Optical imaging, as an important molecular imaging modality, has emerged many attractions in studying the biological or molecular events both in cell level and living subject because of its high resolution and sensitivity, noninvasive manner and low cost [1-4]. Among the optical imaging materials, conjugated polyelectrolytes (CPs), with the properties of strong light-harvesting ability, high quantum yield and excellent photostability [5], were endowed bright potentials and have been widely used in biochemical and biological imaging and tracing research [6-14]. Our group has reported the cationic polythiphene for cisplatin tracing and apoptosis imaging [6, 7]. CPs also was studied for imaging fibronectin fibrils in live fibroblast cells [8]. Besides the direct utility, Liu et al. fabricated oligofluorene nanoparticles to enhance the cell nucleus imaging [9]. Modification the side chains of CPs with targeted small molecular, selective cell or cell membrane imaging was achieved [10, 11]. Antibody, affibody and peptide were also introduced to the surface of CP nanoparticles for simultaneous discrimination of different cancer cells [12-14].
Each deeper cognizance of CPs had offered researchers new approaches for biological study and enormously expanded the application range. Along with the understanding of the endocytosis mechanism of CPs, extensive researches have focused on the designation of multifunctional CPs, such as selective organelle imaging and drug delivery [15], and cell imaging and nucleic transfection [16-18]. Further investigation of CPs revealed the ability of CPs to sensitize the oxygen to singlet oxygen under irradiation, and then CPs were widely reported as multifunctional materials for simultaneous imaging and therapy in the antibacterial and anticancer studies [19-23].
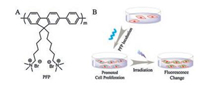
Thus herein we bring novel insights into a water-soluble conjugated polyelectlyte by deeply studying its properties in cells. Poly(9, 9-bis(6'-N, N, N-trimethylammonium hexyl)fluorene phenylene) (PFP) (molecule structure is showed in Scheme 1A), a good biosensing material [24, 25], was studied in this paper. The biocompatibility of PFP was investigated in different cells, and cell cycle analysis was carried out to explore the reasons of different biocompatibility of PFP to cells. After irradiation, fluorescence enhancement of blue emission and turn-on of long-wavelength emission of PFP in HepG2 cells was observed, which was first reported as far as we know (Scheme 1B). The differentiated biocompatibility of PFP and its particular imaging properties in cancer cells can help to guide the application of conjugated polymers in cells and provide a new dimension in designing sensitive and responsive imaging materials.
|
Download:
|
| Scheme 1. The structure of conjugated polyelectrolyte PFP and schematic of the enhanced cell proliferation and particular imaging property of PFP in cells. | |
The derivate of PFP has been reported to be toxic to certain cells [7]. However, the universality and mechanism of the toxicity of PFP to cells were not clearly studied. The biocompatibility of PFP was first evaluated in four cancer cell lines from different organs: human hepatoblastoma G2 cell (HepG2), pulmonary adenocarcinoma cell (A549), renal cell carcinoma (A498) and cervical cancer cell (HeLa). The cells wereincubatedwith PFP in serum-free DMEM for 48h, and MTT method was used to evaluate the cell viability of four cell lines. Surprisingly, differentiated biocompatibility of PFP to the four cell line was observed. PFP exhibited obvious cell viability promotion to HepG2 cells, while good biocompatibility to A549 cell line and significant cytotoxicity to HeLa and A498 cells (Fig. 1A). The unique growth effect of PFP to HepG2 cells was further confirmed by cell counting method (Fig. 1B) and cell imaging (Fig. 1C). This phenomenon indicated the complex influence of cationic conjugated polyelectrolyte to cancer cells [26].

|
Download:
|
| Fig. 1. Differentiated biocompatibility for conjugate polymer PFP to four cell lines. (A) Cell viability as a function of concentrations of PFP in repeat units (RU). HepG2, A549, Hela and A498 cells were treated with PFP in the serum-free culture medium for 48h. (B) Cell counts of HepG2 cells and A549 cells as a function of concentrations of PFP after | |
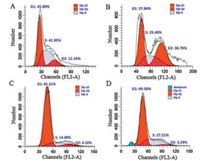
To investigate the mechanism of differentiated biocompatibility of PFP, the growth promoted HepG2 cell and growth inhibited A498 cell were selected for further cell cycle analysis using flow cytometry. Through analyzing the contents of propidium iodide (PI)-stained DNA, the percentages of cells in different period were determined and calculated. Percentage changes of first gap (G1), synthesis (S) and second gap (G2) phase would deeply reflect the influence of PFP on the physiological activities of the cells. When cultured in serum-free medium without PFP, HepG2 cells was arrested in S phase due tothe nutritional deficiencies (Figs. 2 A and S1 in Supporting information). Nevertheless, the addition of PFP in culture medium drove HepG2 cells from S phase to G2 phase, with a decrease of S phase from 41.95% to 25.40% and an increase of G2 phase from 12.16% to 36.76%, which signifies stronger cell division (Fig. 2B). Thus, the growth promotion of PFP to HepG2 cell was achieved by stimulating the cells to overcome the barriers and enter G2 phase and division phase from S phase. In contrast, the incubation of A498 cells with PFP resulted in no evident change on the percentage of G2 phase but apparent percentage increase of cells in S phase from 14.68% to 27.21%, which means the PFP treatment aggravated the S phase cell cycle arrestof A498 cells and subsequently caused the cell apoptosis (Fig. 2C and D). These data were consistent with cell viability of MTT assay and gave deeper insights for differentiated biocompatibility of PFP, which will tutor the design of conjugated polyelectrolytes and their application in complex biosystem.
|
Download:
|
| Fig. 2. Cell cycle analysis of HepG2 cells and A498 cells. PFP incubation increased the percentage of HepG2 cells in G2 phase (A, B) while induced S phase arrest in A498 cells (C, D). (A) HepG2 cells cultured in serum-free DMEM as control; (B) HepG2 cells cultured with PFP in serum-free DMEM for 24h; (C) A498 cells cultured in serum-free DMEM as control; (D) A498 cells cultured with PFP in serum-free DMEM for 24h. | |
Although PFP is a kind of excellent fluorescent agent with good photophysical property, it has not been applied directly for cell imaging. Since it is biocompatible to HepG2 cells, the cell imaging of PFP in HepG2 cells was studied (Fig. 3). It was clear that PFP can be uptaken and then located in the cell plasma after incubation.

|
Download:
|
| Fig. 3. Fluorescence imaging of HepG2 cells with PFP. The type of light filter was D380/30 nm exciter, 420 nm beam splitter and D460/50 nm emitter. Scale bar, 100 μm. | |
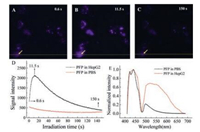
Dramatically, we found that the fluorescence of PFP in HepG2 cells could be enhanced within several seconds upon exposure to light. HepG2 cells were treated in medium containing 10 μmol/L PFP for 24h following in PFP-free medium for another 24h. When irradiated at the wavelength range of 380/30nm, the fluorescence imagesof HepG2 cells at a series of irradiationtimes were recorded by collecting the blue emission of PFP from 435nm to 485nm. Fluorescence intensity of PFP underwent significant change from low to high and then of a decrease (Fig. 4A–C), and the change was clarified with the signal intensitydata of theyellowline (Fig. S2A in Supporting information). The intensity at the point (in the yellow line pointed with yellow arrows) as a function of irradiation time further confirmed the changing process (Fig. 4D). It is easy to understand the laterchangesof fluorescence intensity from high to low, because the irradiation with so strong light of short wavelength would definitely cause the photo bleaching of PFP. However, compared to the intensity at 0.6 s, it is quite interesting that the fluorescence intensity was enhanced about 3 times after irradiation for another ~11 s. Comparing, the control group (PFP in PBS solution) showed no fluorescence enhancement with the same irradiation treatment (Fig. 4D). This phenomenon of irradiation induced fluorescence enhancement must be referred to the interaction of PFP with certain cell components.
|
Download:
|
| Fig. 4. Fluorescence intensity and spectrum of PFP in cancer cells was changed after light irradiation. (A–C) Fluorescence images of HepG2 cells after irradiation for different time. (D)The fluorescence intensity of the point (pointed with yellow arrows on the yellow line of A–C) changed with the increasing of irradiation time. The type of light filter was D380/30 nm exciter, 420 nm beam splitter and D460/ 50 nm emitter. (E) Fluorescence spectrum of PFP uptaken by HepG2 cells (red line) after 10 s irradiation. PFP solution in PBS after the same irradiation was also scanned as control. These data were measured on CLSM with an excitation wavelength at 405 nm. | |
PFP had been reported to be used as a fluorescent membrane marker and the PFP can interact well with model membranes [27]. Thus, we speculated that the membrane component of cells may play a crucial role for the irradiation-induced fluorescence enhancement. Liposome (Lipofectamine 2000) was used to imitate the cell membrane to investigate the reason of fluorescence enhancement. The cell membrane mimics were treated with PFP for 1 h, and the fluorescence intensity change of PFP was quite similar with that in HepG2 cells (Fig. S2B). This result demonstrated the interaction of cationic amphiphilic conjugated polymer PFP with negative charged amphiphilic cell membrane components. Thus the mechanism of fluorescence enhancement of PFP may relate to the change of structure or conformation of PFP as well as the interaction with lipid bilayer after irradiation. This phenomenon about enhanced fluorescence of PFP in cells is a newfound amazing property that could increase the sensitivity for sensing and imaging.
Usually, the structure or conformation change of fluorescent molecules always arise the variation of their fluorescent spectra. To further verify the effect of light irradiation to PFP in HepG2 cells, the fluorescence spectrum of conjugated polymer PFP after 10 s' irradiation was scanned by laser confocal fluorescence microscope (CLSM). The fluorescence spectrum of PFP in cells showed obvious appearance of new broad long-wavelength emission band from 480 nm to 700 nm (Fig. 4E) and was very different from that without irradiation. It is reported that the interaction of PFP with lipid and its analogues would not greatly change the fluorescence spectrum of PFP [27], thus the new generated green emission should be contributed to the light irradiation. Meanwhile, the changed spectrum was quite familiar with the fluorenonecontaining polyfluorenes [28], and this result agreed with the effect of photo-oxidation of the polyfluorene and poly(p-phenylenevinylene) films [29]. That is, it was quite possible that PFP experienced an oxidization process via light irradiation. In this course, the fluorenone defects were introduced, and molecular structure of PFP was thus changed along with consequence that low-energy emission was turned on. In contrast, when PFP was in PBS solution, only weak long-wavelength emission was observed after irradiation, which indicated the significant role of lipid membrane. The structure change of PFP in HepG2 cells must alter the interaction of PFP with membrane components in cells, which should attribute to the stronger emission in short wavelength of PFP. The great difference of long wavelength emission between before and after irradiation implied that light irradiation can be regard as a key factor to turn on the long-wavelength emission of PFP in cells.
In summary, the biocompatibility of PFP to cells and its fluorescence properties were studied. Differentiated biocompatibility was observed, and the influence of PFP on the cell cycle was verified as the essential mechanism of growth promotion to HepG2 cells. This result will guide the designation of conjugated polyelectrolytes and the application in complex biosystem. Further investigation on the imaging properties of PFP in cells was carried out. After irradiation, fluorescence intensity in short-wavelength of endocytosed PFP was enhanced, which can increase the sensitivity of PFP in biosensing or bioimaging; long-wavelength emission was notably generated, which demonstrates the quick turn-on property of PFP in cells. The present discoveries made conjugated polymers great potential in light responsive bioresearches and provide people new insights for the bio-application of conjugated polymers.
AcknowledgmentsThe authors are grateful to the National Natural Science Foundation of China (Nos. 21473220 and 51573002), the Major Research Plan of China (No. 2013CB932800) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB12030300).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.002.
| [1] |
H. Liu, M. Zhao, Q. Qiao, et al., Chin. Chem. Lett. 25(2014) 1060-1064. DOI:10.1016/j.cclet.2014.05.010 |
| [2] |
I.L. Medintz, H.T. Uyeda, E.R. Goldman, H. Mattoussi, Nat. Mater. 4(2005) 435-446. DOI:10.1038/nmat1390 |
| [3] |
C.H. Qin, S.P. Zhu, J. Tian, Curr. Pharm. Biotechnol. 11(2010) 620-627. DOI:10.2174/138920110792246519 |
| [4] |
J. Tan, W. Chen, J. Guo, Chin. Chem. Lett. 27(2016) 1405-1411. DOI:10.1016/j.cclet.2016.06.050 |
| [5] |
T.M. Swager, Acc. Chem. Res. 31(1998) 201-207. DOI:10.1021/ar9600502 |
| [6] |
H.W. Tang, C.F. Xing, L.B. Liu, et al., Small 7(2011) 1464-1470. DOI:10.1002/smll.201002189 |
| [7] |
L.B. Liu, M.H. Yu, X.R. Duan, S. Wang, J. Mater. Chem. 20(2010) 6942-6947. DOI:10.1039/c0jm01078b |
| [8] |
R.L. Mcrae, R.L. Phillips, I.B. Kim, U.H.F. Bunz, C.J. Fahrni, J. Am. Chem. Soc. 130(2008) 7851-7853. DOI:10.1021/ja8007402 |
| [9] |
K.Y. Pu, K. Li, B. Liu, Adv. Mater. 22(2010) 643-646. DOI:10.1002/adma.v22:5 |
| [10] |
R. Hu, S. Li, H. Bai, et al., Chin. Chem. Lett. 27(2016) 545-549. DOI:10.1016/j.cclet.2016.02.001 |
| [11] |
B. Wang, C.L. Zhu, L.B. Liu, et al., Poly. Chem. 4(2013) 5212-5215. DOI:10.1039/c3py00097d |
| [12] |
K. Li, R.Y. Zhan, S.S. Feng, B. Liu, Anal. Chem. 83(2011) 2125-2132. DOI:10.1021/ac102949u |
| [13] |
L.H. Feng, C.L. Zhu, H.X. Yuan, et al., Chem. Soc. Rev. 42(2013) 6620-6633. DOI:10.1039/c3cs60036j |
| [14] |
L.H. Feng, L.B. Liu, F.T. Lv, G.C. Bazan, S. Wang, Adv. Mater. 26(2014) 3926-3930. DOI:10.1002/adma.201305206 |
| [15] |
G.M. Yang, L.B. Liu, Q. Yang, F.T. Lv, S. Wang, Adv. Funct. Mater. 22(2012) 736-743. DOI:10.1002/adfm.201101764 |
| [16] |
P. Maturavongsadit, X. Bi, T.A. Gado, et al., Chin. Chem. Lett. 27(2016) 1473-1478. DOI:10.1016/j.cclet.2016.03.012 |
| [17] |
X.L. Feng, F.T. Lv, L.B. Liu, et al., Adv. Mater. 24(2012) 5428-5432. DOI:10.1002/adma.v24.40 |
| [18] |
R.C. Jiang, X.M. Lu, M.H. Yang, et al., Biomacromolecules 14(2013) 3643-3652. DOI:10.1021/bm401000x |
| [19] |
S. Chemburu, T.S. Corbitt, L.K. Ista, et al., Langmuir 24(2008) 11053-11062. DOI:10.1021/la8016547 |
| [20] |
A. Parthasarathy, S. Goswami, T.S. Corbitt, et al., ACS Appl. Mater. Interfaces 5(2013) 4516-4520. DOI:10.1021/am400282p |
| [21] |
C.F. Xing, Q.L. Xu, H.W. Tang, L.B. Liu, S. Wang, J. Am. Chem. Soc. 131(2009) 13117-13124. DOI:10.1021/ja904492x |
| [22] |
C.L. Zhu, Q. Yang, L.B. Liu, et al., Adv. Mater. 23(2011) 4805-4810. DOI:10.1002/adma.201102850 |
| [23] |
B. Wang, H.X. Yuan, C.L. Zhu, et al., Sci. Rep. 2(2012) 766. DOI:10.1038/srep00766 |
| [24] |
X.L. Feng, L.B. Liu, S. Wang, D.B. Zhu, Chem. Soc. Rev. 39(2010) 2411-2419. DOI:10.1039/b909065g |
| [25] |
X.R. Duan, L.B. Liu, F.D. Feng, S. Wang, Acc. Chem. Res. 43(2010) 260-270. DOI:10.1021/ar9001813 |
| [26] |
G.M. Yang, L.B. Liu, F.T. Lv, S. Wang, Sci. Rep. 3(2013) 1702. DOI:10.1038/srep01702 |
| [27] |
Z. Kahveci, Martinez-Tome M.J., R. Mallavia, C.R. Mateo, Biomacromolecules 14(2013) 1990-1998. DOI:10.1021/bm400348n |
| [28] |
A.P. Kulkarni, X.X. Kong, S.A. Jenekhe, J. Phys. Chem. B 108(2004) 8689-8701. DOI:10.1021/jp037131h |
| [29] |
X. Gong, P.K. Iyer, D. Moses, et al., Adv. Funct. Mater. 13(2003) 325-330. DOI:10.1002/adfm.200304279 |
 2017, Vol. 28
2017, Vol. 28 


