe was prepared via two steps. The synthesis and characterizations of upconversion nanoparticles (UCNPs) and CoOOH were studied. Firstly, NaYF4: 18% Yb, 2% Er nanocrystals (β-phase) were prepared for morphology study of the UCNPs via transmission electron microscopy (TEM). As shown in Fig. 1A, the oleic acid capped UCNPs (OA-UCNPs) with an average diameter of 23 nm were obtained with further modifications. In order to enhance the OA-UCNPs' surface hydrophilicity, the carbon-carbon double bond on the OA-UCNPs was oxidized to carboxyl group by Lemieux-von Rudloff (5.7 mmol/L KMnO4, 0.105 mol/L NaIO4). Fourier transform infrared (FTIR) spectra (Fig. S1 in Supporting information) demonstrate the OA's C=C stretching vibration characteristic peak at 3007 cm-1 (black line) completely disappeared after the treatment (red line), indicating the OA-UCNPs were fully oxidized to azelaic acid capped-upconversion nanoparticles (OX-UCNPs). It was further proved by the TEM that the OX-UCNPs display uniform hexagonal morphology similar to OAUCNPs (Fig. 1B), meaning the NPs retain stable against oxidation and only surface ligands changed. Moreover, the diffraction patterns of both OA-UCNPs and OX-UCNPs are consistent with hexagonal NaYF4 phase (JCPDS card 16-0334) owing to its strong inoxidizability (Fig. S2 in Supporting information). In addition, the optical property was also evaluated through fluorescence spectroscopy with emission at 540 and 660 nm, and excitation at 980 nm. The restored fluorescence intensities of both NPs display exactly same emission, suggesting the NPs' optical property have not been affected by oxidizing agents (Fig. S3 in Supporting information).
|
Download:
|
| Fig. 1. The TEM of OA-UCNPs (A), OX-UCNPs (B), CoUCNPs (C). | |
Nanoflakes of cobalt oxyhydroxide (CoOOH) were synthesized with further modification. Typically, an aqueous CoCl2 solution was added into NaClO solution with the presence of NaOH, so that amorphous CoOOH nanoflakes can be formed as the Co2+ oxidized by NaClO under basic condition. During the synthesis, a new UV–vis absorption band centered at 410 nm was emerged while the maximum absorption of CoCl2 was at 510 nm completely gone (Fig. S4 in Supporting information), indicating a fully oxidation of Co2+. The main peak of X-ray photoelectron spectroscopy (XPS) clearly shows the oxidation state of Co (Ⅲ) at 781.29 eV (Fig. S5 in Supporting information), suggesting the successfully synthesis of CoOOH nanoflakes.
The negative surface of the OX-UCNPs was modified with the CoOOH nanoflakes (CoUCNPs). Normally, NaOH and NaClO solution was mixed with OX-UCNPs solution, and then the CoCl2 solution was added to form the CoUCNPs. According to TEM (Fig. 1C) and XPS (Fig. S6 in Supporting information), the CoOOH grew on the negatively charged surface of OX-UCNPs which serve as anchor sites that attract Co2+ to form the nanoprobes. The UV–vis absorption spectroscopy (Fig. S7 in Supporting information) exhibits the only characteristic absorption of CoOOH at 410 nm, indicating the successful conjugation of CoOOH nanoflakes onto OX-UCNPs. To investigate the feasibility of CoUCNPs, the optical properties of CoOOH and UCNPs were studied. Since the CoOOH nanoflakes' broad absorption band (200–800 nm) well overlaps with the two emission wavelengths (540 nm and 660 nm) of OX-UCNPs (Fig. S8 in Supporting information), the upconversion luminescence (UCL) can be efficiently quenched by the CoOOH nanoflakes due to the generation of Förster resonance energy transfer (FRET). Photoluminescence spectra of OX-UCNPs, CoOOH mixed with OX-UCNPs, and CoUCNPs were carried out (Fig. S9 in Supporting information), which demonstrates that only the CoUCNPs leads to a significant upconversion luminescence intensity decrease. Therefore, it is assumed that the positively charged CoOOH nanoflakes attached to the OX-UCNPs' negative charged surface by electrostatic force.
As luminescence quenching degrees influence the FRET, different ratios of CoOOH nanoflakes modified UCNPs were applied for optimization. In Fig. S10 in Supporting information, the increased CoCl2 concentrations from 0 to 1.2 mol/L lead to a dramatic decrease of the nanoprobes' fluorescence intensities, and the maximum quenching efficiency raise up to 95.75%. Corresponding pictures of the samples display a tunable solution color change as a function of CoCl2 concentration. Considering the fluorescence intensities changed more slowly with the concentration over 0.9 mmol/L, so 0.9 mmol/L was chosen as the most suitable concentration.
Afterwards, the CoUCNPs' fluorescence responses to AA in aqueous solutions were evaluated (Fig. 2A). As different concentrations (7.5 μmol/L to 100 μmol/L) of AA were added into the nanoprobe solution, a well linear relationship between recovery of the fluorescence intensities and the concentrations was observed. The regression equation was ΔF = 57.011 × C-434.877 with a significant low detection limit of ~3.32 μmol/L (Fig. 2B). The recovery of upconversion emission was attributed to the decomposition of the CoOOH from UCNPs, since the AA reduces CoOOH to Co2+ (Equation S1 in Supporting information). Afterwards, the kinetic performance of the nanoprobes toward the oxidationreduction reaction was also investigated. As AA (final concentration at 100 μmol/L) added into the nanoprobes' aqueous solution, the fluorescence intensity changes were recorded as a function of time. In Fig. S11 in Supporting information, the fluorescence intensity of CoUCNPs enhanced immediately to equilibrium and then continues with a little change lasted for the next 5 min. Therefore, the instantaneous reaction of AA and CoUCNPs with outstanding stability can be applied for chemical sensing applications.

|
Download:
|
| Fig. 2. (A) The fluorescence spectra changes of the nanoprobe to different amounts of AA. (B) Linear correlation between the fluorescence intensity and AA concentrations. | |
Since selectivity plays a significant role in the nanoprobe's applications, investigating the nanoprobe's selectivity of the AA towards other interfering reactive species in the biological system can specifically reduce the false positive possibilities. Therefore, before applying to the biological assay, the nanoprobe was firstly revealed by monitoring the fluorescence intensity change upon exposing to electrolytes and weakly reducing bioagents. In Fig. S12 and S13 in Supporting information, the nanoprobes' responses to AA exhibit highly selectivity other than the interfering agents, further ensuring the practicality of the proposed strategy to monitor AA in biological sample analysis. In addition, the colloid stability of OX-UCNPs and OX-UCNPs-CoOOH were also studied, which was another important factor for AA detection. As shown in Fig. S14 in Supporting information, those data of dynamic light scattering measurement confirmed that OX-UCNPs and OX-UCNPs-CoOOH nanoparticles can retain their morphology in water for two days without any aggregation or degradation.
Due to the importance of AA in human system, food's AA concentration determination helps human building healthy diet. In this regard, fruit juice and pepper extract were chosen as two typically AA nutriments. Since the two samples are rich in various fluorescent substances, such as fluorescein and pigment, external excitation intensively interferes bio-related auto-fluorescence. Hence, the effect of different light sources for excitation on the background fluorescence of both samples was measured via fluorescence spectrometer (Fig. 3). Compared with UV–vis light, the 980 nm laser was used since its unique properties with less background noise, light scattering, and sample damage. By applying the CoUCNPs nanoprobe into the two existing samples, the concentration of AA was assessed in comparison to concentrated standard AA solution for verifying the feasibility. The actual concentration of AA was calculated in theory according to the purchased AA instruction. Table S1 in Supporting information summarizes the AA concentrations which demonstrate outstanding agreement with the predicted values and standard addition recovery rate of 106.5% ± 16%. Afterwards, 10 μL of the both samples were added into the 1 mL nanoprobe solution respectively, and the fluorescence intensity at 541 nm was recorded before and after the addition of samples with the excitation of a CW 980 nm laser. At last, Table 1 showed analytical results that 100 mL fruit juice contained 59.2 mg AA and 100 mL pepper extract contained 135.5 mg AA. It is found that the proposed sensor can accurately determine the AA concentration in foods. Such sensing strategy provides a promising detection route for other reducing reactive species, such as glutathione and H2S, based on their specific oxidation-reduction reaction with the nanoprobe. It is believed that this sensing platform will find great application in practical biological analysis.

|
Download:
|
| Fig. 3. (A) The background of orange juice at the excitation of different light sources. (B) The background of capsicum extracts at the excitation of different light sources. | |
|
|
Table 1 The AA content of orange juice and capsicum extracts (mg/100 mL). |
In conclusion, a turn-on fluorescent nanoprobe based on CoUCNPs by applying the specific redox reaction between AA and CoOOH was created, which takes full advantage of the excellent optical property of 980 nm laser for rapid screening of AA in biological sample analysis. After being hybridized with CoOOH nanoflakes to form CoUCNPs, the luminescence of UCNPs can be efficiently quenched by CoOOH via FRET. Due to the instantaneous reaction between the enediol group of AA and hexagonal CoOOH nanoflakes, the nanoflakes can be reduced into Co2+ by AA and result a fluorescence recovery. The method demonstrated here possesses an improved sensitivity toward AA detection and effectively reduces background noise and scattering of light from biological samples produced by various fluorescent substances when in situ excitation. We anticipate that the current strategy can provide an opportunity for a better understanding of the AA-involved biologically antioxidants investigation.
AcknowledgmentsThis work was supported by 973 Program (No. 2013CB933800) and National Natural Science Foundation of China (Nos. 21390411, 21535004, 21422505, 21375081), and Natural Science Foundation for Distinguished Young Scholars of Shandong Province (No. JQ201503).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.024.
| [1] |
E.R. Stadtman, B.S. Berlett, Chem. Res. Toxicol. 10(1997) 485-494. DOI:10.1021/tx960133r |
| [2] |
Q. Chen, M.G. Espey, A.Y. Sun, et al., Proc. Natl. Acad. Sci. 105(2008) 11105-11109. DOI:10.1073/pnas.0804226105 |
| [3] |
G. Gegelashvili, A. Schousboe, Brain Res. Bull. 45(1998) 233-238. DOI:10.1016/S0361-9230(97)00417-6 |
| [4] |
M.E. Rice, Trends Neurosci. 23(2000) 209-216. DOI:10.1016/S0166-2236(99)01543-X |
| [5] |
A. Bossi, S.A. Piletsky, E.V. Piletska, P.G. Righetti, A.P.F. Turner, Anal. Chem. 72(2000) 4296-4300. DOI:10.1021/ac000185s |
| [6] |
J. Fu, X.H. Tan, Y.H. Li, X.J. Song, Chin. Chem. Lett. 27(2016) 1541-1546. DOI:10.1016/j.cclet.2016.07.007 |
| [7] |
X.M. Fu, Z.J. Liu, S.X. Cai, et al., Chin. Chem. Lett. 27(2016) 920-926. DOI:10.1016/j.cclet.2016.04.014 |
| [8] |
N. Li, C.Y. Chang, W. Pan, B. Tang, Angew. Chem. Int. Ed. 51(2012) 7426-7430. DOI:10.1002/anie.201203767 |
| [9] |
L.M. Yang, N. Li, W. Pan, Z.Z. Yu, B. Tang, Anal. Chem. 87(2015) 3678-3684. DOI:10.1021/ac503975x |
| [10] |
M.M. Luan, N. Li, W. Pan, et al., Chem. Commun. 53(2017) 356-359. DOI:10.1039/C6CC07605J |
| [11] |
W. Pan, T.T. Zhang, H.J. Yang, et al., Anal. Chem. 85(2013) 10581-10588. DOI:10.1021/ac402700s |
| [12] |
N. Li, W. Diao, Y.Y. Han, et al., Chem. Eur. J. 20(2014) 16488-16491. DOI:10.1002/chem.201404625 |
| [13] |
Y.L. Xu, X.Y. Niu, H.L. Chen, S.G. Zhao, X.G. Chen, Chin. Chem. Lett. 28(2017) 338-344. DOI:10.1016/j.cclet.2016.10.003 |
| [14] |
G.F. Wang, A.X. Guan, C.Y. Zhou, et al., Chin. Chem. Lett. 27(2016) 1788-1792. DOI:10.1016/j.cclet.2016.07.012 |
| [15] |
Y. Li, Y.M. Miao, M.Q. Yang, Y.X. Wu, G.Q. Yan, Chin. Chem. Lett. 27(2016) 773-778. DOI:10.1016/j.cclet.2016.01.006 |
| [16] |
L.L. Xi, H.B. Ma, G.H. Tao, Chin. Chem. Lett. 27(2016) 1531-1536. DOI:10.1016/j.cclet.2016.03.002 |
| [17] |
R. Yu, C.F. Jiang, W. Chu, M.F. Ran, W.J. Sun, Chin. Chem. Lett. 28(2017) 302-306. DOI:10.1016/j.cclet.2016.07.014 |
| [18] |
Y.J. Chen, X.P. Yan, Small 5(2009) 2012-2018. DOI:10.1002/smll.v5:17 |
| [19] |
L.B. Li, C. Wang, K.Y. Liu, et al., Anal. Chem. 87(2015) 3404-3411. DOI:10.1021/ac5046609 |
| [20] |
H.M. Meng, X.B. Zhang, C. Yang, et al., Anal. Chem. 88(2016) 6057-6063. DOI:10.1021/acs.analchem.6b01352 |
| [21] |
N. Li, Y.H. Li, Y.Y. Han, et al., Anal. Chem. 86(2014) 3924-3930. DOI:10.1021/ac5000587 |
| [22] |
Z.Z. Yu, W. Pan, N. Li, B. Tang, Chem. Sci. 7(2016) 4237-4244. DOI:10.1039/C6SC00737F |
| [23] |
Z.Z. Yu, Q.Q. Sun, W. Pan, N. Li, B. Tang, ACS Nano 9(2015) 11064-11704. DOI:10.1021/acsnano.5b04501 |
| [24] |
J.L. Liu, Y. Liu, Q. Liu, et al., J. Am. Chem. Soc. 133(2011) 15276-15279. DOI:10.1021/ja205907y |
 2017, Vol. 28
2017, Vol. 28 



