b School of Biomedical Sciences, The University of Queensland, Brisbane, QLD 4072, Australia;
c Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455, USA
In the past several decades, with their ability to form various types of nanoparticles, amphiphilic block copolymers have been driving investigators' attention. These copolymers displayed great potential in drug delivery applications [1-3]. These materials are generally comprised of both hydrophobic polymer blocks and hydrophilic blocks. The resulting copolymers will display different affinities for an aqueous solvent. To date, a large number of amphiphilic block copolymers with different block combinations and block chain lengths have been synthesized [4, 5]. Bearing both hydrophobic and hydrophilic blocks within same polymer chain, amphiphilic copolymers can self-assemble and form different types of nanoparticular structures in an aqueous medium [6-8]. According to literature reports, the nanoparticular structures that amphiphilic block copolymer could form included polymersomes or micelles [3, 9, 10]. Generally, two distinct components can commonly be found in these nanoparticles: a) an inner hydrophobic region where hydrophobic compounds are readily incorporated with high loading capacity [11]; b) a hydrophilic polymer layer as the outer layer of nanoparticles to improve the stability of nanoparticles and circulation time without the need for additional stabilizers [12]. Accordingly, these nanoparticles seem to be potential carriers for poorly water soluble drugs [13, 14].
PCEC copolymers are among the most popular amphiphilic triblock copolymers [15-17]. They are comprised of FDA approved biodegradable hydrophobic PCL blocks and a hydrophilic biocompatible PEG block. In aqueous media, the PCEC copolymers can selfassemble and form micelles or polymersomes. According to literature reports, the mass or volume fraction of the PEG component of the PCEC copolymer (f) is a decisive parameter to affect the morphology of the self-assembled system [3, 18, 19]. As a general rule, polymersomes are favoured when of PEG (PEG) is between 10% and 40%. When fPEG falls between 45% and 55%, it is likely to form cylindrical micelles, and when fPEG = 55%-70%, the structure of spherical micelles will be predominant.
Here, a series of amphiphilic PCEC triblock copolymers were synthesized with various mass ratios of PCL/PEG, which were characterized by 1H NMR, FT-IR, and GPC. Then, paclitaxel (PTX) is utilized as a model hydrophobic anticancer drug to be encapsulated [20]. Polymeric micelles and polymersomes loaded with PTX were fabricated from PCEC copolymer with appropriate ratio of hydrophilic blocks to hydrophobic blocks. Morphology and structure of the nanoparticles were characterized by TEM and DLS, and drug loading content and encapsulation efficiency were characterized by HPLC. In vitro cellular uptake of the nanoparticles was evaluated by confocal laser scanning microscopy (CLSM). Based on the high drug-loading efficiency and cellular uptake efficiency of these nanoparticles, PTX-loaded polymeric micelles and polymersomes showed promising potential as drug delivery vehicles to solubilize hydrophobic drugs. PEG (Mn = 4000, 8000, and 12, 000), ε-caprolactone (ε-CL), and stannous octoate (Sn (Oct)2) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Paclitaxel (PTX, >99.5% purity) was purchased from Sichuan Jiufeng Natural Pharmaceutical Co., Ltd. (Sichuan, China). Fetal bovine serum and cell culture media were bought from Invitrogen (Grand Island, NY, U.S.A.). All other chemicals were of analytical grade and were used without further purification. A series of amphiphilic PCEC triblock copolymers were synthesized by a ringopening polymerization of ε-CL in the presence of HO-PEG-OH with Sn(Oct)2 as catalyst [21]. In brief, certain amounts ofε-CL and HO-PEG-OH were introduced into a dry polymerization tube under a nitrogen atmosphere, and several drops of Sn(Oct)2 was added. Then the polymerization tube was immersed in an oil bath at 120 ℃ with magnetic stirring for 48 h. After cooling to room temperature, the crude copolymers were dissolved in methylene dichloride and precipitated in an excess amount of cold methanol to remove the un-reacted ε-CL monomers and HO-PEG-OH homopolymers. The purification process was repeated twice more. The purified precipitates were vacuum-dried to constant weight and kept in a desiccator.
In current work, the triblock copolymers were named as PCLX-PEGY-PCLX and listed in Table 1, where X represented the number average molecular weight (Mn) of PCL block and Y represented Mn of PEG block, respectively. Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet IS10 FTIR spectrophotometer (Thermo Scientific, U.S.A.) for identifying the chemical structure of PCEC copolymers. The molecular weight and chemical composition of the copolymers were characterized by 1H NMR spectra using CDCl3 as solvent and recorded by an AVANCE III spectrometer (Faellanden, Switzerland) using tetramethylsilane as an internal standard. GPC (Agilent 110 high-performance liquid chromatography, U.S.A.) was utilized to determine the Mw, Mn, and molecular weight distribution of these synthesized copolymers using THF as the eluent at a rate of 1 mL/min and polystyrene as the calibration standard. The thermal behaviours of the different PCEC copolymers were investigated by differential scanning calorimeter (DSC, Q2000, TA Instruments, U.S.A.). Approximately 5.0 mg of dry copolymer specimen was placed in a standard aluminum pan and sealed. The samples were heated from 0 ℃ to 80 ℃ at a rate of 5 ℃/min under nitrogen atmosphere, and then cooled to 0 ℃ at the same rate.
|
|
Table 1 The characterization results of the PCEC copolymers synthesized. |
PTX-loaded polymeric nanoparticles were prepared by the thinfilm hydration and ultrasonic dispersion method [22, 23]. Briefly, 100 mg of PCEC copolymers and 11.11 mg of PTX were dissolved in 10 mL of methylene dichloride in an eggplant-shaped flask. The solvent was removed using an Eyela N-1001 rotary evaporator (Rikakikai Co., Ltd., Japan) and keeping temperature at 35 ℃ for about 1 h. Residual methylene dichloride remaining in the formed thin-film layer was evaporated and removed overnight in vacuum condition. The thin-film layer was incubated in water bath at 65 ℃ and the samples formed gel-like and displayed transparent. Deionized water was added to the resultant thin film as an aqueous phase and kept in hydration for 5 h at 65 ℃. Then the mixture was put in an ice bath and sonicated using a VCX-130-PBsonicator (Sonics & Material Inc., U.S.A.) at the output of 40 kW for an appropriate time to obtain a clear suspension. The particle suspension was frozen and lyophilized to obtain the product. Blank PCEC nanoparticles were prepared following the similar procedures without the addition of PTX. And the fluorescent nanoparticles were prepared according to the above method while using Nile Red instead. In this article, a series of blank and paclitaxel-loaded PCEC nanoparticles were denoted as NPsX and PTX-NPsX, respectively, where X represented the code of PCEC copolymer used in the first column of Table 1.
The morphological characterizations of the nanoparticles were investigated by Tecnai-F20 TEM (FEI Co., the Netherlands). One drop of the suspension was deposited onto a carbon film-coated copper grid (400 mesh), and then removed the excess solution with a strip of filter paper. The sample was dried at room temperature for the TEM observation. The particle size and distribution, and zeta potential of blank and PTX-loaded nanoparticles were measured by a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) after equilibration for 2 min. Each particle suspension was diluted to the appropriate mass concentration with ultrapure water before measurement. The amount of PTX loaded in the lyophilized particles was measured by HPLC (Agilent LC 1100; Agilent Technologies, U.S.A.) [24]. Briefly, 5 mg of PTX-NPs were dissolved in 2.5 mL of acetonitrile under vigorous vortexing, and then diluted with ultrapure water to 5 mL. The samples were analyzed by HPLC, which was equipped with a revers ε-phase column (Symetry, 150 mm × 4.6 mm, 5 μm) and with a UV detector at 40 ℃. A mixture solution of acetonitrile and water at 50/50 (v/v) was used as the mobile phase of HPLC test. The experiment was carried out at a flow rate of 1.0 mL/min and the column effluent was measured at 227 nm with a UV-vis detector. The drug-loading (DL) and encapsulation efficiency (EE) were calculated according to the following formula:
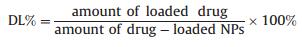
|
(1) |

|
(2) |
In the cellular uptake experiment, Nile Red was used as a model fluorescent probe loaded in nanoparticles for the qualitative investigation on cellular uptake by EMT-6 cells. EMT-6 cells were cultured in RPMI 1640 media supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin under a humidified atmosphere of 95% air and 5% CO2 at 37 ℃. Prior to the experiment, cells were cultured till reached 80% confluence. The EMT-6 cells were seeded into a 35 mm culture dish with a density of 1.0 ×105 and incubated in 1 mL of RPMI 1640 containing 10% FBS for 24 h at 37 ℃ to let cell adhesion and spread. Then, the medium was removed and replaced with the growth medium with Nile Red-loaded nanoparticles at the concentration of 0.250 mg/mL for 4 h and 24 h, respectively. The cells were then washed with PBS buffer three times, fixed by immunostaining fix solution at 25 ℃ for 10 min. Subsequently, immunostaining wash buffer was used to wash the cells three times, and the cells were stained with Actin-Tracker Green for 1 h. After washing with immunostaining wash buffer, DAPI solution was added to stain cell nucleus for 10 min at room temperature. PBS buffer was used to wash the stained cells three times thus removing free DAPI. The cells were observed by confocal laser scanning microscopy (LSM 710, Carl Zeiss Meditec AG).
A series of amphiphilic PCEC triblock copolymers were successfully prepared with various weight ratios of PCL/PEG by ring-opening polymerization of HO-PEG-OH and ε-CL monomer with a trace amount of Sn(Oct)2 as catalyst. Triblock copolymers with different chain lengths were synthesized by changing the PEG precursors with different molecular weight and controlling the ratio of ε-CL/PEG. Table 1 gave the details on the feed molar ratio and targeted molecular weight of the obtained copolymers. The results of FTIR, 1H NMR and GPC further confirmed the preparation and structure of the PCEC triblock copolymers by altering the feeding ratio.
FTIR spectra of PCL and PEG8000 homopolymers, PCL4000-PEG8000-PCL4000 and PCL8000-PEG8000-PCL8000 triblock copolymers are shown in Fig. S1 (Supporting information). The PCL4000-PEG8000-PCL4000 and PCL8000-PEG8000-PCL8000 represent the obtained copolymers with the ε-CL/PEG weight ratios of 1:1 and 2:1, respectively. The presence of a strong peak appeared at 1725 cm-1 was attributed to the characteristic absorption of the C=O stretching vibrations of the ester carbonyl group of the CL unit. The absorption bands at 2944 cm-1 and 2868 cm-1 corresponded to the C-H stretching vibrations of PCL and PEG, respectively. Moreover, the 3436 cm-1 absorption band was assigned to be the terminal hydroxyl groups from the PCEC copolymers and PEG homopolymers. Finally, the absorption peak at 1107 cm-1 showed that there was a characteristic absorption of C-O-C stretching vibration of repeated -OCH2CH2 units of PEG. It was clear that PCL-PEG-PCL copolymers exhibited characteristic peaks of both PCL and PEG components. These signals indicated that the PCEC triblock copolymers were synthesized [25].
Fig. S2 (Supporting information) is the 1H NMR spectrum of the synthesized PCEC copolymers. The sharp single peak at 3.63 ppm arose from the methylene protons of the oxyethylene unit from the PEG block. In addition, the other four peaks at δ 1.40, 1.64, 2.30, and 4.04 were assigned to the protons from methylene groups of the oxycarboxy-1, 5-pentamethylene unit of the PCL blocks [26, 27]. All of the six synthesized copolymers have the similar peaks in 1H NMR spectrum. The number average molecular weight (Mn) of PCEC copolymers and PCL/PEG ratio in these copolymers were obtained based on the equation Mn = (1 + R) × MPEG, where R is the ratio of integration area of PCL to PEG and MPEG is the number average molecular weight of PEG [28]. The ratio was calculated by comparing the integration area of the peak at δ 4.04 (from the PCL block) to that of the peak at δ 3.63 (from the PEG block). In each GPC chromatogram, unimodal curve indicated that the polymers were free of impurities. All of the products had relatively higher polydispersity indexes of molecular weight. Mn and PCL/PEG block ratios calculated from 1H NMR spectra and GPC analysis were listed in Table 1. According to Table 1, the results from NMR spectra agree well with the theoretical values calculated from the feeding ratio.
The thermal properties of the synthesized copolymers, PCL and PEG homopolymers with different molecular weights was evaluated by DSC, which was showed as Fig. S3 (Supporting information). Fig. S3A displays the heating process of the series of PCEC copolymers, in which the PCL block and PEG block clearly demonstrated two endothermic peaks. The melting transition temperatures of PCL and PEG homopolymers were higher than their segments in the copolymers, which can be illustrated as the interaction between the melting of PCL block and PEG segment. During the cooling process (Fig. S3B), the copolymers with the PCL/ PEG/PCL ratios of 1:2:1 were observed two exothermic peaks at 10-40 ℃, and the copolymers with the PCL/PEG/PCL ratios of 1:1:1 were observed one exothermic peak at 10-40 ℃. It is probably because when the length of PCL block is half of that of PEG block, the crystallization temperature of PEG block is obviously higher than PCL block, then forming two peaks. But when the molecular weight of PEG block is close to that of PCL block, their peaks overlaps and became an exothermic peak. The conclusion that crystallization temperature is positive correlation to the block length can also be seen from that the crystallization temperature of PEG is higher when its block length is longer. The crystallization temperature of PCL and PEG segment in the copolymers was both lower than PEG homopolymers. We can conclude that crystallization of PEG and PCL block is not better than the homopolymers, there might be some compatibility. According to the above results, the polymers obtained were PCEC copolymers, but not the mixture of PEG and PCL homopolymers.
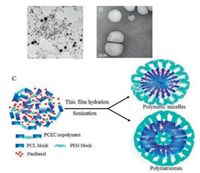
Due to their amphiphilic properties, amphiphilic triblock copolymers have been known to form nanoparticles with coreshell structure in aqueous media. In the current work, a series of blank or drug loaded PCEC nanoparticles were prepared by thinfilm hydration and ultrasonic dispersion method. The morphology of the prepared blank nanoparticles was characterized by TEM. TEM results (Fig. 1) showed that most of the nanoparticles demonstrated a discrete spherical shape with uniform size. It can be seen that, when the PCL/PEG block ratio was 1 (e.g., PCL4000-PEG8000-PCL4000) and 2 (e.g., PCL8000-PEG8000-PCL8000), the PCEC copolymers can form polymeric micelles (Fig. 1A) and polymersomes (Fig. 1B), respectively. It is in agreement with the literature reports. The PCEC polymeric micelles demonstrated an apparent corε-shell structure. The inner and hydrophobic part is the PCL core, which was surrounded by a "mushroom" hydrophilic PEG corona. Both terminals of the PEG blocks are anchored at the core/ shell interfaces, which makes the surface closed and consequently limits the possibility of opsonization. Hydrophobic drugs, such as PTX, can be easily encapsulated into the hydrophobic core of PCL segment of the polymeric micelles via hydrophobic interactions. The PCEC polymersomes demonstrated a different kind of morphology with hollow structures, compared with that of polymeric micelles. A bilayered lamellar structure is typical for the wall of the polymersomes. The thick membranes of the polymersomes allow for loading of hydrophobic compounds (e.g., PTX) into polymersome membranes. The schematic structure of the polymeric micelles and polymersomes are represented in Fig. 1C.
|
Download:
|
| Fig. 1. TEM micrograph of (A) PCL4000-PEG8000-PCL4000 polymeric micelles (scale bar = 200 nm) and (B) PCL8000-PEG8000-PCL8000 polymersomes (scale bar = 100 nm). (C) The illustration of the formation of PTX-loaded polymeric micelles and polymersomes, respectively. | |
The size and distribution, zeta potential of blank or drug loaded nanoparticles were measured by DLS in aqueous media. The characterization results are summarized in Table 2. It can be seen that these blank nanoparticles have uniform diameters less than 200 nm except NPs6. For two kinds of nanoparticles with a fixed PEG length, the size increased when increasing the length of the hydrophobic PCL block. And for a series of nanoparticles with fixed ratios of PCL/PEG block, increasing the molecular weight of the triblock copolymers would increase the diameters of nanoparticles. After drug loading, the size of nanoparticles increased. It was reasonable that the volume of the nanoparticles wouldincreaseafter incorporating PTX into the hydrophobic PCL cores. Remarkably, PTXNPs1 has the largest size, polydispersity and the highest zeta potential. The reason may be that the hydrophobic PCL block of NPs1 is too short to load so much PTX, and the excess PTX present in the solution is crystalline form. Zeta potential reflects the surface charge of nanoparticles. Nanoparticles with high surface charge generally repel each other strongly and result in a stable dispersion. From Table 2, it is found that all the nanoparticles have negative zeta potentials. After drug loading, the result from zeta potential test did not show obvious changes.
|
|
Table 2 Physicochemical characterization of blank or PTX-loaded PCEC nanoparticles. |
Drug loading capacity and encapsulation efficiency are critical factors for nanoparticles. High drug loading capacity and encapsulation efficiency are always demanded for a good drugdelivery system. Table 2 lists the drug loading capacity and encapsulation efficiency of PCEC polymeric nanoparticles. Both polymeric micelles and polymersomes demonstrated high level of drug-loading efficiency owing to the strong hydrophobic interaction. From Table 2, for two series of PTX-loaded nanoparticles with fixed PEG length, the PTX loading efficiency of polymersomes is higher than that of polymeric micelles. The above results showed that nanoparticles formed by copolymers with longer PCL chains have higher drug-loading efficiency.
To investigate the internalization of the nanoparticles into cells, cellular uptake experiments of the polymeric micelles and polymersomes were performed using EMT-6 breast cancer cells. Hydrophobic Nile Red (water solubility less than 1 μg/mL) was chosen as the lipophilic stain in this study. The internalization of Nile Red-loaded polymeric micelles (Nile Red-NPs2) or polymersomes (Nile Red-NPs5) incubated for 4 h and 24 h were visualized by CLSM. To visualize subcellular trafficking of the nanoparticles, we stained the nucleus with DAPI (blue), and actin with ActinTracker Green (green). The fluorescence from the polymeric micelles and polymersomes internalized in EMT-6 cells were shown in Fig. 2. Row 1 and 2 displayed the cells incubated with micelles for 4 h and 24 h, and row 3 and 4 show the polymersomes for 4 h and 24 h, respectively. The figures showed that the nucleus (blue, DAPI) was circumvented by internalized Nile Red loaded nanoparticles (red) in cytoplasm. Concurrently, the Actin-Tracker Green labeled cytoskeleton of EMT-6 cells could be obviously distinguished. Therefore, we confirmed the cellular uptake of nanoparticles qualitatively by CLSM. As shown in Fig. 2, the red fluorescence of nanoparticles incubated for 24 h (row 2 and 4) is much brighter than that for 4 h (row 1 and 3) in the cytoplasm. And the red fluorescence of micelles in the cytoplasm (row 1) is not as bright as that of the polymersomes (row 3) after 4 h incubation. Nevertheless, after incubating for 24 h, there was no significant difference of intracellular red fluorescence intensity between the micelles and the polymersomes. The findings clearly illustrated that both polymeric micelles and polymersomes have high cellular uptake efficiency. Depending on shape and particle size, nanoparticles could utilize a nonspecific endocytosis pathway to internalize through lipid-bilayered cellular membranes. Accordingly, these polymeric micelles and polymersomes might be used in drug delivery systems.

|
Download:
|
| Fig. 2. CLSM images EMT6 cells after incubation with Nile Red-loaded polymeric micelles (Nile Red-NPs2) and polymersomes (Nile Red-NPs5) for 4 and 24 h. Row 1 and 3, nanoparticles were incubated for 4 h. Row 2 and 4, nanoparticles were incubated for 24 h. In row 1 and 2, micelles were used while in row 3 and 4, polymersomes were used. Abbreviations: FITC, fluorescein isothiocyanate; CLSM, confocal laser scanning microscopy; Scale bar = 20μm. | |
In present work, a series of amphiphilic PCEC triblock copolymers were synthesized with various weight ratios of PCL/ PEG by ring-opening polymerization of PEG and ε-CL monomer with Sn(Oct)2 as catalyst. The results by FT-IR, 1H NMR, GPC and DSC indicated that PCEC copolymers were synthesized successfully. The chemical synthesis process can be controlled to obtain the desirable chain length of the copolymers and PCL/PEG block ratio. In our work, both series of blank or drug-loaded PCEC polymeric micelles and polymersomes were fabricated by the thin-film hydration and ultrasonic dispersion method. As the ratio of PCL/ PEG block weight was less than 2, the PCEC copolymers formed polymeric micelles and polymersomes, respectively, which was in good agreement with literature reports. TEM image confirmed that most of the nanoparticles have a discrete spherical shape and a uniform size. The particle size and distribution, and zeta potential of blank and drug-loaded nanoparticles were measured by DLS. These results indicated that the particle sizes of nanoparticles were dependent on the lengths of PCL and PEG blocks. All the nanoparticles have negative zeta potentials. The results of drug loading capacity and cellular uptake experiment showed that both polymeric micelles and polymersomes have high drug-loading efficiency and cellular uptake efficiency. Based on above findings, we conclude that PTX-loaded polymeric micelles and polymersomes may display great potential as the drug delivery vehicles for solubilization of hydrophobic drugs.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 81571793, 81671806 and 51373199), CAMS Innovation Fund for Medical Sciences, Tianjin Municipal Natural Science Foundation (No. 15JCZDJC38300) and Science and Technology Support Program of Tianjin (No. 15RCGFSY00146).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.020.
| [1] |
C.S. Patrickios, T.K. Georgiou, Curr. Opin. Colloid Interface Sci. 8(2003) 76-85. DOI:10.1016/S1359-0294(03)00005-0 |
| [2] |
H. Li, X. Li, C. Zhang, et al., J. Biomed. Nanotechnol. 12(2016) 1258-1269. DOI:10.1166/jbn.2016.2247 |
| [3] |
K. Letchford, H. Burt, Eur. J. Pharm. Biopharm. 65(2007) 259-269. DOI:10.1016/j.ejpb.2006.11.009 |
| [4] |
H. Xu, X.L. Li, H. Kong, et al., J. Biomed. Nanotechnol. 12(2016) 1699-1707. DOI:10.1166/jbn.2016.2293 |
| [5] |
L. Lv, K.F. Qiu, X.X. Yu, et al., J. Biomed. Nanotechnol. 12(2016) 973-985. DOI:10.1166/jbn.2016.2231 |
| [6] |
Y.Y. Song, B. Lou, J. Cheng, et al., J. Biomed. Nanotechnol. 12(2016) 2083-2096. DOI:10.1166/jbn.2016.2314 |
| [7] |
L.H. Zhang, Z. Chen, H. Wang, et al., RSC Adv. 6(2016) 54727-54737. DOI:10.1039/C6RA04687H |
| [8] |
Q. Yang, X.J. Yu, T. Li, et al., J. Biomed. Nanotechnol. 16(2016) 6734-6740. |
| [9] |
H.K. Cho, I.W. Cheong, J.M. Lee, Korean J. Chem. Eng. 27(2010) 731-740. DOI:10.1007/s11814-010-0216-5 |
| [10] |
D.H. Levine, P.P. Ghoroghchian, J. Freudenberg, et al., Methods 46(2008) 25-32. DOI:10.1016/j.ymeth.2008.05.006 |
| [11] |
Z. Dai, Y. Tu, L. Zhu, J. Biomed. Nanotechnol. 12(2016) 1199-1210. DOI:10.1166/jbn.2016.2249 |
| [12] |
Y.P. Wu, Z.Y. Wang, G. Liu, et al., J. Biomed. Nanotechnol. 11(2015) 1247-1260. DOI:10.1166/jbn.2015.2068 |
| [13] |
N. Nishiyama, K. Kataoka, Pharmacol. Ther. 112(2006) 630-648. DOI:10.1016/j.pharmthera.2006.05.006 |
| [14] |
Y. Hu, J.W. Xie, Y.W. Tong, C.H. Wang, J. Control Release 118(2007) 7-17. DOI:10.1016/j.jconrel.2006.11.028 |
| [15] |
M.L. Gou, L. Zheng, X.Y. Peng, et al., Int. J. Pharm. 375(2009) 170. DOI:10.1016/j.ijpharm.2009.04.007 |
| [16] |
Y.G. Chang, S. Shuai, W.D. Peng, et al., J. Pharm. Sci. 98(2009) 4684-4694. DOI:10.1002/jps.21780 |
| [17] |
R.L. Feng, Z.M. Song, G.X. Zhai, Int. J. Nanomed. 7(2012) 4089-4098. |
| [18] |
Y.Y. Won, F.S. Bates, Science 283(1999) 960-963. DOI:10.1126/science.283.5404.960 |
| [19] |
J.S. Lee, J. Feijen, J. Control Release 161(2011) 473-483. |
| [20] |
Y.L. Wang, H. Zhao, J.R. Peng, et al., J. Biomed. Nanotechnol. 12(2016) 2097-2111. DOI:10.1166/jbn.2016.2319 |
| [21] |
L.H. Zhang, Y.N. He, G.L. Ma, C.X. Song, H.F. Sun, Nanomedicine 8(2012) 925-934. DOI:10.1016/j.nano.2011.11.005 |
| [22] |
H.Y. Yu, Z.H. Tang, M.Q. Li, et al., J. Biomed. Nanotechnol. 12(2016) 69-78. DOI:10.1166/jbn.2016.2152 |
| [23] |
L.H. Zhang, D.W. Zhu, X. Dong, et al., Int. J. Nanomedicine 10(2015) 2101-2114. |
| [24] |
E. Bernabeu, L. Gonzalez, M.J. Legaspi, M.A. Moretton, D.A. Chiappetta, J. Biomed. Nanotechnol. 15(2015) 160-170. |
| [25] |
K. Tanaka, T. Kanazawa, Y. Shibata, et al., Int. J. Pharm. 396(2010) 229-238. DOI:10.1016/j.ijpharm.2010.06.028 |
| [26] |
B. Xu, J.F. Yuan, T. Ding, Q.Y. Gao, Polym. Bull. 64(2010) 537-551. DOI:10.1007/s00289-009-0157-5 |
| [27] |
F. Lu, L. Lei, Y.Y. Shen, et al., Int. J. Pharm. 419(2011) 77-84. DOI:10.1016/j.ijpharm.2011.07.020 |
| [28] |
L.H. Piao, Z.L. Dai, M.X. Deng, X.S. Chen, X.B. Jing, Polymer 44(2003) 2025-2031. DOI:10.1016/S0032-3861(03)00087-9 |
 2017, Vol. 28
2017, Vol. 28