b Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China
Intracellular pH (pHi), as a significant physiological parameter, plays a critical role in living cells [1-3], which can be influenced by variety of physiological and pathological processes. Quantitatively measuring pH is vital for cellular analysis or diagnosis. For instance, the pH of 4.5 to 5.5 within lysosomes provides the basic requirement for the action of lysosomal hydrolases to degrade proteins, DNA, RNA, polysaccharides, and lipids [4]. Disruptive variation in the lysosomal pH causes defects in lysosomal function and consequently leads to many lysosomal storage diseases [5]. Therefore, it is important to track lysosomal pH in living cells to understand its physiological and pathological processes.
Fluorescence imaging has become an essential tool in the study of biological molecules, pathways, and processes in living cells because of its high spatial and temporal resolution [6-11]. Particularly, because of its ability of providing quantitative measurements, the ratiometric fluorescence technique is regarded as an ideal method for accessing intracellular pH [12]. Although considerable efforts have been devoted to the development of pHresponsive ratiometric sensors based on organic molecules [13-17] and nanophosphors [18-24], only a limited number of them have been applied to subcellular labeling and pH sensing. Thus, it is still challenging to fabricate ratiometric fluorescent sensors for subcellular pH quantification.
Our previous studies demonstrated that 1, 8-naphthalimide derivatives are useful fluorophores to design colorimetric and fluorescent probes because the photophysical properties are easily influenced by reagents [25-27]. Based on photo-induced electron transfer (PET) mechanism, we recently reported a class of 4-substituted 1, 8-naphthalimide-based fluorescent off-on probes [28, 29]. These observations encouraged us to make use of such PET process to design pH-responsive 1, 8-naphthalimide-based fluorescent sensors. However, the off-on mode is not ideal for the cellular application.
On the other hand, due to their small size, tunable surface functionalities and good photostability, carbon nanodots (CDs) have many excellent properties such as good water solubility, biocompatibility, excellent cell membrane permeability and low cost [30-37]. Such features make CDs especially useful for fluorescent biosensing or imaging [38-44]. However, exploration of CDs as fluorescent sensors to monitor and imagine an analyte in living cells or biological process still remains at an early stage and very few reports about CDs fluorescent sensors toward pH has been reported.
In this paper, based on 1, 8-naphthalimide derivative (1), CDs, and lysosome-targeted unit (morpholine, Lyso), two ratiometric fluorescent nanosensors toward pH were fabricated. In these sensors, the CDs not only serve as fluorescence out mode, but also as the anchoring site for the sensor, a 1, 8-naphthalimide derivative and the lysosome-targeted unit. The schematic illustration for the sensing of pH by the nanosensors is shown in Scheme 1. The sensors based on this CDs-organic molecule architecture demonstrate several advantages: first, they are ratiometric fluorescent sensors toward pH which can ensure more accurate detection especially in biosystems; second, the nanosensors exhibit excellent anti-disturbance ability, reversible pH sensing ability, and a linear response range in a wide pH range; lastly, one of the two sensors is able to target lysosome and it is also capable of permeating the cell membrane and realizing pH imaging in living cells.

|
Download:
|
| Scheme 1. Schematic illustration for the sensing of pH by the hybrid nanosensors. | |
For the fabrication of the sensing system, first we synthesized amino and carboxyl coated CDs by a simple hydrothermal method using citric acid and ethylenediamine as the precursors. Fig. 1 shows the TEM images of CDs, revealing that the nanoparticles have a diameter ranging from 3 nm to 4 nm. FT-IR spectrum (Fig. S1 in Supporting information) reveals that the CDs mainly contain carbon, nitrogen, oxygen, and the surfaces of the CDs are functionalized with amino, hydroxyl, and carboxylic/carbonyl moieties. Fig. S2 in Supporting information shows the TEM images of 1-CDs (3.0 ×10-5 mol/L 1, 8-naphthalimide derivative 1 and 0.02 mg/mL CDs) and 1-CDs-Lyso (3.0 × 10-5 mol/L of 1, 8-naphthalimide derivative 1, 0.02 mg/mL of CDs, and 3.0 ×10-5 mol/L of lysosome-targeted unit morpholine), revealing that there is agglomeration behavior in the hybrid nanomaterials. The zeta potential of CDs is -10.4 mV, which is ascribed to the surface negative electricity of the CDs. Further experiments demonstrate that the zeta potentials of 1-CDs and 1-CDs-lyso are -7.16 mV and 3.59 mV, respectively. The zeta potential difference of the three kinds of nanomaterials may be ascribed to the interaction between the CDs and organic molecules.

|
Download:
|
| Fig. 1. TEM images of as-prepared CDs. The scale bar is 50 nm (a), 10 nm (b), 5 nm (c), and is 2 nm (d), respectively. | |
1, 8-Naphthalimide derivative (sensor 1) was selected as the sensing unit, which was synthesized in a high yield starting from 4-bromo-1, 8-naphthalic anhydride through four steps (Scheme 2). The reaction of 4-bromo-1, 8-naphthalic anhydride and monoethanolamine gives the product 4 in a 69.2% yield. Compound 3 was obtained with a yield of 73.9% by the reaction of compound 4 and piperazine. The reaction of 3 and propargyl bromide gives the product 2 in a yield of 76.5%. Sensor 1 was obtained by the reaction of compound 2 and succinic anhydride in a yield of 68.9%, which was characterized by 1H NMR, 13C NMR and HRMS (Figs. S3–S5 in Supporting information). Experiment details please see Supporting information.

|
Download:
|
| Scheme 2. Synthesis of sensor 1. | |
As shown in Fig. 2a, with pH increasing of the medium from 4.0 to 8.0 and upon excitation at 380 nm, the emission peak at 527 nm decreased gradually, which was due to the molecular change from 1H to 1 (Scheme 1). In the same condition, no obvious absorption spectral change was found (Fig. 2b), indicating that pH change cannot result in the excitation state change. However, the PL spectrum of the CDs without (Fig. 2c) or with lysosome-targeted unit (Fig. 2d) hardly changes with pH changing. Besides, there is a good overlap between the absorption spectrum of sensor 1 and the PL spectrum of CDs (Fig. 2b). Considering the results of Fig. 2, it is easy to conclude that the PL spectrum of CDs can be used as a reference if we use the hybrid material of compound 1, and CDs as a pH-sensor (1-CDs). Therefore, it is possible to select a 380 nm wavelength laser to excite CDs and compound 1 in the hybrid sensor. Under the excitation at 380 nm and in acidic condition, the emission of CDs was partially absorbed by compound 1 and the nanohybrid system exhibits a very strong emission peak at 527 nm and a very weak emission peak at 450 nm. However, under the same excitation and in neutral condition, no emission at 527 nm was found yet the emission at 450 nm still existed. If a lysosometargeted unit such as morpholine was introduced to the above system, a lysosome-targeted pH hybrid sensor (1-CDs-Lyso) could be fabricated.

|
Download:
|
| Fig. 2. (a) Fluorescence spectra of sensor 1 (3.0 × 10-5 mol/L) in DMSO-HEPES buffer solutions (1:100, v/v) of different pHapp values determined upon excitation at 380 nm. (b) Absorption spectra of sensor 1 (3.0 × 10-5 mol/L) in 0.01 mol/L different pH HEPES solution (red, green, and blue lines) and luminescence spectra of CDs (0.02 mg/mL) with | |
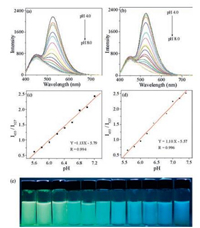
With pHapp increasing from 4.0 to 8.0 and upon excitation at 380 nm, the emission at 527 nm of 1-CDs (Fig. 3a) and 1-CDs-lyso (Fig. 3b) decreased and that at 455 nm hardly changed in intensity with increasing pHapp. The emission intensity increase at 527 nm in acidic solution can be ascribed to the structural transformation from compound 1 to 1H (Scheme 1) and the inhibition of PET process [45]. The apparent pKa' was determined to be 6.47 ± 0.22 and 6.28 ± 0.17 for 1-CDs and 1-CDs-lyso respectively via fitting the pH titration profile based on the normalized I455/I527 (Figs. S6 and S7 in Supporting information) [46]. The linear ranges for the ratiometric response from emission spectra of 1-CDs and 1-CDslyso are pHapp of 5.6 to 7.2 (Fig. 2c) and 5.6 to 7.4 (Fig. 2d), respectively. Fig. 3e demonstrates the fluorescence color change of 1-CDs-lyso in different pH solutions. In addition, the enhanced I455/I527 value of 1-CDs and 1-CDs-lyso at pHapp 7.8 and 7.4 can be recovered to the solution original I455/I527 value at pHapp 5.6 and 5.0 respectively, and this reversible ratiometric pH sensing ability can be retained for at least 10 cycles (Fig. 4).
|
Download:
|
| Fig. 3. Fluorescence spectra of sensor 1-CDs (a) and 1-CDs-Lyso (b) in DMSO-HEPES buffer solutions (1:100, v/v) of different pHapp values determined upon excitation at 380 nm. The linear relationship for the ratiometric response from emission spectra of 1-CDs (c) and 1-CDs-Lyso (d) to different pHapp. (e) Fluorescent photographs of sensor 1-CDs-Lyso in DMSO-HEPES buffer solutions (1:100, v/v) of different pHapp values determined upon excitation at 365 nm from a portable lamp. The pH values from left to right are 4.0, 4.4, 4.8, 5.2, 5.6, 6.0, 6.4, 6.8, 7.2, 7.6, and 8.0. | |

|
Download:
|
| Fig. 4. Emission intensity ratio I455/I527 of 1-CDs (a) and 1-CDs-Lyso (b) in the same medium determined over consecutive pHapp cycles. | |
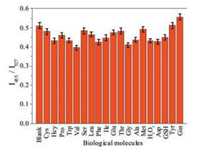
To further assess the utility of 1-CDs and 1-CDs-Lyso as pH ratiometric hybrid nanosensors, their emission spectral response to pH 7.4 in the presence of other species including biological molecules, metal ions, and anions (Fig. 5, and Figs. S8–S12 in Supporting information) was also tested. The results demonstrated that all of the selected species have no interference in sensing of pHapp. This result strongly indicates that 1-CDs and 1-CDs-Lyso could be excellent ratiometric fluorescent sensors toward pH with strong anti-interference ability.
|
Download:
|
| Fig. 5. Emission spectral response of 1-CDs-Lyso in DMSO-HEPES buffer solution (1:100, v/v, pHapp = 5) upon addition of different species (3 equiv. of species relative to 1), the excitation wavelength was 380 nm. I455 and I527 represent the emission intensity at 455 and 527 nm. The species used were blank, Cys, Hcy, Pro, Trp, Val, Ser, Leu, Phe, lle, Glu, Thr, Gly, Ala, Met, H2O2, Asp, GSH, Tyr, and Gin. | |
An ideal sensor ought to have good thermal stability and photostability. As shown in Fig. 6a, with irradiation (λex= 380 nm) time extending, the emission at 527 nm of compound 1 only increased, indicating that compound 1 do not have good photostability. With temperature increasing from 30 ℃ to 48 ℃, the emission of compound 1 hardly changes (Fig. 6b), indicating good thermal stability. The photostability and thermal experiments of the nanohybrid sensors 1-CDs (Fig. 6c and d) and 1-CDs-lyso (Fig. 6e and f) were also operated, an excellent result was obtained under the same condition as that of compound 1, the emission intensity ratio I455/I527 does not change with time extending or temperature changing. The results demonstrate that the nanohybrid materials as pH sensors have good thermal stability and photostability.

|
Download:
|
| Fig. 6. Emission of compound 1 in DMSO-HEPES buffer solution (1:100, v/v, pHapp 7.4, 3.0 ×10-5 mol/L) with irradiation (lex = 380 nm) time extending (a) and temperature changing (b). Emission intensity ratio I455/I527 of 1-CDs in DMSO-HEPES buffer solution (1:100, v/v, pHapp 7.4) with irradiation time extending (λex = 380 nm) (c) and temperature changing (d). Emission intensity ratio I455/I527 of 1-CDs-Lyso in DMSO-HEPES buffer solution (1:100, v/v, pHapp 5) with irradiation (λex = 380 nm) time extending (e) and temperature changing (f). | |
The cytotoxicity of sensors 1-CDs and 1-CDs-Lyso toward Hela and hepatocyte cells were measured using MTT assay to evaluate the potential application of these two sensors in living cell imagining. The cellular viability was estimated to be greater than 95% after 1 h at the sensing concentration for the two sensors, suggesting the low cytotoxicity of 1-CDs and 1-CDs-Lyso (Fig. S13 in Supporting information).
The intrinsic ability of 1-CDs-Lyso to target lysosome was investigated in living Hela cells. The cells stained with 1-CDs-Lyso (30 min, 25 ℃) were co-stained further with the commercially available lysosome-Tracker Cys deep red (1 mmol/L, 30 min) in the culture medium. The imaging results show that the green image for 1-CDs-Lyso channel obtained upon excitation at 630 nm is almost identical to the red image for the lysosome-Tracker channel obtained upon excitation at 633 nm (Fig. 7). The overlay between the fluorescence images of 1-CDs-Lyso and lysosome-Tracker discloses a Pearson's correction coefficient of 0.75, suggesting the ability of 1-CDs-Lyso to target lysosome.

|
Download:
|
| Fig. 7. Confocal fluorescence images of Hela cells stained with 1-CDs-Lyso for 15 min (a) and lysosome-Tracker (2.0 mm) for 15 min (b). (c) Merged image of (a) and (b). (d) Bright-field image. (e) Intensity profile of regions of interest (ROIs) across Hela cells. (f) Correlation plot of the intensities of 1-CDs-Lyso and lysosome-Tracker (Rr = 0.75). | |
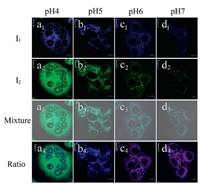
The practical ratiometric pHi imaging ability of 1-CDs-Lyso was further applied in monitoring acidic pHi upon different stimulation (Fig. 8, Fig. S14 in Supporting information). In this study, the intracellular pH calibration was carried out in living HeLa cells using a standard procedure followed by a further incubation with high K+ buffers of different pH, also containing 1 mmol/L nigericin. As shown in Fig. 8, the ratio of Iblue to Igreen gradually increased with pH changing from 4.0 to 7.0. The change in the ratio of Iblue to Igreen shows the ability of sensor 1-CDs-Lyso to measure a pHdependent signal change. The ratio value of Iblue/Igreen is 1.97, 8.19, 34.67 and 51 from pH 4 to pH 7.
|
Download:
|
| Fig. 8. Confocal microscopy images of 1-CDs-Lyso in living HeLa cells clamped at pH 4 (a1–a4), 5 (b1–b4), 6 (c1–c4), and 7 (d1–d4). The excitation wavelength was 405 nm. The images were collected at blue channel with 482/35 filter (first row), green channel with 525/50 filter (second row) and overlay of first row and second row (third row). Ratio images obtained from the two above channels (fourth row). The ratio value of Iblue/Igreen is 1.97, 8.19, 34.67 and 51 from pH 4 to pH 7. | |
Two ratiometric fluorescent CDs-organic molecule hybrid nanosensors with single excitation wavelength was synthesized for sensing acidic pH with strong anti-disturbance ability, good photostability and thermal stability. The linear ratiometric pH response range in a wide range, the reversible pH sensing ability, the lysosome-targeted capacity and the cell membrane permeability make this sensor especially suitable for the practical tracking of acidic pHi in living cells via ratiometric imaging.
AcknowledgmentsWe are grateful for the financial supports from National Natural Science Foundation of China (Nos. 21601158, U1504203 and J1210060), Key Laboratory of Photochemical Conversion and Optoelectronic Materials and Zhengzhou University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.027.
| [1] |
Y.H. Li, Y.J. Wang, S. Yang, et al., Anal. Chem. 87(2015) 2495-2503. DOI:10.1021/ac5045498 |
| [2] |
J.R. Casey, S. Grinstein, J. Orlowski, Nat. Rev. Mol. Cell Biol. 11(2010) 50-61. DOI:10.1038/nrm2820 |
| [3] |
K.J. Zhou, H.M. Liu, S.R. Zhang, et al., J. Am. Chem. Soc. 134(2012) 7803-7811. DOI:10.1021/ja300176w |
| [4] |
J.P. Luzio, P.R. Pryor, N.A. Bright, Nat. Rev. Mol. Cell Biol. 8(2007) 622-632. DOI:10.1038/nrm2217 |
| [5] |
T. Fukuda, L. Ewan, M. Bauer, et al., Ann. Neurol. 59(2006) 700-708. DOI:10.1002/ana.v59:4 |
| [6] |
Fernandez-Suarez M., A.Y. Ting, Nat. Rev. Mol. Cell Biol. 9(2008) 929-943. DOI:10.1038/nrm2531 |
| [7] |
Y.L. Xu, X.Y. Niu, H.L. Chen, S.G. Zhao, X.G. Chen, Chin. Chem. Lett. 28(2017) 338-344. DOI:10.1016/j.cclet.2016.10.003 |
| [8] |
M.J. Liu, Z.Q. Ye, C.L. Xin, J.L. Yuan, Anal. Chim. Acta 761(2013) 149-156. DOI:10.1016/j.aca.2012.11.025 |
| [9] |
Q.J. Luo, Y.X. Li, M.Q. Zhang, P. Qiu, Y.H. Deng, Chin. Chem. Lett. 28(2017) 345-349. DOI:10.1016/j.cclet.2016.10.024 |
| [10] |
L. Shang, S.J. Dong, G.U. Nienhaus, Nano Today 6(2011) 401-418. DOI:10.1016/j.nantod.2011.06.004 |
| [11] |
H.R. Zheng, L.Y. Niu, Y.Z. Chen, et al., Chin. Chem. Lett. 27(2016) 1793-1796. DOI:10.1016/j.cclet.2016.04.023 |
| [12] |
X.H. Li, X.H. Gao, W. Shi, H.M. Ma, Chem. Rev. 114(2014) 590-659. DOI:10.1021/cr300508p |
| [13] |
Y.C. Chen, C.C. Zhu, J.J. Cen, et al., Chem. Sci. 6(2015) 3187-3194. DOI:10.1039/C4SC04021J |
| [14] |
Q.Q. Wan, S.M. Chen, W. Shi, L.H. Li, H.M. Ma, Angew. Chem. Int. Ed. 53(2014) 10916-10920. DOI:10.1002/anie.201405742 |
| [15] |
S.J. Chen, Y.N. Hong, Y. Liu, et al., J. Am. Chem. Soc. 135(2013) 4926-4929. DOI:10.1021/ja400337p |
| [16] |
L. Yuan, W. Lin, Z. Cao, J. Wang, B. Chen, Chem. Eur. J. 18(2012) 1247-1255. DOI:10.1002/chem.201101434 |
| [17] |
T. Myochin, K. Kiyose, K. Hanaoka, et al., J. Am. Chem. Soc. 133(2011) 3401-3409. DOI:10.1021/ja1063058 |
| [18] |
S. Sun, X. Ning, G. Zhang, et al., Angew. Chem. Int. Ed. 55(2016) 2421-2424. DOI:10.1002/anie.201509515 |
| [19] |
J.J. Peng, W. Xu, C.L. Teoh, et al., J. Am. Chem. Soc. 137(2015) 2336-2342. DOI:10.1021/ja5115248 |
| [20] |
J. Hu, G. Liu, C. Wang, et al., Biomacromolecules 15(2014) 4293-4301. DOI:10.1021/bm501296d |
| [21] |
M.H. Lee, J.H. Han, J.H. Lee, et al., Angew. Chem. Int. Ed. 52(2013) 6206-6209. DOI:10.1002/anie.201301894 |
| [22] |
A.M. Dennis, W.J. Rhee, D. Sotto, S.N. Dublin, G. Bao, ACS Nano 6(2012) 2917-2924. DOI:10.1021/nn2038077 |
| [23] |
M.J. Marin, F. Galindo, P. Thomas, D.A. Russell, Angew. Chem. Int. Ed. 51(2012) 9657-9661. DOI:10.1002/anie.v51.38 |
| [24] |
K. Zhou, Y. Wang, X. Huang, et al., Angew. Chem. Int. Ed. 50(2011) 6109-6114. DOI:10.1002/anie.v50.27 |
| [25] |
W. Du, J. Xu, H. Li, et al., RSC Adv. 5(2015) 15077-15083. DOI:10.1039/C5RA00596E |
| [26] |
C. Liu, J. Xu, F. Yang, et al., Sens. Actuators B 212(2015) 364-370. DOI:10.1016/j.snb.2015.02.010 |
| [27] |
W.Y. Zhang, X.J. Liu, H.Y. Zhang, et al., J. Mater. Chem. C 3(2015) 8248-8254. DOI:10.1039/C5TC01363A |
| [28] |
M.M. Yu, W.W. Du, W. Zhou, et al., Dyes Pigments 126(2016) 279-285. DOI:10.1016/j.dyepig.2015.12.001 |
| [29] |
X. Liu, W. Zhang, C. Li, et al., RSC Adv. 5(2015) 4941-4946. DOI:10.1039/C4RA13262A |
| [30] |
S.Y. Lim, W. Shen, Z.Q. Gao, Chem. Soc. Rev. 44(2015) 362-381. DOI:10.1039/C4CS00269E |
| [31] |
O.S. Wolfbeis, Chem. Soc. Rev. 44(2015) 4743-4768. DOI:10.1039/C4CS00392F |
| [32] |
C.Q. Ding, A.W. Zhu, Y. Tian, Acc. Chem. Res. 47(2014) 20-30. DOI:10.1021/ar400023s |
| [33] |
C. Wu, C. Wang, T. Han, et al., Adv. Healthcare Mater. 2(2013) 1613-1619. DOI:10.1002/adhm.v2.12 |
| [34] |
R. Ye, C. Xiang, J. Lin, et al., Nat. Commun. 4(2013) 2943. |
| [35] |
S.N. Baker, G.A. Baker, Angew. Chem. Int. Ed. 49(2010) 6726-6744. DOI:10.1002/anie.200906623 |
| [36] |
S.J. Zhu, Q.N. Meng, L. Wang, et al., Angew. Chem. Int. Ed. 52(2013) 3953-3957. DOI:10.1002/anie.v52.14 |
| [37] |
S.N. Qu, X.Y. Wang, Q.P. Lu, X.Y. Liu, L.J. Wang, Angew. Chem. Int. Ed. 51(2012) 12215-12218. DOI:10.1002/anie.v51.49 |
| [38] |
H. Ali, S.K. Bhunia, C. Dalal, N.R. Jana, ACS Appl. Mater. Interfaces 8(2016) 9305-9313. DOI:10.1021/acsami.5b11318 |
| [39] |
P.C. Hsu, H.T. Chang, Chem. Commun. 48(2012) 3984-3986. DOI:10.1039/c2cc30188a |
| [40] |
Q. Qu, A. Zhu, X. Shao, G. Shi, Y. Tian, Chem. Commun. 48(2012) 5473-5475. DOI:10.1039/c2cc31000g |
| [41] |
W. Shi, X. Li, H. Ma, Angew. Chem. Int. Ed. 51(2012) 6432-6435. DOI:10.1002/anie.201202533 |
| [42] |
H. Nie, M. Li, Q. Li, et al., Chem. Mater. 26(2014) 3104-3112. DOI:10.1021/cm5003669 |
| [43] |
J. Shangguan, D. He, X. He, et al., Anal. Chem. 88(2016) 7837-7843. DOI:10.1021/acs.analchem.6b01932 |
| [44] |
Y. Wang, L. Lu, H. Peng, et al., Chem. Commun. 52(2016) 9247-9250. DOI:10.1039/C6CC02874H |
| [45] |
Z.X. Li, L.F. Zhang, X.Y. Li, et al., Dyes Pigments 94(2012) 60-65. DOI:10.1016/j.dyepig.2011.11.007 |
| [46] |
B. Shi, Y. Gao, C. Liu, et al., Dyes Pigments 136(2017) 522-528. DOI:10.1016/j.dyepig.2016.08.058 |
 2017, Vol. 28
2017, Vol. 28 


