b State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
Intracellular biothiols are closely related to the pathology of many diseases. Abnormal levels of biothiols have been shown to be associated with human diseases such as slow growth, liver damage, skin lesions [1, 2]. Particularly, cysteine (Cys), which is a precursor amino acid of glutathione (GSH), plays crucial roles in mitochondrial processes as a significant antioxidant reservoir to regulate the oxidative stresses [3]. The levels of Cys in mitochondria are very important and sensitive to oxidative stress of related diseases, including type 2 diabetes, Alzheimer's and Parkinson's diseases [4-6]. Therefore, it is crucial to develop mitochondrial Cys-selective probes for real-time in vivo monitoring intracellular biothiols in living cells.
Near-infrared (NIR) fluorescent bioimaging would be of greater interest because the long wavelength emission allows deep-tissue imaging, reduces auto-fluorescence and decreases light scattering [7-10] In particular, NIR fluorescent probe has attracted increasing attention due to their unique advantages, noninvasive and realtime in vivo detection with high sensitivity and selectivity. In general, there are two main difficulties in the detection of mitochondria Cys: specific mitochondria targeting and selective detection of Cys over Hcy and GSH. To solve these problems, the combinational strategy of Michael addition and cyclization reaction proposed by Strongin and co-workers was employed to improve the selectivity of Cys over Hcy and GSH [11]. More recently, Yoon and co-workers built a novel NIR probe for high selective detection of Cys, and demonstrated its excellent in vivo bioimaging properties for monitoring Cys in living cells [12]. However, until now, there is still a challenge for NIR fluorescent probe for Cys with the high specific mitochondria targeting ability [13-16].
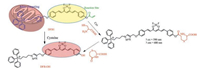
As well known, curcumin features a π-conjugated system and a central β-diketone unit with low dark toxicity and multiple therapeutic effects, including therapy effects for cancers [17-21]. Notably, Moore and co-workers recently reported the application of curcumin difluoroboron complexes as a promising NIR fluorescent dye for in vivo amyloid-β plaque-specific studies [22]. Undoubtedly, curcuminoid difluoroboron is a typical high-performance fluorophore with great potential application in bioimaging. In this work, we developed a mitochondrial-targetable dualchannel NIR fluorescent probe DFB1 based on curcuminoid difluoroboron scaffold for the detection of Cys (Scheme 1). In the DFB1, the acryloyl functionality group served as a reaction unit for selective detection of Cys, and the triphenylphosphine (TPP) moiety enables specifically target mitochondria in the living cells. The Michael addition to the acryloyl group in DFB1 afforded a sevenmembered ring in the presence of Cys, which was followed by cleavage of the ester bond to form DFB-OH (Scheme 1). Specifically, the probe has initial strongly fluorescence at 560 nm with the addition of Cys, while a new NIR emission band at 680 nm significantly appears and concurrently the emission at 560 nm rapidly decreases. Notably, Cys could be quantitatively detected by monitoring two fluorescence channels with this transformation from DFB1 to DFB-OH. More importantly, DFB1 showed excellent cell membrane permeability in living cells. Compared with other reported probes, this dual-channel NIR probe could provide more accurate in situ and in vivo information of Cys during the sensing process. We believe that this NIR probe DFB1 will make significant advances in the study of the biological functions of mitochondrial thiols.
|
Download:
|
In our probe design, the acryloyl functionality groups as a reaction unit for selective detection of Cys, and a cationic moiety as a mitochondrial-targeting unit were included. As shown in Scheme 2, we synthesized curcumin analogs DFB1 containing two functional groups, an acryloyl group as the specific trigger moiety for biothiols and a TPP group as a mitochondria-specific targeted unit. The key intermediates were prepared from the treatment of the key intermediate curcumin precursor 2 gave the final product DFB1 by three steps [23, 24]. Specifically, 3 was synthesized by the reaction of 2 with propargyl bromide in DMF, which an alkynyl group was decorated with 2. Then, 4 was obtained by the reaction of 3 with acryloyl chloride. The chemical structures of all compounds including DFB1 were characterized by 1 H NMR, 13C NMR, 19F NMR and HRMS in Supporting information. The details of synthetic procedures and the structure information are described in Supporting information. Specifically, the characteristic coupling constant (J = 15.6 Hz) of alkene protons at δ 7.96 and 6.92 and are indicative of the predominated trans isomer in the Supporting information.

|
Download:
|
| Scheme 2. Synthetic route for DFB1. | |
To meet the requirements of NIR fluorescent probe for Cys, herein the product which interacted with Cys was a NIR fluorescent curcuminoid difluoroborond dye DFB-OH with a phenolic hydroxyl group as an electron donor (Scheme 1). It will display significantly emission shift from 560 nm to 680 nm by the generation of DFB-OH following the reaction between DFB1 and Cys. The mechanism is based on Michael addition and intramolecular cyclization reactions by the following two steps. Firstly, the addition of Cys to the acryloyl group generates thioether product, then undergoes a rapid intramolecular cyclization to produce DFB-OH accompanied by the release of the cyclization byproduct. As a result, obviously changes in the UV–vis spectra and fluorescent emission spectra could be observed.
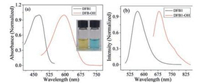
We firstly investigated the photophysical properties of DFB1 and DFB-OH. As shown in Fig. 1, DFB1 exhibited a large blue shift ca. 120 nm in contrast with that of DFB-OH in the absorption spectra, while they have similar molar absorption coefficients (ε = 4100 L mol-1 cm-1). It should be attributed to the incorporation acryloyl moiety with DFB1 weakening the electron-donating ability of the phenolic group [12, 25-26]. As depicted in Scheme 1, the reaction of Cys with DFB1 was proceeded by involving two steps: Conjugation addition to generate thioethers and then intramolecular cyclization to yield DFB-OH. The electron-donating ability alteration between DFB-OH and DFB1 made an obviously shift in the absorption peak, resulting in distinct color changed from yellow to blue in 5 min (Inset of Fig. 2a). With the titration of Cys (200 mmol/ L) to DFB1 (20 mmol/L), the absorption peak at 470 nm decreased sharply and a new band at 590 nm appeared with an isosbestic point at 510 nm (Fig. 2a). These facts indicate that DFB1 could serve as a "naked eye" probe for Cys.
|
Download:
|
| Fig. 1. Absorption (a) and fluorescence (b) spectra of DFB1 (20 mmol/L) and DFB-OH (20 mmol/L) in a mixed DMSO/PBS buffer solution (1:1, v/v, 0.01 mol/L, pH 7.4). | |

|
Download:
|
A similar large red shift in the emission spectra of DFB1 was also observed in the presence of Cys. First of all, fluorescence quantum yields of DFB1 and DFB-OH are calculated to 0.19 and 0.11, respectively. As presented in Fig. 2b and 2c, with the titration of Cys (from 0 to 200 mmol/L), a distinct fluorescence decrease at 560 nm for DFB1 was observed upon excitation at 470 nm (Fig. 2b). In the meantime, significant new enhancement fluorescence at 680 nm was also observed upon excitation at 590 nm (Fig. 2c), which could attribute to the product DFB-OH. The above observations demonstrated that upon specific reaction DFB1 with Cys, there was a significant red shift ca. 120 nm in its characteristic emission band and activated the NIR region fluorescent signal. To test the interference with other biological analytes, we firstly investigated the selectivity of DFB1 towards Cys and two similar structure thiols (Hcy and GSH). As shown in Fig. 3a, the timedependent fluorescence response of DFB1 with those three biothiols were monitored at 37 ℃ in a mixed buffer solution. Specifically, with the excitation at 590 nm, the probe DFB1 had initially weak fluorescence, while there was a sharply fluorescent enhancement at 680 nm during 0–10 min and then reached a plateau with the addition of 50 equiv. of Cys. In contrast, the significant responses of DFB1 with Cys over time were not observed for Hcy and GSH. The great superiority of the reaction speed of DFB1 and Cys showed that probe DFB1 could be used to effectively discriminate Cys with from Hcy and GSH. The high selectivity of probe DFB1 for Cys rather than other similar thiols (Hcy and GSH) can be attributed to the kinetic rate of the intramolecular cyclization reactions. The intramolecular cyclization reaction is kinetically favored for Cys to form a sevenmembered ring compared to that of Hcy, which forms an eightmembered ring [12, 27-28]. At the same time the intramolecular cyclization reaction for GSH is hindered by the bulkiness of its tripeptide; as a result, the DFB1-Cys conjugated thioether was generated the most rapidly. The mechanism of this reaction was further proved by ESI-MS analyses. Upon the interaction of Cys with DFB-1, the peak of m/z at 929.32 (corresponding to [DFB1 + Cys]+) was found in the high resolution MS spectrum (in the Supporting information), clearly indicating the existence of the ring transitional state and the correctness of the reaction mechanism. Based on these results, the probe DFB1 exhibited higher reactivity and selectivity for Cys over Hcy and GSH.

|
Download:
|
| Fig. 3. (a) Time-dependent fluorescence intensity changes at 680 nm of DFB1 (20 mmol/L) in the absence (blank) and presence of Cys (50 equiv.), Hcy (50 equiv.), or GSH (300 equiv.) with an excitation at 590 nm. (b) Fluorescence change I/I0 at 680 nm before and after being treated with various analytes (50 equiv.). Inset: DFB1 with Cys displayed an obvious color change from yellow to blue in the presence of various amino acids. All experiments were performed in DMSO/PBS buffer (1:1, v/v, 0.01 mol/L, pH 7.4) at 37 ℃ for 10 min). | |
The fluorescence responses of DFB1 to various other non-thiols amino acids, such as Val, Phe, Arg, Leu, Trp, Gly, Met, Ser, Tyr, Thr, Lys, Pro, Glu, Asn, Asp, His, Ala, Glu, Ile, and nucleophilic biomolecules including hydrogen sulfide (H2S), peroxynitrite (ONOO-), hydrogen disulfide (H2S2) and hypocholrous (ClO-) were also further evaluated [29-32]. Only Cys with DFB1 displayed an obvious color change from yellow to blue in the presence of various amino acids (Inset of Fig. 3b). Correspondingly, the selective turn-on response was also observed at the NIR fluorescent channel in Fig. 3b. All these experiments demonstrated that DFB1 can be applied to selectively detect Cys. Notably, DBF1 possessed dual-channel fluorescent signal responses, particular turn-on NIR fluorescent signals simultaneously in response to Cys concentration. Therefore, we believed that DFB1 would be potential for sensing cellular Cys in bioimaging applications.
Near-infrared dyes are excellent dyes for optical imaging biomolecules in living systems. Due to its selective dual-channel fluorescent signals toward Cys, particular turn-on NIR fluorescent signals, DFB1 was considered a promising tool for imaging cellular thiols. Consequently, the practical utility of DFB1 for Cys detection in confocal luminescence imaging was first carried out in living HeLa cells. As shown in Fig. 4, the red channel represents the initial fluorescence signal of DFB1 and the purple channel represents DFB1 in the presence of Cys, and the mask-colored ratio column was generated by the fluorescence intensity in the purple channel over that of in the red channel. For these experiments, HeLa cells were chosen. After treating Hela cells with glucose-free Dulbecco's modified Eagle medium (DMEM), the intracellular cysteine level was significantly increased during glucose deprivation in parental Hela cells. As shown in Fig. 4d, incubation of Hela cells with DFB1 produced negligible fluorescent ratio signal (0.2), while a strong fluorescent ratio signal (4.8) was observed at 590 nm for Hela cells grown in glucose-free DMEM (Fig. 4i). A sharp fluorescence decrease in the red channel after reacting with cellular cysteine was observed in Fig. 4j. In contrast, it was clearly to observe that the fluorescent ratio became lower after the cells were pretreated with NEM in Fig. 4h, which could lead a lower Cys level in HeLa cells [33, 34]. Clearly, ratiometric imaging can be successfully used to monitor the presence of Cys, which involved using two different imaging signal channels. Based on these results in vivo studies, DFB1 can be used as a dual-channel NIR sensor for bioimaging of endogenous Cys and the correlated oxidative stress in live cancer cells.

|
Download:
|
| Fig. 4. Confocal fluorescence images of living HeLa cells. (a-d) HeLa cells incubated with DFB1 (20 mmol/L) for 2 h at 37 ℃. (e-h) HeLa cells were pretreated with NEM (100 mmol/L) for 30 min and then further incubated with DFB1 (20 mmol/L). (i-l) HeLa cells were treated with glucose-free DMEM incubation and then further incubated with DFB1 (20 mmol/L). (m-p) HeLa cells were pretreated with Cys (200 mmol/L) and then incubated with DFB1 (20 mmol/L). In the red channel, the excitation wavelength is at 470 nm and the emission was collected at 530–600 nm; in the purple channel, the excitation wavelength is at 590 nm and the emission was collected at 650–730 nm. The ratiometric images were obtained by the image analysis software Image J. | |
Considering a positively charged TPP moiety in DFB1, it would have a high tendency to localize in mitochondria of a cell due to the large negative membrane potential of the inner mitochondrial membrane. To further examine the intracellular localization of DFB1, colocalization experiments were carried out by the costaining with Mito-Tracker green, which is a widely used commercially available mitochondrial dye (Fig. 5). The fluorescence of Mito-Tracker (green, Fig. 5b and Fig. 5f) was perfectly colocalized with DFB1 in the absence (red, Fig. 5c) and presence (purple, Fig. 5g) of Cys. The Pearson's correlation coefficient in the merged images (Fig. 5d and Fig. 5h) are 0.94 and 0.91, respectively. This indicated that DFB1 was stably localized in mitochondria during the sensing process and did not show significant escape. These results confirmed that DFB1 can specifically target the mitochondria and detect Cys in living HeLa cells.

|
Download:
|
| Fig. 5. Confocal fluorescence images of living HeLa cells. (A) HeLa cells were pretreated with NEM (100 mmol/L) for 30 min and then further incubated with DFB1 (20 mmol/L) in DMSO/PBS (1:99 v/v, 0.01 mol/L, pH 7.4) solution for 2 h at 37 ℃; (B) HeLa cells were pretreated with Cys (200 mmol/L) and then incubated with DFB1 (20 mmol/L). The overlay images (d, h) were obtained by the image analysis software Image J. | |
In summary, we have developed a highly selective dual-channel NIR fluorescent probe (DFB1) based on curcuminoid difluoroboron, which could discriminate Cys over GSH, Hcy and other amino acids. The high specificity of DFB1 for Cys derives from the carefully secific recognition group and sophisticated reaction mechanism in the detection process. As expected, the curcumin analog framework endowed the probe ideal biocompatibility and long wavelength fluorescence, thus the sensor had good membrane permeability and it could assess the Cys level in mitochondria through its NIR fluorescence signal, this makes it capable of detecting mitochondrial Cys. As highly selective and sensitive NIR fluorescence probes for the detection of small biological molecules in vitro and in vivo are very limited, we hope that this curcuminoid difluoroboron fluorophore will be of great benefit for many researchers engaged in the study of biomolecules and bioimaging.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China for Excellent Young Scholars (No. 21622602), Distinguished Young Scholars (No. 21325625), Oriental Scholarship, Fok Ying Tong Education Foundation (No. 142014), the Fundamental Research Funds for the Central Universities (Nos. WJ1616008, WK1013002), the State Key Laboratory of Fine Chemicals (No. KF1509).
| [1] |
J.M. Xavier, C.M.P. Rodrigues, S. Solá, Neuroscientist 22(2016) 346-358. DOI:10.1177/1073858415585472 |
| [2] |
V.N. Helin, T.O. Amanda, N. Erik, Biochem. Biophysical Res. Commun 482(2017) 426-431. DOI:10.1016/j.bbrc.2016.11.088 |
| [3] |
M. Wasilewski, K. Chojnacka, A. Chacinska, Biochim. Biophys. Acta 1864(2017) 125-137. DOI:10.1016/j.bbamcr.2016.10.019 |
| [4] |
M. Celia, M.S. Ross, Am. J. Med. 118(2005) 1174-1175. |
| [5] |
Y.D. Wang, J. Fang, C.H. Li, et al., Mitochondrion 6(2006) 263-288. |
| [6] |
P. Back, P.B. Braeckman, F. Matthijssens, Oxid. Med. Cell. Longe 201(2012) 608478. |
| [7] |
S.A. Hilderbrand, R. Weissleder, Curr. Opin. Chem. Biol 14(2010) 71-79. DOI:10.1016/j.cbpa.2009.09.029 |
| [8] |
L. Yuan, W. Lin, K. Zheng, L. He, W. Huang, Chem. Soc. Rev 42(2013) 622-661. DOI:10.1039/C2CS35313J |
| [9] |
Z. Guo, S. Park, J. Yoon, I. Shin, Chem. Soc. Rev. 43(2014) 16-29. DOI:10.1039/C3CS60271K |
| [10] |
X. Wang, Z.B. Zeng, J.H. Jiang, Y.T. Chang, L. Yuan, Angew. Chem. Int. Ed. 55(2016) 13658-13699. DOI:10.1002/anie.201510721 |
| [11] |
X.F. Yang, Y.X. Guo, R.M. Strongin, Angew. Chem. Int. Ed. 50(2011) 10690-10693. DOI:10.1002/anie.201103759 |
| [12] |
Z.Q. Guo, S.W. Nam, S. Park, J. Yoon Chem. Sci. 3(2012) 2752-2760. |
| [13] |
W. Niu, L. Guo, Y. Li, et al., Anal. Chem. 88(2016) 1908-1914. DOI:10.1021/acs.analchem.5b04329 |
| [14] |
Y. Chen, T. Wei, Z. Zhang, et al., Chin. Chem. Lett. (2017), doi: http://dx.doi.org/10.1016/j.cclet.2017.05.010.
|
| [15] |
C.M. Han, H.R. Yang, M. Chen, et al., ACS Appl. Mater. Inter. 7(2015) 27968-27975. DOI:10.1021/acsami.5b10607 |
| [16] |
H. Qua, Z. Zhang, N. Wang, et al., Chin. Chem. Let. 26(2015) 1249-1254. DOI:10.1016/j.cclet.2015.06.016 |
| [17] |
X. Zhang, Y. Tian, P. Yuan, et al., Chem. Commun. 50(2014) 11550-11553. DOI:10.1039/C4CC03731F |
| [18] |
X. Zhang, Y. Tian, Z. Li, et al., J. Am. Chem. Soc. 135(2013) 16397-16409. DOI:10.1021/ja405239v |
| [19] |
C. Ran, X. Xu, S. Raymond, et al., J. Am. Chem. Soc. 131(2009) 15257-15261. DOI:10.1021/ja9047043 |
| [20] |
X. Zhang, Y. Tian, C. Zhang, et al., PNAS 112(2015) 9734-9739. DOI:10.1073/pnas.1505420112 |
| [21] |
G. Bai, C. Yu, C. Cheng, et al., Org. Biomol. Chem. 12(2014) 1618-1626. DOI:10.1039/C3OB42201A |
| [22] |
C. Ran, X. Xu, S. Raymond, et al., J. Am. Chem. Soc. 131(2009) 15257-15261. DOI:10.1021/ja9047043 |
| [23] |
G. Canard, Ponce-Vargas M., D. Jacquemin, et al., RSC Adv. 7(2017) 10132-10142. DOI:10.1039/C6RA25436E |
| [24] |
M. Halik, H. Hartmann, J. Chem Eur 5(1999) 2511-2517. DOI:10.1002/(ISSN)1521-3765 |
| [25] |
H.L. Wang, G.G. Zhou, H.W. Gai, X.Q. Chen, Chem. Commun. 48(2012) 8341-8343. DOI:10.1039/c2cc33932c |
| [26] |
H.Y. Lee, Y.P. Choi, S. Kim, et al., Chem. Commun. 50(2014) 6967-6969. DOI:10.1039/c4cc00243a |
| [27] |
C. Galli, L. Mandolini, Eur. J. Org. Chem. 18(2000) 3117-3125. |
| [28] |
P. Blondeau, R. Gauthier, C. Berse, D. Gravel, Can. J. Chem. 49(1971) 3866-3876. DOI:10.1139/v71-645 |
| [29] |
J. Peng, A. Samanta, L. Yuan, X. Liu, Y.T. Chang, Angew. Chem. Int. Ed. 129(2017) 4229-4233. DOI:10.1002/ange.201612020 |
| [30] |
L. Chen, D. Wu, C.S. Lim, et al., Chem. Commun. 53(2017) 4791-4794. DOI:10.1039/C7CC01695F |
| [31] |
B.C. Dickinson, D. Srikun, C.J. Chang, Curr. Opin. Chem. Biol. 14(2010) 50-56. DOI:10.1016/j.cbpa.2009.10.014 |
| [32] |
D. Cheng, Y. Pan, L. Wang, et al., J. Am. Chem. Soc. 139(2017) 285-292. DOI:10.1021/jacs.6b10508 |
| [33] |
M. Kota, A. Bicza, Med. Chem. 23(2008) 514-520. |
| [34] |
Y.J. Lee, J.C. Chen, A. Amoscato, et al., J. Cell. Sci. 114(2001) 677-684. |
 2017, Vol. 28
2017, Vol. 28 


