Mitochondria, the double-membrane-bound subcellular organelles, are the major energy sources in living cells, and play the significant roles for cellular vital processes [1, 2]. A series of physiological processes are taken place in their inner membrane due that the various proteins are contained within mitochondria [3]. On the other hand, lysosomes are also of great importance for living cells especially in the cellular degradation processes, and they are well-known as the digestive organs with singlemembrane-bound structure [4, 5]. Therefore, it is of great significance to investigate mitochondria and lysosomes in subcellular levels in vitro and in vivo. Small-molecule fluorescent probes are attractive for studying biological systems thanks to their several considerable merits, which include high specificity, simple operation and low cost, among others. With the recent development of imaging techniques, researchers now are able to utilize the fluorescent probes as powerful tools to sense and visualize analytes microscopically [6-11].
To date, many small-molecule fluorescent probes have been used to respond to biomolecules (including ions, reactive oxygen species (ROS), reactive sulfur species (RSS) and reactive nitrogen species (RNS)) and changes inside micro-environment (e.g., pH, viscosity and polarity) [6-25]. However, the reported ones for monitoring and visualizing aforementioned analytes in mitochondria and lysosomes are still limited [26-39], and most of reported fluorescent probes are designed as one-photon (OP) fluorescent probes by utilizing one-photon microscopy (OPM), which requires excitation with short-wavelength light (ca. 350-550 nm) that limits their application in subcellular organelles and deep-tissue, owing to the shallow penetration depth (less than 80 μm) as well as to photobleaching, photodamage, and cellular autofluorescence. In comparison with OP fluorescent probes, two-photon (TP) fluorescent probes with the assistance of two-photon microscopy (TPM) [40, 41], which can be excited by two-photon absorption (TPA) in the near-infrared (NIR) wavelength, have been considered as the powerful tools to overcome the problems originated from the OP fluorescent probes. In recent years, reviews of smallmolecule two-photon fluorescent probes were reported by Cho, Kim, Ahn, and Belfield's group, etc. [42-45], still, further development of mitochondria-and lysosomes-targeted smallmolecule two-photon fluorescent probes is in demand. Considering Kim and Cho have summarized the small-molecule TP probes published prior to December 2014 [45], in this review, the smallmolecule TP probes have been categorized according to their sensing characteristics into different sections, including the visualization of mitochondria and lysosomes (e.g., mitotracker and lysotracker), the selective detection of biomolecules (e.g., ions, ROS and RSS) and changes inside micro-environment (e.g., pH, viscosity and polarity) in mitochondria and lysosomes with the assistance of two-photon microscopy during the published dates from January 2015 to May 2017
2. Designs and tactics of mitochondria and lysosomes-targeted small-molecule two-photon fluorescent probesFirstly, we should retrospect the basic conceptions of twophoton absorption materials. TPA is the necessary photophysical feature for those molecules that could be induced to an excited state via absorbing two photons simultaneously. The TPA process was predicted early in 1931 by Göppret-Mayer [46]. TPA crosssection (δ) is a significant parameter for evaluating the merits of TP fluorescent probes. Besides, TP action cross-section (δΦ), an arithmetic product of the TP cross-section (δ) and the fluorescence quantum yield (Φ), is the most common factor for estimating TP characteristic especially in TP bio-imaging [43, 45, 47].
In most cases, the TP fluorescent probes are initially projected with huge conjugated structures. On the basis of many outstanding fluorophores (e.g., coumarin, quinoline, fluorene and carbazole) [48-51], a series of TP fluorescent platforms possessing impressive TP action cross-section with enormous conjugation (such as the electron donor-acceptor (D-A) dipoles, the donor-conjugateddonor (D-π-D) and donor-acceptor-donor (D-A-D) quadrupoles) were exploited and utilized in bio-imaging field. In addition, the TP fluorescent probes were rationally designed to be incorporated with a wide range of recognition groups for selective and sensitive response to various analytes in living system.
In order to investigate substances in subcellular levels within living cells, the organelle-targeted small-molecule TP fluorescent probes are eagerly needed. A targeting group should be added to the TP fluorescent skeleton, which aims to selectively be stained into mitochondria or lysosomes. Mitochondria, as the energy source for eukaryocyte cells, are related to several physiological activities, such as metabolism, signal transduction, and apoptosis. These complicated processes are also associated with diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD), and atherosclerosis, etc. In addition, the negative membrane potential (-180 mV) within the mitochondrial inner membrane are generated during the cellular respiration process [52]. In response to the negative membrane potential of mitochondria, a smart strategy for designing mitochondria-targeted fluorescent probes is linking the fluorophores with positive lipophilic cation moieties, such as triphenylphosphine cation (TPP), methyl pyridinium cation, indole cation and so on (Scheme 1). Such small organic positive unit could promote the probe to move across phospholipid bilayers and further facilitate its accumulation into the mitochondrial matrix [53-58]. Lysosomes, well-known as the catabolic organelles in eukaryocyte cells, are full of more than 60 kinds of degradative enzymes. Lysosomes are responsible for intracellular digestion degradation, metabolism of substances as well as endocrine regulation. Variations of lysosomes' quantities, distributions and morphologies caused by self-aberrance or environmental fluctuations, would result in a number of illnesses, such as PD, AD and cancers [59-61]. In consideration of the acidic pH range (4.5-5.5) of lysosomes that are filled with a number of acid hydrolases [62-64], the alkaline moieties, such as morpholine, pyridine, dimethylamino group and so on (Scheme 1), are quite fit for linking with the probes as lysosomal targeting groups. In general, mitochondria-and lysosomes-targeted TP small-molecule fluorescent probes were designed by containing three parts as follows, the TP fluorophore, the recognition group and the targeting moiety (cation groups for mitochondria and organic base groups for lysosomes).
|
Download:
|
| Scheme 1. General designed structures of mitochondria-and lysosomes-targeted small-molecule two-photon fluorescent probes. | |
3. Mitochondria-targeted small-molecule two-photon fluorescent probes 3.1. Tracker
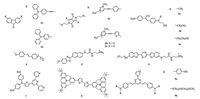
Ciccarella et al. reported two fluorenone-based fluorescent probes 1a and 1b (Fig. 1) specifically localizing mitochondria in MCF-7 cells [65]. Due to the increased conjugated system, 1a presented larger TP action cross-section than that of 1b in a wide wavelength range from 700 nm to 1000 nm. Moreover, 1a (94%) exhibited higher co-localization coefficients with MitoTracker Red than that of 1b (85%). Compared with MitoTracker Red, both 1a and 1b showed better photostability and long-term retention in living cells with low cytotoxicity. Gryko et al. synthesized three diketopyrrolopyrrole-imidazolium salts as small-molecule fluorescent probes (2a, 2b and 2c) selectively staining mitochondria (Fig. 1) [66]. In DMSO solvent, 2a, 2b and 2c exhibited the maximum TPA cross-sections with the values of 380 GM at 720 nm, 4320 GM and 3430 GM at 740 nm, respectively. Compared with MitoTracker Green, 2a, 2b and 2c showed good co-localization for mitochondria in living cells. Wu and her co-workers designed three novel two-photon fluorescent probes 3a, 3b and 3c with large two-photon absorption cross-sections as well as specifically targeting mitochondria in HepG2 cells (Fig. 1) [67]. A new mitochondria-localized two-photon fluorescent probe 4 with high permeability and qualified photostability was synthesized by Yu and his fellows (Fig. 1) [68]. A pair of two-photon fluorescent probes 5 and 6 were synthesized by Cho and his co-workers for mitochondria imaging in living cells (Fig. 1) [69]. Both probes 5 and 6 owned big efficient TP action cross-section values of 1860 GM and 1950 GM, respectively. Tian et al. reported a novel two-photon fluorescent probe 7 with large TP action across-section (~370 GM at 850 nm) successfully staining at mitochondria under low working concentration (10 nmol/L) (Fig. 1) [70]. A water-soluble two-photon fluorescent probe 8 with triarylborane chromophores was designed by Marder et al. (Fig. 1) [71]. Furthermore, probe 8 showed great co-localization property with MitoTracker Red in mitochondria, on the other hand, the large TPA across-section value of 268 GM at 800 nm in water makes it suitable for TP microscopy. Tian et al. reported two small water-soluble pyrimidine hexafluorophosphate derivatives 9a and 9b, which were both used as highly efficient two-photon mitochondria trackers in comparison with the commercial MitoTracker (Fig. 1) [72].
|
Download:
|
| Fig. 1. Chemical structures of probes 1-9. | |
3.2. Metal ions
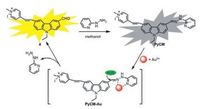
Meng et al. designed a novel ratiometric two-photon fluorescent probe 10 on the basis of quinolone platform to monitor the fluctuation of zinc ions in mitochondrial field (Fig. 2) [73]. With the addition of Zn2+, emission of probe 10 was shifted from 420 nm to 488 nm resulting in ca. 8-fold dramatical enhancement in the ratio of fluorescence intensity (I488 nm/I420 nm). Probe 10 also exhibited large TPA cross-section value of 150 GM at 720 nm. In addition, it was as well as an efficient mitochondria-localized fluorescent probe in comparison of MitoTracker Red. Taking advantage of carbazole fluorophore, Feng and her co-workers developed a twophoton fluorescent probe 11 for gold ions detection in mitochondria (Fig. 2) [74]. As an excellent water-soluble fluorescent probe, it showed an apparent emission enhancement response to Au3+. The "turn on" fluorescence response was attributed to the gold ionsinduced C=N bond hydrolysis sensing mechanism (Fig. 3). The large TPA cross-section (1321 GM) at 860 nm exhibited its proficiency on two-photon biological imaging with the help of TP microscopy. The co-localization coefficient of 0.85 between 11 and MitoTracker Red revealed that 11 was predominantly present in mitochondria.
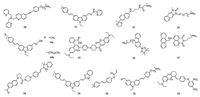
|
Download:
|
| Fig. 2. Chemical structures of probes 10-22. | |
|
Download:
|
| Fig. 3. Synthesis and Au3+-induced hydrolysis of probe PyCM (probe 11). Copied with permission [74]. Copyright 2015, Elsevier B. V. | |
3.3. ROS and RSS
In order to detect hypochlorous acid (HClO) in mitochondria, Chang et al. reported a novel mitochondria-targeted two-photon fluorescent probe 12 for rapidly (within seconds) and ultrasensitively (less than 20 nmol/L) monitoring HClO (Fig. 2) [75]. It's worth noticing that probe 12 was the first two-photon fluorescent "turn-on" probe for HClO in mitochondria. Furthermore, probe 12 can be used to monitor HClO in mitochondria of lipopolysaccharides (LPS)/phorbol 12-myristate 13-acetate (PMA)-stimulated macrophage cells and mitochondria of macrophage cells during inflammation conditions in a murine model. Another two-photon fluorescent probe 13 for HClO in mitochondria was designed by Yoon and his fellows (Fig. 2) [76]. The detection limit of probe 13 was calculated to be 0.21 mmol/L. According to the co-staining experiment of probe 13 and MitoTracker Red in HeLa cells with two-photon excitation at 700 nm, the probe showed great Pearson's co-localization coefficient of 0.83. Two water-soluble two-photon fluorescent probes 14a and 14b based on carbazole skeleton were synthesized by Feng et al. (Fig. 2) [77]. In those two molecular structures, alkyl pyridinium moieties were used as the mitochondria-targeted functional groups. Owing to the larger TPA cross-section value of 14b (1642 GM) in comparison with that of 14a (980 GM) at 860 nm and the better fluorescent response to hypochlorite, 14b was approved as the efficient two-photon fluorescent probe for hypochlorite in mitochondrial imaging with assistance of TP microscopy.
Yuan et al. synthesized a novel mitochondria-targeted twophoton fluorescent probe 15 for selectively visualization of the endogenous peroxynitrite (ONOO-) via fluorescence resonance energy transfer (FRET) ratiometric fluorescence signals (Fig. 2) [78]. Upon addition of ONOO- from 0 to 7.5 μmol/L, a 93-fold fluorescence ratio (I473 nm/I651 nm) enhancement was observed along with a low detection limit of 11.3 nmol/L. Moreover, probe 15 was capable of monitoring ONOO- produced by LPS stimulation in the inflamed mouse model. Li et al. reported a two-photon fluorescent probe 16 for mitochondrial hydrogen peroxide (H2O2) detection and imaging on the basis of carbazole fluorophore (Fig. 2) [79]. The probe exhibited a "turn on" fluorescent response to H2O2 as well as a detection limit of 1.96 μmol/L. Co-localization experiments clearly demonstrated that probe 16 could selectively accumulate in mitochondria. Zhang et al. developed an efficient two-photon fluorescent probe 17 for monitoring singlet oxygen (1O2) in mitochondria (Fig. 2) [80]. Upon photoirradiation of coincubated photosensitizer Chlorin e6, the absorbance of the probe showed a gradual decrease at 400 nm, accompanied by a 17.8-fold fluorescence enhancement at 536 nm. In addition, the probe 17 can monitor intracellular 1O2 particularly during the photodynamic therapy (PDT) process in living cells and tissues with TP bioimaging.
Yang et al. reported the first two-photon probe 18 based on acedan-merocyanine dyads for visualization of endogenous sulfur dioxide derivatives (SO2) via TP-FRET ratiometric fluorescence response in mitochondria (Fig. 2) [81]. The probe was potentially suitable for quantitative determination of HSO3-/SO32- concentrations due to the excellent linear relationship between [I500 nm/ I590 nm]n/[I500 nm/I590 nm]0 and the concentration of HSO3- in the range from 0 to 10.0 mmol/L with a detection limit of 50 nmol/L. Meanwhile, probe 18 exhibited high TP action cross-section (100 GM) at 760 nm. The probe can be utilized to study the metabolism of sulfur-containing species in biomedical research under TP microscopy. Feng and her co-workers also reported a novel mitochondria-targeted colorimetric and two-photon fluorescent probe 19 for visualization of biological SO2 derivatives (Fig. 2) [82]. Probe 19 showed good linear relationship between fluorescence intensity and HSO3- from 0 to 1.0 equiv. with a low detection limit of 2.21 nmol/L. Large TP action cross-section (ca. 194 GM) was presented at 840 nm. The co-localization coefficient of 0.89 between probe 19 and MitoTracker Red indeed favored the probe could be as a useful tool of sensing and bio-imaging of SO2 derivatives in mitochondria. Li and Dong reported a two-photon fluorescent probe 20 for selectively ratiometric sensing and imaging mitochondrial cysteine (Cys) on the basis of a merocyanine as the fluorophore and an acrylate moiety as the biothiol reaction site (Fig. 2) [83]. Upon addition of Cys, the intensity of fluorescence emission located at 452 nm decreased gradually with a concomitant increase in fluorescence intensity at 518 nm. The large TP action cross-sections of 65.2 GM at 740 nm and 72.6 GM at 760 nm in aqueous medium both for the probe and its respective fluorophore were obtained. According to those feature, the probe can be applied to selectively detect mitochondrial Cys and monitor Cys concentration change in live cells and living tissues by TP microscopy. By utilizing an aldehyde as the response site, Lin et al. designed a dual-site two-photon fluorescent probe 21 for visualizing thiol-containing amino acids (RSH) in mitochondria (Fig. 2) [84]. Under the excitation at 365 nm, the fluorescence intensity located at 410 nm had an evident increase with the addition of Cys. And the detection limit of Cys was calculated to be 0.2 μmol/L within 10 min response time. The probe was proved to target in mitochondria efficiently with the Pearson's co-localization co-efficient of 0.90.
Zhou et al. reported a mitochondria-localized two-photon fluorescent probe 22 for gaseous signal molecules hydrogen sulfide (H2S) with near-infrared fluorescence emission (665 nm) (Fig. 2) [85]. The fluorescence intensity at 665 nm increased linearly with the concentration of H2S ranging from 0 to 5.0 μmol/L along with the detection limit of 83 nmol/L. It also possessed a large TP action cross-section of ca. 160 GM at near 800 nm as well as a high fluorescence quantum yield of 15%. This probe was also applied for two-photon imaging in mitochondria and tissues slices of nude mice.
Thus, exploring mitochondrial ROS and RSS by utilizing the two-photon fluorescent probes has received extensive attentions. A list of photophysical characteristics of mitochondria-targeted TP fluorescent probes for ROS and RSS can be found in Table 1 for convenient comparison. Correspondingly, a summary of photophysical properties of lysosomes-targeted TP fluorescent probes for ROS and RSS was listed in Table 2.
|
|
Table 1 Photophysical characteristics of mitochondria-targeted TP fluorescent probes for ROS/RSS. |
|
|
Table 2 Photophysical characteristics of lysosomes-targeted TP fluorescent probes for ROS/RSS. |
3.4. Micro-environment
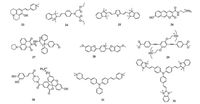
Zhang et al. reported a novel two-photon fluorescent probe 23 based on a rotor mechanism for selectively staining mitochondria both in immortalized and normal cells (Fig. 4) [86]. The probe was nonluminous in non-viscous medium due to the free rotation around the substituted vinyl bond. Both its single-photon excited fluorescence (SPEF) (λex = 530 nm) and two-photon excited fluorescence (TPEF) (λex = 900 nm) emissions at red-emitting wavelength 620 nm were dramatically enhanced in viscous glycerol (Gly), providing high signal-to-noise ratio (SNR). Probe 23 also possessed high photostability as well as excellent counterstain compatibility. Moreover, upon addition of nystatin, the probe can monitor viscosity change in mitochondria with an obvious increasing in fluorescence. Tian et al. developed a water-soluble two-photon fluorescent probe 24 for ratiometric imaging of microviscosity in mitochondria (Fig. 4) [87]. A good linear relationship between the fluorescence intensity ratio (I569 nm/I384 nm) and the logarithm of the viscosity (log η) was presented in water-glycerol system with the excitation wavelength at 320 nm. It was found that the probe showed the maximum TP action cross-section value at 700 nm, which was increased with the enlargement of solution viscosity. Furthermore, it was successfully applied for monitoring the viscosity distinction in normal and nystatin treated HepG2 cells. A new two-photon fluorescent probe 25 was designed for sense viscosity by Yoon and his co-worker (Fig. 4) [88]. A typical rotatable conjugated fluorophore in 25 can be considered as the response group of viscosity based on the intramolecular charge transfer (ICT) effect. Compared with MitoTracker Deep Red, the probe showed a unique ability for selective and robust staining of mitochondria with high photostability within ca. 50 min. This dye was consequently utilized in high resolution imaging of mitochondrial transport in primary cortical neurons, where the rapid transport of tubule-like mitochondria along the dendrites and axons was clearly visualized.
|
Download:
|
| Fig. 4. Chemical structures of probes 23-32. | |
Kim et al. reported a novel ratiometric two-photon fluorescent probe 26 for quantitative imaging of mitochondrial pH values (Fig. 4) [89]. As the mitochondrial pH is known to be alkaline (near 8.0), the pKa value was the key design factor of mitochondrial pH probe. The probe was excellently suitable for mitochondrial pH monitoring due to its ideal pKa value in 7.86 ± 0.05. A good fluorescence emission ratio (I604 nm/I540 nm) response for pH at the range of 3.0 to 11.0 was exhibited along with a robust reversible sensing ability (pH 5.0-9.0).
Xiao and his co-workers developed a multifunctional and bipolar two-photon fluorescent probe 27 for tracing local depolarization in mitochondria (Fig. 4) [90]. In the probe design, the hydrophilic anchoring unit was chemically immobilized on a membrane protein with assistance of a reactive aldehyde group into TPP, while the lipophilic fluorophore could be inserted deep into the phospholipid layer of the inner mitochondrial membrane. The probe showed a double-exponential decay corresponding to two lifetimes. One (τ1) could be ascribed to the ICT state, while the other (τ2) was not so sensitive and could be attributed to the locally excited (LE) state. The applicability of the probe has been confirmed by TP fluorescence lifetime imaging investigation on carbonyl cyanide 3-chlorophenylhydrazone (CCCP)-induced mitochondrial depolarization.
3.5. OthersLim et al. reported a mitochondria-targeted two-photon fluorescent probe 28 to measure the mitochondria/nucleus ratios in human colon tissues (Fig. 4) [91]. The probe 28 showed great colocalization property under two-photon excitation with MitoTracker Red in mitochondria. The probe 28 (600-700 nm) and the nucleus-stained probe (400-475 nm) were co-labelled for stimultaneously detecting nuclei/mitochondria in human colon tissues by dual-colour TPM imaging. Fan et al. developed an inner saltshaped small molecular photosensitizer 29 with extremely enhanced two-photon absorption for mitochondria-targeted photodynamic therapy (Fig. 4) [92]. And it exhibited 3260 GM in methanol solvent. Probe 29 facilitated the transfer of singlet-totriplet excited states for triplet photosensitized 1O2, and the probe possessed a high 1O2 quantum yield of 72.8%. The probe was certified to selectively target mitochondria according to the colocalization experiment between probe 29 and MitoTracker Red.
Superoxide (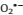
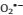
On the basis of fluorenone, Ciccarella et al. designed a new twophoton fluorescent probe 33 for lysosomes imaging with a large TP action cross-section value (150 GM) (Fig. 5) [65]. Co-localization experiments exhibited that this probe was an admired lysosomes tracker with a Pearson's co-localized coefficient of 0.89. Yu et al. reported another two-photon fluorescent probe 34 with pyridine working as recognizing group for imaging lysosomes in living cells (Fig. 5) [68]. The probe showed strong fluorescence in buffer solutions with pH values ranging from 5.5 to 4.0. Moreover, the probe showed large co-localization coefficient of 0.87. An efficient two-photon lysosomes tracker 35 was developed by Cho and his co-workers with the enormous TP action cross-section value of 2110 GM at 740 nm (Fig. 5) [69]. Moreover, it was also utilized in the simultaneous visualization of lysosomes deep inside a live tissue by TPM imaging. A unique family of two-photon full-color-tunable fluorescent probes 36 a-e for lysosomes imaging were reported by Lin et al (Fig. 5) [95]. On the basis of the proposed "hybridization" strategy, those lysosomes-targeted functional fluorescent probes displayed full-color-tunable emission from blue to near-infrared region in solutions. Those probes could all be used in lysosomes imaging.
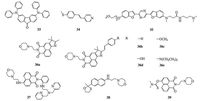
|
Download:
|
| Fig. 5. Chemical structures of probes 33-39. | |
4.2. Metal ions
Cho et al. developed a new two-photon fluorescent probe 37 for zinc ions imaging in lysosomes (Fig. 5) [96]. The probe showed a good linear relationship with Zn (Ⅱ) in the low concentration region from 0 to 1.0 μmol/L along with the detection limit of 0.18 μmol/L, offering a very high sensitivity toward Zn (Ⅱ). The probe also presented great co-localization property with lysosomes, and the Pearson's co-localization coefficient was calculated to be 0.87.
4.3. ROS and RSSChang et al. synthesized the first small-molecule two-photon fluorescent probe 38 for lysosomal hypochlorous acid (HClO) imaging in living cells (Fig. 5) [75]. A novel thiocarbamate-based two-photon fluorescent probe 39 was designed for imaging native HClO in lysosomes by Tang and his fellows (Fig. 5) [97]. The probe exhibited "turn on" emission response at 547 nm towards HClO. In addition, a good linear relationship between the fluorescence intensities and the HClO concentrations (0-1.5 μmol/L) was obtained with the detection limit of 7.6 pmol/L. This probe was utilized to monitor the distribution of HClO in lysosomes and in deep tissues.
Lin et al. developed a novel dual-site two-photon fluorescent probe 40 for visualizing lysosomal hydrogen sulfide (H2S) (Fig. 6) [98]. Under the acidic conditions, the pyridine moiety was protonated, which exhibited a red-shift fluorescence emission (625 nm). On the other hand, in the presence of both H2S and H+, a blue-shift emission (550 nm) was induced. The Mander's overlap coefficient and Pearson's colocalization coefficient were calculated to be 0.96 and 0.87, respectively. A ratiometric two-photon fluorescent probe 41 was designed by Liu et al. for lysosomal H2S imaging (Fig. 6) [99]. Upon the addition of H2S (using Na2S as a donor) to the probe, an 80-fold fluorescence ratio (I541 nm/I475 nm) enhancement was observed at a wide linear range of 25-2500 μmol/L with the detection limit of 0.70 μmol/L under twophoton excitation of 840 nm. The co-localization experiment showed its good targeted property with the Pearson's correction coefficient of 0.85. A lysosomes-specific two-photon fluorescent probe 42 based on 1, 8-naphthalimide towards lysosomal thiols by Fan and her co-workers (Fig. 6) [100]. Upon addition of Cys, the fluorescence emission signal became stronger at 540 nm, along with a strong linear relationship between the fluorescence intensity and the concentrations of Cys ranging from 30 to 160 μmol/L. The detection limits were calculated as low as 0.26 μmol/L for Cys, 2.41 μmol/L for GSH and 4.87 μmol/L for Hcy, respectively. Cell-imaging and tissue experiments indicated that probe 42 can be used as a new lysosomes-specifc and twophoton probe for thiols.

|
Download:
|
| Fig. 6. Chemical structures of probes 40-48. | |
4.4. Micro-environment
Zhou et al. reported a novel ratiometric two-photon fluorescent probe 43 for lysosomal pH detection, which utilized through bond energy transfer (TBET) strategy based on naphthalimide and rhodamine B moieties (Fig. 6) [101]. With the pH values increasing from 3.0 to 7.4, a ratiometric (I515 nm/I595 nm) fluorescent signal detection and imaging of pH was achieved. Tian and Wu developed a pair of novel two-photon excited fluorescent pH probes 44 and 45 for intracellular pH mapping (Fig. 6) [102]. The probe 44 exhibited a good linear relationship with remarkable emission ratio enhancement (I460 nm/I580 nm) at the pH range from 4.33 to 6.73, while the probe 45 exhibited a good linear relationship (I465 nm/ I540 nm) at the pH range from 2.99 to 5.28. In addition, the pKa value of the probes 44 and 45 were 5.12 and 3.85, respectively. According to the co-localization experiments, both the probes 44 and 45 showed great co-localized properties with a commercially available lysosome-targeted dye at lower pH. A novel lysosomes-targeted FRET based two-photon pH probe 46 was synthesized by Liu and his fellows (Fig. 6) [103]. There were two fluorescence signals at 461 nm and 555 nm, which represented the typical emission peak of the coumarion and naphthalimide, respectively. When pH increased from 5.0 to 11.0, an apparent 15-fold enhancement of the ratio value (I461 nm/I555 nm) was displayed from 0.36 to 5.28. Co-localization experiment showed its good targeting property to lysosomes with a Pearson's coefficient of 0.95 in living cells. Furthermore, the probe could also be utilized in realtime monitoring chloroquine-induced pH changes with ratiometric analysis in living HeLa cells.
Meng et al. developed a two-photon fluorescent probe 47 for polarity in lysosomes (Fig. 6) [104]. This probe was the first TP probe for monitoring autophagy by detection of the change in lysosomal polarity. With the increase in solution polarity, the fluorescent emission intensity presented a good linear relationship, as well as the quantum yield and lifetime behaviors. The TP action cross-section (δΦ) of the probe was detected in the H2O/THF mixtures, and the maximum δΦ values at 760 nm was decreased from 102 GM (10% of water content) to 4.4 GM (80% of water content) by degrees. The probe displayed excellent co-localization trait compared to the commercial lysosome-targeted dye both in the MCF-7 (0.91) and HeLa cells (0.89), respectively. In addition, this probe could also be utilized in investigating the changes of lysosomal polarity during autophage in living cells (Fig. 7).

|
Download:
|
| Fig. 7. (a) Fluorescence confocal imaging of MCF-7 cells stained with Lyso-OC (Probe 47) before being induced by HBSS. (b) Fluorescence confocal imaging of MCF-7 cells stained with Lyso-OC in autophagy. λex = 760 nm, λem = 490-550 nm. The scale bar represents 20 mm. Copied with permission [104]. Copyright 2017, Royal Society of Chemistry. | |
4.5. Others
Wang et al. developed a novel lysosomes-targeted two-photon fluorescent probe 48 for endogenous β-galactosidase (β-gal) imaging (Fig. 6) [105]. β-Gal is known as an overexpressed enzyme in primary ovarian cancer, which could be utilized as a meritorious biomarker of ovarian cancer. With addition of β-gal, the probe displayed "turn on" fluorescence response at 560 nm (>1000 fold). Upon addition with β-gal from 0 U/L to 50 U/L, the ratio of I560 nm/I450 nm showed a good linear relationship along with the detection limit of 4.0 ×10-5 U/mL. Moreover, the probe can be used for endogenous β-gal imaging in living cells.
5. Conclusion and outlookHerein, we summarized the recent advances of mitochondriaand lysosomes-targeted small-molecule two-photon fluorescent probes. The review is focused on the probes which can target and visualize two kinds of specific subcellular organelles (e.g., mitotracker and lysotracker), selectively and sensitively detect the concentration and activities of biomolecules, and changes inside micro-environment in mitochondria and lysosomes.
It is noticed that the reported small-molecule TP probes for mitochondria and lysosomes were relatively limited in comparison with the OP ones. In the meantime, the most mitochondria-and lysosomes-targeted TP fluorescent probes for ROS/RSS are reaction-based ones, which cannot visualize dynamic concentration changes of the analytes due to the irreversibility of these reactions. Therefore, more research studies should be focused on designing the small-molecule two-photon fluorescent probes for the quantitative and specific detection of ROS/RSS in mitochondria and lysosomes. On the other hand, the key development of mitochondria-and lysosomes-targeted small-molecule TP fluorescent probes in future should be their promising clinical applications for disease analysis and diagnosis, as well as drug screening. This part of work will need the closer cross-cooperation of organic chemists, biologists and clinical scientists. Therefore, the study of small-molecule TP probes, which can specially locate in mitochondria and lysosomes, will have a bright application prospect.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (Nos. 21778001, 21372005), the Anhui Provincial Natural Science Foundation (No. 1608085MB39), the Natural Science Foundation of Education Department of Anhui Province (No. KJ2015A047) and the 211 Project of Anhui University.
| [1] |
S. Fulda, L. Galluzzi, G. Kroemer, Nat. Rev. Drug Discov. 9(2010) 447-464. DOI:10.1038/nrd3137 |
| [2] |
R.A.J. Smith, R.C. Hartley, H.M. Cochemé, M.P. Murphy, Trends Pharmacol. Sci. 33(2012) 341-352. DOI:10.1016/j.tips.2012.03.010 |
| [3] |
H.M. McBride, M. Neuspiel, S. Wasiak, Curr. Biol. 16(2006) 551-560. DOI:10.1016/j.sbi.2006.06.011 |
| [4] |
M. Aridora, L.A. Hannan, Traffic 1(2000) 836-851. DOI:10.1034/j.1600-0854.2000.011104.x |
| [5] |
Q.L. Liang, X.S. Ouyang, L. Schneider, J.H. Zhang, Mol. Neurodegener. 6(2011) 37-48. DOI:10.1186/1750-1326-6-37 |
| [6] |
Y.B. Ding, Y.Y. Tang, W.H. Zhu, Y.S. Xie, Chem. Soc. Rev. 44(2015) 1101-1112. DOI:10.1039/C4CS00436A |
| [7] |
L. Yuan, W.Y. Lin, K.B. Zheng, S.S. Zhu, Acc. Chem. Res. 46(2013) 1462-1473. DOI:10.1021/ar300273v |
| [8] |
Z.Q. Guo, S. Park, J. Yoon, I. Shin, Chem. Soc. Rev. 43(2014) 16-29. DOI:10.1039/C3CS60271K |
| [9] |
M. Vendrell, D.T. Zhai, J.C. Er, Y.T. Chang, Chem. Rev. 112(2012) 4391-4420. DOI:10.1021/cr200355j |
| [10] |
M. Beija, C.A.M. Afonso, J.M.G. Martinho, Chem. Soc. Rev. 38(2009) 2410-2433. DOI:10.1039/b901612k |
| [11] |
T. Terai, T. Nagano, Pflugers Arch. Eur. J. Physiol. 465(2013) 347-359. DOI:10.1007/s00424-013-1234-z |
| [12] |
Y. Zhang, Y.Y. Fu, D.F. Zhu, et al., Chin. Chem. Lett. 27(2016) 1429-1436. DOI:10.1016/j.cclet.2016.05.019 |
| [13] |
X.M. Li, R.R. Zhao, Y.L. Wei, et al., Chin. Chem. Lett. 27(2016) 813-816. DOI:10.1016/j.cclet.2016.04.001 |
| [14] |
X.D. Jiang, H.F. Yu, J.L. Zhao, et al., Chin. Chem. Lett. 26(2015) 1241-1245. DOI:10.1016/j.cclet.2015.07.002 |
| [15] |
X.H. Yang, S. Li, Z.S. Tang, et al., Chin. Chem. Lett. 26(2015) 129-132. DOI:10.1016/j.cclet.2014.09.025 |
| [16] |
Y. Zhang, Y.G. Gao, Y.D. Shi, et al., Chin. Chem. Lett. 26(2015) 894-898. DOI:10.1016/j.cclet.2015.05.032 |
| [17] |
Y. Long, J. Zhou, M.P. Yang, B.Q. Yang, Chin. Chem. Lett. 27(2016) 205-210. DOI:10.1016/j.cclet.2015.09.003 |
| [18] |
H.R. Qu, Z.Y. Zhang, N. Wang, et al., Chin. Chem. Lett. 26(2015) 1249-1254. DOI:10.1016/j.cclet.2015.06.016 |
| [19] |
L. Song, X.D. Sun, Y. Ge, et al., Chin. Chem. Lett. 27(2016) 1776-1780. DOI:10.1016/j.cclet.2016.05.007 |
| [20] |
L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27(2016) 330-334. DOI:10.1016/j.cclet.2015.12.012 |
| [21] |
Q. Wang, Y. Feng, J. Jiang, W.J. Wang, et al., Chin. Chem. Lett. 27(2016) 1563-1566. DOI:10.1016/j.cclet.2016.02.021 |
| [22] |
P. Ning, P.Y. Dong, Q. Geng, et al., J. Mater. Chem. B 5(2017) 2743-2749. DOI:10.1039/C7TB00136C |
| [23] |
Y.K. Yue, F.J. Huo, P. Ning, et al., J. Am. Chem. Soc. 139(2017) 3181-3185. DOI:10.1021/jacs.6b12845 |
| [24] |
W. Zhang, K.X. Xu, L.X. Yue, et al., Dyes Pigm. 137(2017) 560-568. DOI:10.1016/j.dyepig.2016.11.002 |
| [25] |
Z.G. Yang, J.F. Cao, Y.X. He, et al., Chem. Soc. Rev. 43(2014) 4563-4601. DOI:10.1039/C4CS00051J |
| [26] |
W. Feng, Q.L. Qiao, S. Leng, et al., Chin. Chem. Lett. 27(2016) 1554-1558. DOI:10.1016/j.cclet.2016.06.016 |
| [27] |
Y.H. Tang, X.Q. Kong, Z.R. Liu, A. Xu, W.Y. Lin, Anal. Chem. 88(2016) 9359-9363. DOI:10.1021/acs.analchem.6b02879 |
| [28] |
B.L. Dong, X.Z. Song, C. Wang, et al., Anal. Chem. 88(2016) 4085-4091. DOI:10.1021/acs.analchem.6b00422 |
| [29] |
K. Sreenath, Z. Yuan, J.R. Allen, M.W. Davidson, L. Zhu, J. Chem. Eur. 21(2015) 867-874. DOI:10.1002/chem.v21.2 |
| [30] |
M.G. Ren, B.B. Deng, J.Y. Wang, Z.R. Liu, W.Y. Lin, J. Mater. Chem. B 3(2015) 6746-6752. DOI:10.1039/C5TB01184A |
| [31] |
G. Zhang, J.J. Gruskos, M.S. Afzal, D. Buccella, Chem. Sci. 6(2015) 6841-6846. DOI:10.1039/C5SC02442K |
| [32] |
K. Li, J.T. Hou, J. Yang, X.Q. Yu, Chem. Commun. 53(2017) 5539-5541. DOI:10.1039/C7CC01679D |
| [33] |
H.B. Xiao, P. Li, X.F. Hu, et al., Chem. Sci. 7(2016) 6153-6159. DOI:10.1039/C6SC01793B |
| [34] |
C.Y. Kim, H.J. Kang, S.J. Chung, et al., Anal. Chem. 88(2016) 7178-7182. DOI:10.1021/acs.analchem.6b01346 |
| [35] |
Z.C. Dai, L. Tian, B. Song, X.L. Liu, J.L. Yuan, Chem. Sci. 8(2017) 1969-1976. DOI:10.1039/C6SC03667H |
| [36] |
W. Xu, Z.B. Zeng, J.H. Jiang, Y.T. Chang, L. Yuan, Angew. Chem. Int. Ed. 55(2016) 13658-13699. DOI:10.1002/anie.201510721 |
| [37] |
H. Zhu, J.L. Fan, J.J. Du, X.J. Peng, Acc. Chem. Res. 49(2016) 2115-2126. DOI:10.1021/acs.accounts.6b00292 |
| [38] |
N. Roopa, V. Kumar, Kumar M.Bhalla, Chem. Commun. 51(2015) 15614-15628. |
| [39] |
Z. Xu, L. Xu, Chem. Commun. 52(2016) 1094-1119. DOI:10.1039/C5CC09248E |
| [40] |
W.R. Zipfel, R.M. Williams, W.W. Webb, Nat. Biotechnol. 21(2003) 1369-1377. DOI:10.1038/nbt899 |
| [41] |
F. Helmchen, W. Denk, Nat. Methods 2(2005) 932-940. DOI:10.1038/nmeth818 |
| [42] |
H.M. Kim, B.R. Cho, Acc. Chem. Res. 42(2009) 863-872. DOI:10.1021/ar800185u |
| [43] |
S. Yao, K.D. Belfield, Eur. J. Org. Chem. 2012(2012) 3199-3217. DOI:10.1002/ejoc.v2012.17 |
| [44] |
D. Kim, H.G. Ryu, K.H. Ahn, Org. Biomol. Chem. 12(2014) 4550-4566. DOI:10.1039/C4OB00431K |
| [45] |
H.M. Kim, B.R. Cho, Chem. Rev. 115(2015) 5014-5055. DOI:10.1021/cr5004425 |
| [46] |
M. Göppert-Mayer, Ann. Phys. 401(1931) 273-294. DOI:10.1002/(ISSN)1521-3889 |
| [47] |
H.M. Kim, B.R. Cho, Chem. Commun(2009), 153-164. |
| [48] |
H.J. Yin, B.C. Zhang, H.Z. Yu, et al., J. Org. Chem. 80(2015) 4306-4312. DOI:10.1021/jo502775t |
| [49] |
X.M. Wang, X.H. Wang, Y. Feng, et al., Dalton Trans. 44(2015) 6613-6619. DOI:10.1039/C5DT00012B |
| [50] |
X.L. Yue, Z. Armijo, K. King, et al., ACS Appl. Mater. Interfaces 7(2015) 2833-2846. DOI:10.1021/am508093p |
| [51] |
D.X. Li, X. Sun, J.M. Huang, et al., Dyes Pigm. 125(2016) 185-191. DOI:10.1016/j.dyepig.2015.10.016 |
| [52] |
J.S. Modica-Napolitano, J.R. Aprille, Adv Drug Deliv. Rev. 49(2001) 63-70. DOI:10.1016/S0169-409X(01)00125-9 |
| [53] |
J.Y. Han, M.S. Han, C.H. Tung, Biochim. Biophys. Acta 1830(2013) 5130-5135. DOI:10.1016/j.bbagen.2013.07.001 |
| [54] |
S. Onoe, T. Temma, Y. Shimizu, M. Ono, H. Saji, Cancer Med. 3(2014) 775-786. DOI:10.1002/cam4.2014.3.issue-4 |
| [55] |
S. Wu, Q.Z. Cao, X.L. Wang, K. Cheng, Z. Cheng, Chem. Commun. 50(2014) 8919-8922. DOI:10.1039/C4CC03296A |
| [56] |
Y.K. Kim, H.H. Ha, J.S. Lee, et al., J. Am. Chem. Soc. 132(2010) 576-579. DOI:10.1021/ja906862g |
| [57] |
W.J. Zhang, R.T.K. Kwok, Y.L. Chen, et al., Chem. Commun. 51(2015) 9022-9025. DOI:10.1039/C5CC02486B |
| [58] |
K.M. Robinson, M.S. Janes, M. Pehar, et al., Proc. Natl. Acad. Sci. U. S. A. 103(2006) 15038-15043. DOI:10.1073/pnas.0601945103 |
| [59] |
T. Kirkegaard, M. Jäättelä, Biochim. Biophys. Acta 1793(2009) 746-754. DOI:10.1016/j.bbamcr.2008.09.008 |
| [60] |
T.M. Cox, M.B. Cachón-González, J. Pathol. 226(2012) 241-254. DOI:10.1002/path.v226.2 |
| [61] |
A. Serrano-Puebla, P. Boya, Ann. N. Y. Acad. Sci. 1371(2016) 30-44. DOI:10.1111/nyas.2016.1371.issue-1 |
| [62] |
G. Kroemer, M. Jäättelä, Nat. Rev. Cancer 5(2005) 886-897. DOI:10.1038/nrc1738 |
| [63] |
M.E. Guicciardi, M. Leist, G.J. Gores, Oncogene 23(2004) 2881-2890. DOI:10.1038/sj.onc.1207512 |
| [64] |
H.B. Yu, Y. Xiao, L.J. Jin, J. Am. Chem. Soc. 134(2012) 17486-17489. DOI:10.1021/ja308967u |
| [65] |
A.L. Capodilupo, V. Vergaro, E. Fabiano, et al., J. Mater. Chem. B 3(2015) 3315-3323. DOI:10.1039/C4TB02116A |
| [66] |
M. Grzybowski, E. Glodkowska-Mrowka, V. Hugues, et al., Chem. Eur. J. 21(2015) 9101-9110. DOI:10.1002/chem.v21.25 |
| [67] |
R.T. Guan, H. Wang, Q. Zhang, J.Y. Wu, Y.P. Tian, Dyes Pigm. 136(2017) 473-479. DOI:10.1016/j.dyepig.2016.09.012 |
| [68] |
M.G. Tian, Y.M. Sun, L.F. Guo, et al., Sens. Actuators B 243(2017) 955-962. DOI:10.1016/j.snb.2016.12.082 |
| [69] |
C.S. Lim, S.T. Hong, S.S. Ryu, D.E. Kang, B.R. Cho, J. Chem Asian, 10(2015) 2240-2249. DOI:10.1002/asia.201500314 |
| [70] |
X.H. Tian, Y.Z. Zhu, M.Z. Zhang, et al., Dyes Pigm. 139(2017) 431-439. DOI:10.1016/j.dyepig.2016.12.052 |
| [71] |
S. Griesbeck, Z.L. Zhang, M. Gutmann, et al., Chem. Eur. J. 22(2016) 14701-14706. DOI:10.1002/chem.201602639 |
| [72] |
Q. Zhang, R.T. Guan, X.H. Tian, et al., RSC Adv. 7(2017) 20068-20075. DOI:10.1039/C7RA00380C |
| [73] |
P. Ning, J.C. Jiang, L.C. Li, et al., Biosens. Bioelectron. 77(2016) 921-927. DOI:10.1016/j.bios.2015.10.061 |
| [74] |
Y. Feng, D.X. Li, Q. Wang, et al., Sens. Actuators B 225(2016) 572-578. DOI:10.1016/j.snb.2015.11.081 |
| [75] |
L. Yuan, L. Wang, B.K. Agrawalla, et al., J. Am. Chem. Soc. 137(2015) 5930-5938. DOI:10.1021/jacs.5b00042 |
| [76] |
Q.L. Xu, C.H. Heo, J.A. Kim, et al., Anal. Chem. 88(2016) 6615-6620. DOI:10.1021/acs.analchem.6b01738 |
| [77] |
D.X. Li, Y. Feng, J.Z. Lin, et al., Sens. Actuators B 222(2016) 483-491. DOI:10.1016/j.snb.2015.08.098 |
| [78] |
D. Cheng, Y. Pan, L. Wang, et al., J. Am. Chem. Soc. 139(2017) 285-292. DOI:10.1021/jacs.6b10508 |
| [79] |
K. Zhang, W. Wu, Y.H. Li, et al., RSC Adv. 6(2016) 115298-115302. DOI:10.1039/C6RA21260C |
| [80] |
H.W. Liu, S. Xu, P. Wang, et al., Chem. Commun. 52(2016) 12330-12333. DOI:10.1039/C6CC05880A |
| [81] |
X.G. Yang, Y.B. Zhou, X.F. Zhang, et al., Chem. Commun. 52(2016) 10289-10292. DOI:10.1039/C6CC05254A |
| [82] |
Q. Wang, W.J. Wang, S.Q. Li, et al., Dyes Pigm. 134(2016) 297-305. DOI:10.1016/j.dyepig.2016.07.030 |
| [83] |
W.F. Niu, L. Guo, Y.Y. Li, et al., Anal. Chem. 88(2016) 1908-1914. DOI:10.1021/acs.analchem.5b04329 |
| [84] |
F.F. Meng, Y. Liu, X.Q. Yu, W.Y. Lin, New J. Chem. 40(2016) 7399-7406. DOI:10.1039/C6NJ00330C |
| [85] |
L.Y. Zhou, D.Q. Lu, Q.Q. Wang, et al., Biosens. Bioelectron. 91(2017) 699-705. DOI:10.1016/j.bios.2016.12.055 |
| [86] |
G. Zhang, Y.M. Sun, X.Q. He, et al., Anal. Chem. 87(2015) 12088-12095. DOI:10.1021/acs.analchem.5b02807 |
| [87] |
M. Zhao, Y.Z. Zhu, J. Su, et al., J. Mater. Chem. B 4(2016) 5907-5912. DOI:10.1039/C6TB01240J |
| [88] |
Y. Baek, S.J. Park, X. Zhou, et al., Biosens. Bioelectron. 86(2016) 885-891. DOI:10.1016/j.bios.2016.07.026 |
| [89] |
A.R. Sarkar, C.H. Heo, L. Xu, et al., Chem. Sci. 7(2016) 766-773. DOI:10.1039/C5SC03708E |
| [90] |
B.L. Wang, X.F. Zhang, C. Wang, et al., Analyst 140(2015) 5488-5494. DOI:10.1039/C5AN01063B |
| [91] |
C.S. Lim, E.S. Kim, J. Kim, et al., Sci. Rep. 5(2015) 18521-18531. |
| [92] |
W.B. Hu, T.C. He, R.C. Jiang, et al., Chem. Commun. 53(2017) 1680-1683. DOI:10.1039/C6CC09473B |
| [93] |
W. Zhang, X. Wang, P. Li, et al., Anal. Chem. 89(2017) 6840-6845. DOI:10.1021/acs.analchem.7b01290 |
| [94] |
R. Chennoufi, H. Bougherara, N. Gagey-Eilstein, et al., Sci. Rep. 6(2016) 21458-21469. DOI:10.1038/srep21458 |
| [95] |
H. Chen, Y.H. Tang, H.M. Shang, et al., J. Mater. Chem. B 5(2017) 2436-2444. DOI:10.1039/C7TB00174F |
| [96] |
H.J. Lee, C.W. Cho, H. Seo, et al., Chem. Commun. 52(2016) 124-127. DOI:10.1039/C5CC06976A |
| [97] |
B.C. Zhu, P. Li, W. Shu, et al., Anal. Chem. 88(2016) 12532-12538. DOI:10.1021/acs.analchem.6b04392 |
| [98] |
Y. Liu, F.F. Meng, L.W. He, K.Y. Liu, W.Y. Lin, Chem. Commun. 52(2016) 7016-7019. DOI:10.1039/C6CC02368A |
| [99] |
W.Q. Feng, Z.Q. Mao, L.Z. Liu, Z.H. Liu, Talanta 167(2017) 134-142. DOI:10.1016/j.talanta.2017.02.012 |
| [100] |
J.L. Fan, Z.C. Han, Y. Kang, X.J. Peng, Sci. Rep. 6(2016) 19562-19569. DOI:10.1038/srep19562 |
| [101] |
L.Y. Zhou, Y.C. Liu, S.Q. Hu, et al., Tetrahedron 72(2016) 4637-4642. DOI:10.1016/j.tet.2016.06.038 |
| [102] |
S.Y. Chen, M. Zhao, J. Su, et al., Dyes Pigm. 136(2017) 807-816. DOI:10.1016/j.dyepig.2016.09.020 |
| [103] |
W.F. Luo, H.E. Jiang, X.L. Tang, W.S. Liu, J. Mater. Chem. B 5(2017) 4768-4773. DOI:10.1039/C7TB00838D |
| [104] |
J.C. Jiang, X.H. Tian, C.Z. Xu, et al., Chem. Commun. 53(2017) 3645-3648. DOI:10.1039/C7CC00752C |
| [105] |
J.X. Huang, N. Li, Q.Q. Wang, Y.Q. Gu, P. Wang, Sens. Actuators B 246(2017) 833-839. DOI:10.1016/j.snb.2017.02.158 |
 2017, Vol. 28
2017, Vol. 28