b College of Food Science and Light Industry, Nanjing Tech University, Nanjing 211816, China
Reactive oxygen species (ROS), including HOCl [1-3], H2O2 [4, 5], ·O2- [6], ROO· [7-9], 1O2 [10, 11], ·OH [12], are the natural byproducts of the normal metabolism and play vital roles in many physiological and pathological processes [13-16]. As a member of ROS, hypochlorous acid (HOCl) generated from the hydrogen peroxide (H2O2) and chloride (Cl-) ions catalyzed by myeloperoxidase (MPO) in living organisms [17, 18], which is a major oxidant and antimicrobial agent for immune system [19-22]. However, abnormal level of hypochlorous acid is associated with various diseases, including cardiovascular disease, kidney disease, neuron degeneration and cancer [23-25]. Therefore, real-time monitoring the concentration of hypochlorous acid in cellular is rather important for investigation of the pathogenic mechanism of hypochlorite in living systems and human diseases.
Recently, a number of analytical methods, such as colorimetric, electrochemical, chromatographic, luminescent and optical imaging methods, have been developed to detect hypochlorous acid [26-29]. It's noteworthy that fluorescent methods possess many advantages over others due to its high sensitivity, selectivity, specificity, and fast response time, which offering wide application in biological images [30-33]. In the past few years, there are a few fluorescent probes for detecting hypochlorous acid have been reported, but many of them were easy affected by environment or failed to meet good water solubility and real-time detection [34]. It's known that the emission intensity is influenced by many factors, including the concentration of fluorescent probe, excitation intensity, optical path length and detection efficiency. Ratiometric fluorescent probe can eliminate the effect of above factors by its self-calibration, leading to more precise accurate value through measuring the fluorescence intensity ratio at two different emission wavelengths [35-41]. In addition, as mitochondria are considered to be the main source of intracellular ROS, monitoring of HOCl in mitochondria is crucial for investigating the functions of HOCl in living cells. Therefore, developing ratiometric fluorescence probes with the capability of staining mitochondria is highly needed especially for HOCl detection [42-45].
In this work, we designed and synthesized a HOCl-selective fluorescent probe 1, which exhibited blue-shift emission upon the addition of HOCl. The ratiometric fluorescence changes dependent on the concentration of HOCl excludes the disturbance of other species in the environment. Moreover, the probe 1, as the existence of pyridine moiety, possesses the capability of staining mitochon-dria [46-49]. In order to examine whether the probe 1 can detect hypochlorous acid of tap water, the test strips experiments was performed. And the result demonstrated that the probe 1 can detect the hypochlorous acid in tap water accompanied by remarkable color change.
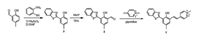
Scheme 1 illustrates the synthetic routes of the probe 1, and compound 2 were synthesized according to our previous report [50, 51]. After the condensation reaction between compound 2 and 1, 4-dimethylpyridin-1-ium, the probe 1 was obtained with a 76% yield. These new compounds were fully characterized by NMR and high resolution FAB mass spectroscopy. The 1H NMR, 13C NMR, and FAB mass spectra were reported in the Supporting information (Figs. S4–S13).
|
Download:
|
| Scheme 1. Synthesis of probe 1. | |
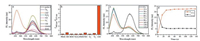
The absorption spectra of probe 1 with and without HOCl were first investigated. When free probe 1 dispersed in the HEPES buffer (10 mmol/L, pH 7.4), three absorption peaks at 315 nm, 398 nm and 500 nm were observed. Upon the addition of NaOCl, the absorption intensity at the above peaks was decreased and a new absorption peak at 225 nm emerged accompanied by the color conversion of the solution from light red to colorless (Fig. S1 in Supporting information). The emission spectra of probe 1 were further examined. As shown in Fig. 1, the initial probe exhibited an emission peaks at 615 nm. With the increasing concentration of NaOCl, the emission of probe 1 at 615 nm was gradually decreased while a new fluorescence band centered at 460 nm emerged, which shown a ratiometric fluorescent change of probe 1 with ClO-. After 150 equiv. of NaOCl was added, the fluorescent intensity had a trend to reach maximum value (Fig. 1).

|
Download:
|
| Fig. 1. Fluorescence spectra (a) and fluorescence intensity ratio (b) of probe 1 (10 mmol/L) upon the titration of NaOCl (0–1.6mmol/L). (c) The plot of fluorescence intensity ratio (I460 nm/I615 nm) versus OCl- concentration in HEPES buffer (10mmol/L, pH 7.4). (d) Effect of pH on fluorescence intensity ratio (I460 nm/I615 nm) of probe 1 and probe 1+NaOCl (0.1mmol/L) at room temperature (lex=398nm. Slit: 5nm/10nm). | |
To investigate the sensitivity of probe 1 towards HOCl, titration of HOCl at low level was performed. According to the titration profiles, the fluorescence intensity ratio (I460 nm/I615 nm) increases steadily with the increasing NaOCl level (Fig. 1c) and there is a good linear relationship between the fluorescence intensity ratio (I460 nm/I615 nm) in the range of 0–10 mmol/L of NaOCl level. In addition, the detection limit (S/N = 3) towards NaOCl was determined to be 0.18 mmol/L, which indicates that the probe 1 is highly sensitive to detect OCl- in aqueous solution.
In addition, the pH titration test reveals that the fluorescence intensity ratio (I460 nm/I615 nm) (Fig. 1d) of the probe 1 nearly remained unchanged at pH ranging from 4 to 9. After the NaOCl (10 equiv.) was added, the fluorescence intensity ratio (I460 nm/I615 nm) indicated remarkable enhancement in pH 4–9. The constant ratio ranges from 5.44 to 7.40, indicating that the probe 1 has the potential to image hypochlorite in biological conditions.
In order to verify whether probe 1 can selectively respond to OCl- under simulated physiological condition, the fluorescent response of probe 1 to other ROS/RNS were performed in HEPES buffer (10 mmol/L, pH 7.4). As shown in Fig. 2a, only hypochlorite (OCl-) leads to a dramatic enhancement of the fluorescent intensity at 460 nm. From the histogram, the fluorescence ratio value (I460 nm/I615 nm = 6.84) of probe with hypochlorite is much higher than that with other ROS/RNS, including H2O2, NO, ·O2-, ONOO-, ROO, 1O2, ·OH, exhibiting the high selectivity of probe toward hypochlorite (Fig. 2b).
|
Download:
|
| Fig. 2. (A) Fluorescence spectra of probe 1 (10 mmol/L) upon adding NaClO(500 mmol/L) and otherROS/RNS, including H2O2, NO, ·O2-, ONOO-, ROO·, 1O2, ·OH (1mmol/L). (B) Fluorescence intensity ratio of probe 1 (10 mmol/L) mixed with NaOCl (500 mmol/L) and other ROS/RNS (1mmol/L). (C) Time-dependent fluorescence spectra for probe 1 while adding ClO- (500 mmol/L). (D) Plot of fluorescence intensity at 460nm and 615nm of probe 1 while adding ClO- (500 mmol/L) in HEPES buffer (10mmol/L, pH 7.4) at room temperature (λex=398nm. Slit: 5nm/10nm). | |
The time course of the probe 1 with NaOCl was further carried out. As shown in Fig. 2c, upon the addition of NaOCl (50 equiv.), the fluorescence intensity exhibits a dramatic enhancement at 460 nm and displays a remarkable decrease at 615 nm within reaction time 0–30 s, Increasing with the reaction time, the fluorescence intensity remains little changes both at 460 nm and 615 nm after 30 s, Corresponding to the trend of fluorescence intensity changes in Fig. 2d, which displays the rapid response rate of probe 1 toward HOCl.
In Scheme S2 (Supporting information), the probe 1 itself possesses excited state intramolecular proton transfer (ESIPT) effect between the dyad of benzothiazole moiety and hydroxyl group, leading to an emission wavelength is about 460 nm with blue fluorescence [50]. However, the probe 1 exhibited red fluorescence with an emission wavelength at 615 nm when excited at 398 nm, which is attributed to the AIE property of probe 1. After excess ClO- was added, probe 1 exhibited a ratiometric fluorescence change in aqueous solution, accompanied by strong blue emission (460 nm) based on suppression of ESIPT effect. To further investigate the sensing mechanism of probe 1, the HPLC-MS analyses were used to study the oxidation reaction of probe 1 and OCl-. As shown in Fig. S4 in Supporting information, after probe 1 mixed with NaOCl, there are two main peaks at m/z 283.0704 (calcd. 283.0303) and m/z 306.0519 (calcd. 306.0201) were observed. We speculated that the probe 1 was oxidized to a possible organic compound of 1-ClO by ClO- [52], and the above mentioned peaks corresponded to [1-OCl+H]+ and [1-OCl+Na]+, respectively.
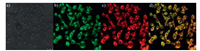
A standard MTT assay was employed to estimate cytotoxicity of probe 1 in HeLa cells with 0, 2, 4, 6 and 8 mmol/L probe 1 for 24 h. The result shows that cell viability was little changed when the concentration of probe 1 below 8 mmol/L, which indicates low cytotoxicity of probe 1 (Fig. S2 in Supporting information). To demonstrate the intracellular localizations of probe 1, a commercial mitochondrial organelle tracker, MT Green FM, was employed for fluorescence colocalization experiments. As illustrated in Fig. 3, the probe 1 signal overlaid very well with the fluorescence of MT Green FM. It is noteworthy that a high Pearson's co-localization coefficient we have obtained was calculated to be 0.97, which confirmed that probe 1 was site-specifically internalized in mitochondria in living cells.
|
Download:
|
| Fig. 3. HeLa cells were stained with probe 1 (30 mmol/L) and MT Green (1 mmol/L) for 30min, respectively. a): bright field of c; b): MT Green FM. lex=490nm, lem=500– 540nm; c): probe 1, lex=398nm, lem=550–650nm; d): merged of images b and c. Scale bar: 50 mm. | |
Then probe 1 was applied to image HOCl/OCl- in living HeLa cells, and strong fluorescence in the red channel (Fig. 4b) was observed when HeLa cells was incubated with probe 1 for 30 min at 37 ℃, while negligible fluorescence in the blue channel (Fig. 4c) was obtained. When 30 mmol/L of NaOCl was added and incubated for another 30 min at 37 ℃, obvious fluorescence in the blue channel (Fig. 4f) and weak fluorescence in the red channel (Fig. 4e) were observed. This result reveals that probe 1 is capable of detecting HOCl with ratiometric fluorescence changes in the presence of exogenous HOCl in the living HeLa cells.

|
Download:
|
| Fig. 4. Confocal fluorescence imaging of without (a–c) and with (d–f) exogenous NaOCl (0.7mmol/L) in HeLa cells using probe 1 (30 mmol/L) for 30min at 37 ℃. Red channel (b, e), lex=398nm, lem=550–650nm; blue channel (c, f), lex=398nm, lem=420–480nm. Scale bar=50mm. | |
As a member of microbicidal agents, hypochlorous acid is often used for disinfection of tap water. To test whether the probe 1 can detect hypochlorous acid in tap water, we applied probe 1 in test strips. Firstly, the round filter papers we have prepared were immersed in an ethanol solution of probe 1 (1 mmol/L) overnight to ensure that the probe 1 distributed evenly in filter papers, then dried in air to prepare test strips (light yellow). Secondly, the test strips were immersed in tap water and different concentrations (0.01, 0.1, 1, 10 mmol/L) of NaOCl aqueous solution for 10 min. When above test strips dried, the light yellow of test strips became the pink color gradually with the increasing concentration of NaOCl (Fig. S3 in Supporting information). In addition, the color of test slip immersed in tap water also displayed the pale-yellow color, suggesting that the probe 1 can detect the hypochlorous acid in tap water accompanied by remarkable color change.
In summary, we have designed and synthesized an excellent water-solubility fluorescent probe 1 that exhibited significant ratiometric (I460nm/I615 nm) response and high selectivity towards OCl- over other ROS/RNS. Moreover, probe 1 was mitochondrialspecific (Pearson's co-localization coefficient: 0.97) and successfully applied to detect HOCl/OCl- in a ratiometric fluorescence change. Finally, test strips experiment suggests that the probe 1 can detect the hypochlorous acid in tap water accompanied by remarkable color change. We anticipate that probe 1 will play a promising role in exploring of HOCl/OCl- in biological and environment systems.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21376117, 21406109 and 31401588), the Jiangsu Natural Science Funds for Distinguished Young Scholars (No. BK20140043), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 14KJA150005), the Qing Lan Project and the Project of Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2017.05.010.
| [1] |
S. Kenmoku, Y. Urano, H. Kojima, T. Nagano, J. Am. Chem. Soc. 129(2007) 7313-7318. DOI:10.1021/ja068740g |
| [2] |
J.L. Fan, H.Y. Mu, H. Zhu, et al., Ind. Eng. Chem. Res. 54(2015) 8842-8846. DOI:10.1021/acs.iecr.5b01904 |
| [3] |
X.Q. Chen, X.Z. Tian, I. Shin, J. Yoon, J. Chem. Soc. Rev. 40(2011) 4783-4804. DOI:10.1039/c1cs15037e |
| [4] |
J. Qiao, Z. Liu, Y. Tian, M. Wu, Z.W. Niu, Chem. Commun. 51(2015) 3641-3644. DOI:10.1039/C4CC09120E |
| [5] |
H.S. Wang, W.J. Bao, S.B. Ren, et al., Anal. Chem. 87(2015) 6828-6833. DOI:10.1021/acs.analchem.5b01104 |
| [6] |
H. Zhu, J.L. Fan, J.Y. Wang, H.Y. Mu, X.J. Peng, J. Am. Chem. Soc. 136(2014) 12820-12823. DOI:10.1021/ja505988g |
| [7] |
G.W. Chen, F.L. Song, J.Y. Wang, et al., Chem. Commun. 48(2012) 2949-2951. DOI:10.1039/c2cc17617c |
| [8] |
Y. Zhou, J.Y. Li, K.H. Chu, et al., Chem. Commun. 48(2012) 4677-4679. DOI:10.1039/c2cc30265a |
| [9] |
B.H. Wang, D. Chen, S. Kambam, et al., Dyes Pigments 120(2015) 22-29. DOI:10.1016/j.dyepig.2015.03.022 |
| [10] |
Z.C. Dai, L. Tian, Y.N. Xiao, et al., J. Mater. Chem. B 1(2013) 924-927. |
| [11] |
H.X. Tong, J.W. Du, H. Li, et al., Chem. Commun. 52(2016) 11935-11938. DOI:10.1039/C6CC06439F |
| [12] |
R.L. Zhang, J. Zhao, G.M. Han, et al., J. Am. Chem. Soc. 138(2016) 3769-3778. DOI:10.1021/jacs.5b12848 |
| [13] |
X. Wang, F.L. Song, X.J. Peng, Dyes Pigments 125(2016) 89-94. DOI:10.1016/j.dyepig.2015.10.012 |
| [14] |
J.L. Fan, H.Y. Mu, H. Zhu, J.Y. Wang, X.J. Peng, Analyst 140(2015) 4594-4598. DOI:10.1039/C5AN00777A |
| [15] |
G.H. Cheng, J.L. Fan, W. Sun, et al., Chem. Commun. 50(2014) 1018-1020. DOI:10.1039/C3CC47864E |
| [16] |
J.J. Du, M.M. Hu, J.L. Fan, X.J. Peng, Chem. Soc. Rev. 41(2012) 4511-4535. DOI:10.1039/c2cs00004k |
| [17] |
X.Q. Chen, F. Wang, J.Y. Hyun, et al., Chem. Soc. Rev. 45(2016) 2976-3016. DOI:10.1039/C6CS00192K |
| [18] |
D.P. Li, Z.Y. Wang, X.J. Cao, et al., Chem. Commun. 52(2016) 2760-2763. DOI:10.1039/C5CC09092J |
| [19] |
R. Noubade, K. Wong, N. Ota, et al., Nature 509(2014) 235-239. DOI:10.1038/nature13152 |
| [20] |
M. Yu, X. Wu, B. Lin, et al., Anal. Chem. 87(2015) 6688-6695. DOI:10.1021/acs.analchem.5b00847 |
| [21] |
M.S. Shim, Y.N. Xia, Angew. Chem. Int. Ed. 52(2013) 6926-6929. DOI:10.1002/anie.201209633 |
| [22] |
J. Kim, Y. Kim, Analyst 139(2014) 2986-2989. DOI:10.1039/C4AN00466C |
| [23] |
S. Goswami, S. Paul, A. Manna, Dalton Trans. 42(2013) 10097-10101. DOI:10.1039/c3dt51238j |
| [24] |
Q.A. Best, N. Sattenapally, D.J. Dyer, C.N. Scott, M.E. McCarroll, J. Am. Chem. Soc. 135(2013) 13365-13370. DOI:10.1021/ja401426s |
| [25] |
G.P. Li, D.J. Zhu, Q. Liu, L. Xue, H. Jiang, Org. Lett. 15(2013) 2002-2005. DOI:10.1021/ol4006823 |
| [26] |
L. Yuan, W. Lin, J. Song, Y. Yang, Chem. Commun. 47(2011) 12691-12693. DOI:10.1039/c1cc15762k |
| [27] |
L. Li, C.W. Zhang, G.Y.J. Chen, et al., Nat. Commun. 5(2014) 3276-4276. |
| [28] |
W. Feng, Q.L. Qiao, S. Leng, et al., Chin. Chem. Lett. 27(2016) 1554-1558. DOI:10.1016/j.cclet.2016.06.016 |
| [29] |
X. Chen, K.A. Lee, E.M. Ha, et al., Chem. Commun. 47(2011) 4373-4375. DOI:10.1039/c1cc10589b |
| [30] |
X. Chen, K.A. Lee, X. Ren, J. Yoon, et al., Nat. Protoc. 11(2016) 1219-1228. DOI:10.1038/nprot.2016.062 |
| [31] |
H. Zhu, J.L. Fan, B.H. Wang, X.J. Peng, Chem. Soc. Rev. 44(2015) 4337-4366. DOI:10.1039/C4CS00285G |
| [32] |
Y. Xiao, R. Zhang, Z. Ye, et al., Anal. Chem. 84(2012) 10785-10792. DOI:10.1021/ac3028189 |
| [33] |
J. Lv, F. Wang, T. Wei, X. Chen, Ind. Eng. Chem. Res. 56(2017) 3757-3764. DOI:10.1021/acs.iecr.7b00381 |
| [34] |
S.C. Burdette, G.K. Walkup, B. Spingler, R.Y. Tsien, S.J. Lippard, J. Am. Chem. Soc. 123(2001) 7831-7841. DOI:10.1021/ja010059l |
| [35] |
N. Jiang, J.L. Fan, F. Xu, et al., Angew. Chem. Int. Ed. 54(2015) 2510-2514. DOI:10.1002/anie.201410645 |
| [36] |
X.G. Yang, Y.B. Zhou, X.F. Zhang, et al., Chem. Commun. 52(2016) 10289-10292. DOI:10.1039/C6CC05254A |
| [37] |
A.S. Abdelfattah, V. Rancic, B. Rawal, K. Ballanyi, R.E. Campbell, Chem. Commun. 52(2016) 14153-14156. DOI:10.1039/C6CC06810C |
| [38] |
S.K. Bae, C.H. Heo, D.J. Choi, et al., J. Am. Chem. Soc. 135(2013) 9915-9923. DOI:10.1021/ja404004v |
| [39] |
H.J. Kim, C.H. Heo, H.M. Kim, J. Am. Chem. Soc. 135(2013) 17969-17977. DOI:10.1021/ja409971k |
| [40] |
C. Huang, T. Jia, M.F. Tang, et al., J. Am. Chem. Soc. 136(2014) 14237-14244. DOI:10.1021/ja5079656 |
| [41] |
C.C. Zhao, X.L. Zhang, K.B. Li, et al., J. Am. Chem. Soc. 137(2015) 8490-8498. DOI:10.1021/jacs.5b03248 |
| [42] |
J.L. Fan, M.M. Hu, P. Zhan, X.J. Peng, Chem. Soc. Rev. 42(2013) 29-43. DOI:10.1039/C2CS35273G |
| [43] |
G.H. Cheng, J.L. Fan, W. Sun, et al., Analyst. 138(2013) 6091-6096. DOI:10.1039/c3an01152f |
| [44] |
W.S. Shin, M.G. Lee, P. Verwilst, et al., Chem. Sci. 7(2016) 6050-6059. DOI:10.1039/C6SC02236G |
| [45] |
J.T. Hou, M.Y. Wu, K. Li, et al., Chem. Commun. 50(2014) 8640-8643. DOI:10.1039/C4CC02673J |
| [46] |
Y. Liu, K. Li, M.Y. Wu, et al., Chem. Commun. 51(2015) 10236-10239. DOI:10.1039/C5CC03055B |
| [47] |
H.D. Li, Q.C. Yao, J.L. Fan, et al., Ind. Eng. Chem. Res. 55(2016) 1477-1483. DOI:10.1021/acs.iecr.5b04530 |
| [48] |
Q. Wang, W. Wang, S. Li, et al., Dyes Pigments 134(2016) 297-305. DOI:10.1016/j.dyepig.2016.07.030 |
| [49] |
H.D. Xiao, K. Xin, H.F. Dou, et al., Chem. Commun. 51(2015) 1442-1445. DOI:10.1039/C4CC07411D |
| [50] |
C.C. Chang, F. Wang, J. Qiang, et al., Sens. Actuators B:Chem. 243(2017) 22-28. DOI:10.1016/j.snb.2016.11.123 |
| [51] |
T.T. Chen, T.W. Wei, Z.J. Zhang, et al., Dyes Pigments 140(2017) 392-398. DOI:10.1016/j.dyepig.2017.01.063 |
| [52] |
Y. Yue, F. Huo, C. Yin, et al., RSC Adv. 5(2015) 77670-77672. DOI:10.1039/C5RA16097A |
 2017, Vol. 28
2017, Vol. 28 


