b Department of Forensic Medicine, North Sichuan Medical College, Nanchong 637000, China;
c School of Communication and Information Engineering, University of Electronic Science and Technology of China, Chengdu 610054, China
Fingerprint consists protuberant ridges and recessed furrows on fingertips, which is unique for everyone and thus of great importance for individual identification [1, 2]. When fingertips touch the subjects, there will leave some impressions mainly originated from the secretion from sweat gland. There are three kinds of secretion glands through human body: namely eccrine, sebaceous, and apocrine. Eccrine fingerprints are most representative for individual identification in criminal scene and forensic investigations. Besides, the amount of secretions from eccrine is much less than that from sebaceous glands. In common sense, most fingerprints cannot be observed easily in daylight (the socalled latent fingerprints, LFPs). Therefore, there has been great interest in developing enhanced LFP imaging methods [1, 3-11].
Due to the limit of current imaging technologies and instruments, recognition of fingerprints is mostly based on the level 2 structures (such as ridge termination, bifurcation, crossover, and etc.), which is the basis of the universal recognition. To guarantee the accuracy of identification, the needed number of the level 2 characteristics set by different countries varies from 6 to 17 [12]. However, the distribution of the level 2 structures on fingertips is arbitrary, so it needs relative large fingerprint images to achieve accurate fingerprint recognition or identification. In real-world situations, the collected LFPs may be sometimes incomplete, and even don't have enough characteristics or even don't contain the needed features. Therefore, more characteristics other than level 2 of fingerprints are needed for better recognition.
Besides level 2 structures, the level 3 characteristics (sweat pore) on fingertips are also permanent, immutable and unique, which may help the fingerprint analysis. The Kim group brilliantly used fluorescent and hydrochromic water-sensitive polymers for high-quality mapping of sweat pores on LFPs [13-15]. Physiologically, sweat pores are always breathing and not every pore is active in secreting sweat all the time [16]. Therefore, the performance of LFP recognition based on solely sweat pore-mapping may vary time-to-time. It's thus expected the combination of both level 2 and level 3 structures may be advantageous to improve the accuracy of LFP analysis.
In recent years, there has been a unique twist in the implementation of nanotechnology for advanced fingerprint analysis, due to the novel physical and electronic behavior of various nanomaterials [17-23]. Particularly, fluorescent quantum dots (QDs) provide not only improved contrast but also higher degree of functionality for LFP staining [24-29]. We have previously reported that the use of N-acetylcysteine as surface ligands of QDs could accelerate the mapping of eccrine LFPs [30]. Besides level 2 structures, we also observed the sign of some sweat pores [30]. Therefore, in this work, we proposed the use of Nacetylcysteine-capped, red-emitting QDs for high-performance and high-contrast imaging of eccrine LFPs. The entire level 2 and several level 3 characteristics were simultaneously extracted from the obtained fluorescent images. An algorithm was therefore constructed based on the collected characteristics to achieve fingerprint matching analysis.
In this work, red-emitting CdTe QDs were explored as the medium for LFP staining. N-L-Cys-capped CdTe QDs were prepared via the modified "one-pot" method. As shown in Fig. S2 (Supporting information), the UV–vis absorption spectra of the as-prepared CdTe QDs showed a maximum absorption at 612 nm and a strong emission band from 575 nm to 750 nm (centered at about 664 nm, with chromaticity output of x = 0.6887 and y = 0.3112, CIE 1931). The size of the CdTe QDs, as evaluated from the empirical equation proposed by Peng et al. [31], is about 3.77 nm. The fluorescence lifetime of the QDs is about 28 ns (Fig. S3 in Supporting information), which is also in agreement with that reported previously [32]. The solution of N-L-Cys-capped CdTe QDs displays excellent photostability (Fig. S4 in Supporting information) and bright red fluorescence under a UV lamp (inset in Fig. S2), suggesting a high fluorescence quantum yield of QDs (56.6%, Fig. S5 in Supporting information) readily applicable for fingerprint development.
Owing to the excellent optical properties of CdTe QDs, we intended to take advantage of its strong emission for potential LFPs imaging. Similar to our previous work [30], N-L-Cys-capped CdTe QDs could also quickly stain eccrine LFPs in about 5 s (inset in Fig. S2). A typical fluorescent LFP image stained by N-L-Cys-capped CdTe QDs is given in the inset of Fig. S2. The fluorescent spectra of the QDs on this image is in good agreement with that obtained in solution, indicating the obtained fluorescent contrast is truly from QDs staining.
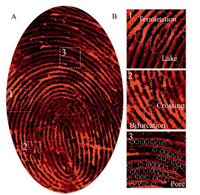
Next, we investigated the performance of N-L-Cys-capped CdTe QDs for imaging of eccrine LFPs. The substructures of LFPs in principle form the basis of fingerprint identification, and their unambiguous imaging is critical for forensic identification of individuals. As shown in Fig. 1A, the eccrine LFP staining with N-L-Cys-capped CdTe QDs displays a well-resolved ridge flow and pattern configuration (level 1). In addition to level 1 details, level 2 (such as ridge termination, bifurcation and crossing) characteristics of the LFP are clearly observed (Fig. 1B). Moreover, a large number of sweat pores (level 3) are also exhibited (Fig. 1B), which is advantageous among the previously reported LFP-staining methods (Table S1 in Supporting information). Particularly, the distribution of the sweat pores is not just a specific area, but in fact all over the obtained images (Figs. 2 A and S6–S9 in Supporting information), demonstrating the high performance of this method in staining both level 2 and level 3 structures of eccrine LFPs.
|
Download:
|
| Fig. 1. (A) Fluorescent image of aluminum foil with eccrine fingerprint after immersing in solution of N-L-Cys-capped CdTe QDs for 5 s, and (B) magnified images (3×, from corresponding areas in A) showing the details of level 2 and level 3 characteristics of LFPs. | |
|
Download:
|
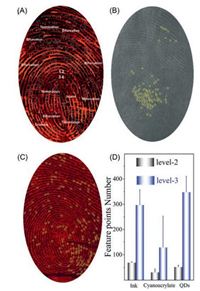
| Fig. 2. Mapping of sweat pores by different imaging agents: (A) N-L-Cys-capped CdTe QDs; (B) cyanoacrylate fuming; (C) ink; and (D) comparison of the mapping performance of level 2 and level 3 characteristics of the LFP from the same source. The statistic summarizing in (D) is obtained by averaging of at least 20 images. | |
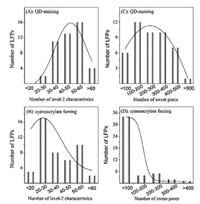
To demonstrate the superiority of N-L-Cys-capped CdTe QDs in mapping of both level 2 and level 3 characteristics of LFPs, we compared the performance of LFP staining with N-L-Cys-capped CdTe QDs and cyanoacrylate fuming (a standard technique being adopted at the forensic crime scene for examination of invisible fingerprints [33]). First, two eccrine LFPs were collected from the same volunteer and stained with N-L-Cys-capped CdTe QDs and cyanoacrylate fuming, respectively. As shown in Fig. S10 (Supporting information), the macrodetails such as friction ridge flow, pattern type, and singular points of the LFP images stained by the above two agents are generally the same. Besides, a lot of similar minutiae points of the two images can be identified (with random 13 points labeled in Fig. S10), implying the accuracy of the QDsstaining on the scale of level 2 characteristics. However, cyanoacrylate fuming for eccrine LFPs may vary between furrow and ridge (Fig. S11 in Supporting information), leading to relatively poor reproducibility. While in this work, QD-staining for eccrine LFPs shows exclusively the furrow (Fig. S12 in Supporting information). Such difference between the two methods results in different numbers of level 2 characteristics obtained (Fig. 2D). When comparing typical images obtained by the above two methods, one can see that the number of the level 2 characteristics by QDstaining that can be identified mostly locates in the range of 30–60 (Fig. 3A), while that by cyanoacrylate fuming is majorly in the range of 20–60 (Fig. 3B). If diving the image into four parts like that in Fig. 2A, the number of the level 2 characteristics in each part of the four by QD-staining is also larger than that of cyanoacrylate fuming (Figs. S13 versus S14 in Supporting information). Considering that in real criminal scene, the collected LFPs may be incomplete, the larger number of level 2 characteristics by QDstaining demonstrates the advantage of QD-staining in real-world applications.
|
Download:
|
| Fig. 3. The number distribution of level 2 (A and B) and level 3 (C and D) characteristics by QD-staining and cyanoacrylate fuming. All the summarizing was collected from 40 images. | |
Next, we compared the performance for sweat pore mapping by QD-staining and cyanoacrylate fuming. As can be seen from Fig. 2A and 2B, the number of sweat pore that can be mapped by QDstaining is even larger than that by cyanoacrylate fuming (Fig. 2D). For typical images obtained by the above two methods, the number of sweat pore by QD-staining that can be identified mostly locates in the range of 100–500 (Fig. 3C), while that by cyanoacrylate fuming is majorly lower than 100 (Fig. 3D). Similarly, the number of sweat pore in each part of the four by QD-staining is also larger than that of cyanoacrylate fuming (Figs. S15 versus S16 in Supporting information). These statistic data clearly demonstrate the superiority of QD-staining over cyanoacrylate fuming.
To check the accuracy of sweat pore mapping, we further used inkpad for capturing eccrine LFPs (Fig. 2C). After careful comparison about the images by QD-staining and inkpad, we found the distribution of the sweat pores on the two images is very similar to each other, indicating high accuracy of N-L-Cys-capped CdTe QDs for mapping of sweat pores. However, it should be noted that inkpad cannot be used for LFP-staining. Since sweat pores are always breathing and notevery pore secrets sweat all the time [16], the mapping results for sweat pores byeither method all have large relative standard deviations (Fig. 2D).
Next, the feasibility of integrating of level 2 and level 3 characteristics extracted from the fluorescent images of eccrine LFPs stained by QDs for fingerprint analysis was explored. A modified Pore Matching algorithm implemented in MATLAB was used for the process (see Supporting information for details). The modified Pore Matching algorithm was employed to compare two fluorescence images (Fig. 4A and B) by QD-staining. The results, displayed as connected lines in Fig. 4C and D, show that the selected fingerprint patterns (about one-sixth of the full fingerprint area) from generated by QD-staining have at least 52 matched points. There are also mismatched points in Fig. 4C and D, but the number (5) is much lower than that of the matched ones. Therefore, the fusion picture (Fig. 4E) of Fig. 4C and D shows that these two pictures are closely match one another. We also conducted the same match program for several other images, the results shown in Fig. S17 and S18 (Supporting information) are generally the same as those in Fig. 4, indicating the robustness the Pore Matching algorithm.

|
Download:
|
| Fig. 4. Fingerprint analysis based on the integration of both level 2 and level 3 features: (A) and (B), random fluorescent eccrine LFP images by QD-staining; (C) and (D), magnified area in (A) and (B), respectively; and (E) fusion image of (C) and (D). The connected lines in (C) and (D) the results obtained from the modified Pore Matching algorithm, with 52 matched and 5 mismatched points. The area of the selected fingerprint patterns is about one-sixth of the full area. | |
Previously, the needed number of level 2 characteristics for individual identification set by different countries varies from 6 to 17. In 1912, Locard proposed that 20–40 pores were sufficient to generate patterns that are required for a human's identity [34]. In this work, we integrated both level 2 and level 3 features of the LFPs for fingerprint analysis. The number of matched points of randomly selected area (one-tenth to one-sixth of the full fingerprint area) is larger than that set by Locard, indicating the superiority of QD-staining for LFP analysis.
In this work, red-emitting N-L-Cys-capped CdTe QDs were explored for eccrine LFPs staining. Similar to other nanocrystalbased imaging agents, here the CdTe QDs can successfully image the level 2 features of fingerprints. Besides, a larger number of level 3 characteristics, namely sweat pores, could also be mapped. When compared to the current standard method (cyanoacrylate fuming), the number of sweat pores that can be mapped by QD-staining is significantly larger. A very preliminary fingerprint matching based modified Pore Matching algorithm was thus developed based on the integration of both level 2 and level 3 characteristics for fingerprint analysis. Satisfactory results of matching were obtained. However, it should be pointed out that here we actually used passive LFP images as the both standard and sample for matching. Therefore, several mismatched points are also obtained. The picture quality (resolution, contrast, and etc.) is one of the bottlenecks for further improving the accuracy of this method. Nevertheless, using QDs as the imaging agent for LFPs for staining of both level 2 and level 3 features may be appealing for future fingerprint analysis.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 21475090 and 21522505).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.027.
| [1] |
L.R. Xu, C.Z. Zhang, Y.Y. He, B. Su, Sci. China-Chem. 58(2015) 1090-1096. DOI:10.1007/s11426-014-5294-5 |
| [2] |
S. Bell, Ann. Rev. Anal. Chem. 2(2009) 297-319. DOI:10.1146/annurev-anchem-060908-155251 |
| [3] |
P. Hazarika, D.A. Russell, Angew. Chem. Int. Ed. 51(2012) 3524-3531. DOI:10.1002/anie.v51.15 |
| [4] |
L.R. Xu, Y. Li, S.Z. Wu, et al., Angew. Chem. Int. Ed. 51(2012) 8068-8072. DOI:10.1002/anie.v51.32 |
| [5] |
J. Wang, T. Wei, X. Li, et al., Angew. Chem. Int. Ed. 53(2014) 1616-1620. DOI:10.1002/anie.201308843 |
| [6] |
K. Song, P. Huang, C.L. Yi, et al., ACS Nano 9(2015) 12344-12348. DOI:10.1021/acsnano.5b05629 |
| [7] |
G. Qin, M.Q. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 24(2013) 173-176. DOI:10.1016/j.cclet.2012.12.017 |
| [8] |
L.R. Xu, Z.Y. Zhou, C.Z. Zhang, et al., Chem. Commun. 50(2014) 9097-9100. DOI:10.1039/C4CC03466J |
| [9] |
Y.Y. He, L.R. Xu, Y. Zhu, et al., Angew. Chem. Int. Ed. 53(2014) 12609-12612. |
| [10] |
K. Li, W.W. Qin, F. Li, et al., Angew. Chem. Int. Ed. 52(2013) 11542-11545. DOI:10.1002/anie.201305980 |
| [11] |
Y. Li, L.R. Xu, Y.Y. He, B. Su, Electrochem. Commun. 33(2013) 92-95. DOI:10.1016/j.elecom.2013.04.033 |
| [12] |
An analysis of standards in fingerprint identification, Federal Bureau of Investigation, U. S. Department of Justice, 1972.
|
| [13] |
M. Pyo, J. Lee, W. Baek, et al., Chem. Commun. 51(2015) 3177-3180. DOI:10.1039/C4CC09085C |
| [14] |
J. Lee, M. Pyo, S.H. Lee, et al., Nat. Commun. 5(2014) 3736. |
| [15] |
D.H. Park, B.J. Park, J.M. Kim, Acc. Chem. Res. 49(2016) 1211-1222. DOI:10.1021/acs.accounts.6b00128 |
| [16] |
O.P. Kreyden, E.P. Scheidegger, Clin. Dermatol. 22(2004) 40-44. DOI:10.1016/j.clindermatol.2003.12.029 |
| [17] |
J. Dilag, H.J. Kobus, A.V. Ellis, Curr. Nanosci. 7(2011) 153-159. DOI:10.2174/157341311794653596 |
| [18] |
O.S. Wolfheis, Angew. Chem. Int. Ed. 48(2009) 2268-2269. DOI:10.1002/anie.200805765 |
| [19] |
M.J. Choi, A.M. McDonagh, P. Maynard, C. Roux, Forensic Sci. Int. 179(2008) 87-97. DOI:10.1016/j.forsciint.2008.04.027 |
| [20] |
A. Becue, C. Champod, P. Margot, Forensic Sci. Int. 168(2007) 169-176. DOI:10.1016/j.forsciint.2006.07.014 |
| [21] |
B.J. Theaker, K.E. Hudson, F.J. Rowell, Forensic Sci. Int. 174(2008) 26-34. DOI:10.1016/j.forsciint.2007.02.030 |
| [22] |
G.F. Wang, A.X. Guan, C.Y. Zhou, et al., Chin. Chem. Lett. 27(2016) 1788-1792. DOI:10.1016/j.cclet.2016.07.012 |
| [23] |
L.L. Xi, H.B. Ma, G.H. Tao, Chin. Chem. Lett. 27(2016) 1531-1536. DOI:10.1016/j.cclet.2016.03.002 |
| [24] |
P. Wu, C.Y. Xu, X.D. Hou, et al., Chem. Sci. 6(2015) 4445-4450. DOI:10.1039/C5SC01497B |
| [25] |
K.K. Bouldin, E.R. Menzel, M. Takatsu, R.H. Murdock, J. Forensic Sci. 45(2000) 1239-1242. |
| [26] |
S. Moret, A. Bécue, C. Champod, Forensic Sci. Int. 224(2013) 101-110. DOI:10.1016/j.forsciint.2012.11.009 |
| [27] |
K.H. Cheng, J. Ajimo, W. Chen, J. Nanosci. Nanotechnol. 8(2008) 1170-1173. |
| [28] |
X.J. Yu, J.J. Liu, S.L. Zuo, et al., Forensic Sci. Int. 231(2013) 125-130. DOI:10.1016/j.forsciint.2013.04.027 |
| [29] |
E.R. Menzel, S.M. Savoy, S.J. Ulvick, et al., J. Forensic Sci. 45(2000) 545-551. |
| [30] |
C.Y. Xu, R.H. Zhou, W.W. He, et al., Anal. Chem. 86(2014) 3279-3283. DOI:10.1021/ac404244v |
| [31] |
W.W. Yu, L.H. Qu, W.Z. Guo, X.G. Peng, Chem. Mater. 15(2003) 2854-2860. DOI:10.1021/cm034081k |
| [32] |
P. Wu, X.P. Yan, Biosens. Bioelectron. 26(2010) 485-490. DOI:10.1016/j.bios.2010.07.068 |
| [33] |
J.S. Day, H.G.M. Edwards, S.A. Dobrowski, A.M. Voice, Spectrochim. Acta Part A 60(2004) 1725-1730. DOI:10.1016/j.saa.2003.09.013 |
| [34] |
E. Locard, Bio. Rev. Sci. de Med. 2(1912) 357-365. |
 2017, Vol. 28
2017, Vol. 28 


