Titanium (Ti) and its alloys have been widely used as implant materials in bio-medicine due to their high biocompatibility, minimal toxicity, good mechanical resistance and outstanding ability to bond to new bones [1-4]. However, in the clinical practice, there are still some problems unsatisfied, which make titanium surface modification a hot topic in recent years [5]. Thus, various techniques have been applied to increase the chemical and morphological properties of titanium, including acid etch technique, anodization, titanium plasma spraying as well as bioactive/ ceramic coating [6-8]. Particularly, calcium-phosphate ceramic nano-coatings, such as hydroxyapatite tricalcium phosphate, biphasic calcium phosphate and calcium pyrophosphate, are classic bone substitute materials in bone regeneration because of their similarity of chemical properties and dissolution characteristics to bone tissue [2, 9-11]. Among these, tricalcium phosphate coating is relatively balanced between material absorption and new bone formation and shows the best osteoinductive capacity.
Nanomaterials have become a rising issue in tissue engineering and regenerative medicine [12-14]. Due to the fact that they may provide a structure that simulates the nature bone, nanomaterials have been a hot issue in bone regeneration field for years [15-19].
Nanoflowers describe a 3D structure which in microscopic view resembles flowers. Their high surface-to-volume ratio which may enhance the efficiency of surface reactions has inspired the interests of scientists [20]. The applications of these nanoflowers are now mainly concentrated in enzyme mimetics, catalysis, dye adsorption, drug delivery systems as well as bone tissue engineering [20-22]. As for calcium-phosphate nanoflowers, several investigations have also been raised. Thereinto, Wang and co-workers described an easy-made chitosan/calcium pyrophosphate hybrid nanoflower [23]. The chitosan/calcium pyrophosphate hybrid nanoflowers were composed of nanosheets and had a porous center.
However, little effort has been paid to discover whether calcium-phosphate nanoflowers could be a promising coating material on the titanium surface. The nanosheets may simulate the bone structure and the flower-like form may provide a better surface-to-volume ratio. Thus, this biocoating may provide a chemical and morphological modification to the titanium surface at the same time.
Therefore, in our present study, we investigated a potential calcium-phosphate flower-like nanocoating on titanium surface. The cytotoxicity of nanoflowers, the morphology of calciumphosphate flower-like coating and their surface wettability are herein discussed.
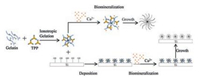
The preparation process of calcium-phosphate nanoflowers and their coatings on titanium surface were illustrated in Fig. 1.
|
Download:
|
| Fig. 1. llustration of the preparation process of calcium-phosphate nanoflowers and their coating on the titanium surface. | |
We first synthesized the calcium phosphate nanoflowers. Three different aqueous solutions were prepared: 2 mg/mL aqueous gelatin, 100 mg/mL sodium tripolyphosphate (TPP) solution, and 11.1 mg/mL aqueous CaCl2. Subsequently, 2 mL of TPP solution (100 mg/mL) was gradually added into 5 mL of gelatin solution (2mg/mL) upon stirring for 10min at room temperature (25 ℃). 7mL of CaCl2 solution (11.1mg/mL) was then slowlyadded into the mixture drop by drop. The liquid mixture was stirred at room temperature for further 20min. The mixture was then centrifuged at 1200rpm for 5min, rinsed in ultrapure water and redispersed. Three cycles after centrifugation/water rinsing/redispersion, the nanoflowers were obtained and lyophilized to keep its morphology.
The principle of synthesis calcium-phosphate nanoflowers was same as organic-inorganic hybrid nanoflowers. Gelatin and tripolyphosphate, which could be positively and negatively charged, respectively, were synthesized into a nanocomplex upon the addition of tripolyphosphate solution to gelatin through ionotropic gelation. The gelatin-tripolyphosphate nanocomplexes may serve as a framework for the formation of nano-petals. Subsequently, CaCl2 solutionwas added tothe mixturetooffer Ca2+ ion and precipitate the growth of the nanoflowers.
Similar to the preparation of nanoflowers, the calciumphosphate flowers were coated onto the titanium surface in a simple approach. In this study, we used commercially pure titanium (Ti) discs with a diameter of 14mm and thickness of 1mm. The discs were wet grounded with #600, #800, and #1200 grits of sandpaper sequentially and then ultrasonically degreased in pure acetone. Ethanol and ultrapure water were used between each step. The alkali treatment was performed by immersing polished samples into 5mol/L NaOH solution at 60 ℃. After 24h, the discs were ultrasonically washed with ultrapure water and dried at 40 ℃ for another 24h. Finally, they were heated to 600 ℃ with a rate of 5 ℃/min, kept at a desired temperature for 1h, and then allowed to cool to room temperature for future use [24, 25]. Once the titanium discs were alkali treated, a mixture of gelatin and tripolyphosphate was first prepared by adding tripolyphosphate solution 2mL into 5mL of aqueous gelatinwhile stirring. The alkali treated Ti discs were then soaked in the mixture for 5min before 7mL CaCl2 solution was added to the mixture. Afterwards, these samples were washed with ultrapure water and air dried at room temperature after laying aside for 30min.
As presented in Fig. 1, the nanoflowercoatings were coatedonto the titanium surface mainly through ionotropic and chemical bonding. After alkali treatment, the titanium surface was hydroxylated and well roughed. The gelatin-tripolyphosphate nanocomplexes and the hydroxylated titanium surface may come into a chemical bonding and deposit onto the surface. Afterwards, Ca2+ ions were added to the system through the addition of CaCl2 solution to the mixture to participate in the growth of the flower coating.
We then looked in to the characteristic of the materials. The surface morphologies of the materials were observed with a field emission scanning electron microscope (FE-SEM, Inspect F) operated at 20.00kV which had a digital camera (MEGA VIEW-II DOCU). In addition, the atomic and molecular structures of the materials were investigated by energy dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD). XRD analysis was performed with an X'Pert Pro X-ray diffractometer (Philips) with Cu Kα radiation (λ=1.5406Aº) with 2θ intervals from 5º to 80º [26]. In order to examine the surface wettability, the contact angle was measured using the sessile drop method by a surface goniometer equipped with image recording software [27].
Moreover, the cytotoxicity measurement was carried by Cell Counting Kit-8 (CCK-8) assay. Primary osteoblast (OB) cell was released from three-days-old SD rats' calvarias [28]. The cells were seeded in a medium consisting of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin and streptomycin, and then cultured at 37 ℃ in 5% CO2. Once the confluence of cells reached 80%, OB cells could be used for further studies. They were seeded into 96-well plates at a density of 104/well with 200μL of media and analyzed with CCK-8 kit after 24h [29]. At the same time, OB cells were seeded at a density of 5 × 104/well with coated titanium discs and cultured at 37 ℃ in 5% CO2 in 24-well plates for 3 days. Afterwards, these samples were rinsed with phosphate buffered saline (PBS) solution, fixed in 2.5% glutaraldehyde for 2h at 4 ℃, dehydrated in a graded ethanol series (50%, 60%, 70%, 80%, 90%, 95% and 100%) and then finally observed with SEM [30].
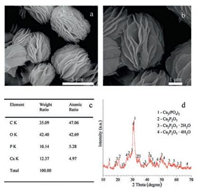
The surface morphologies of the calcium-phosphate nanoflowers were observed with SEM. As indicated in Fig. 2a and b, SEM of calcium-phosphate nanoflowers showed that the nanoflowers were about 5 μm in size and were composed of well-defined nanosheets within 100nm. These nanosheets were grown around the frame provided by gelatin-tripolyphosphate nanocomplexes and were assembled into a spherical morphology. Moreover, the center of these hybrid calcium-phosphate nanoflowers was porous, according to Wang and co-workers, which may give the nanoflowers a higher surface-to-volume ratio. These characteristics ensures the calcium-phosphate nanoflowers a promising specific surface area, which creates a suitable environment for osteoblasts' migration and proliferation and provides a number of potential binding sites for bioactive factors in further studies.
|
Download:
|
| Fig. 2. SEM electron micrographs, EDS analyses and XRD analyze of calciumphosphate nanoflowers: (a) (b) SEM micrographs of calcium-phosphate nanoflowers, (c) EDS examination (d) XRD analyze. | |
Energy dispersive spectroscopy (EDS) analysis was brought to analyze the elemental contents of nanoflowers (Fig. 2c). The result manifested the existence of element C, O, P and Ca. Moreover, the atomic ratio of P and Ca was about 1.06. In order to further characterize the crystal structure, X-ray diffraction was employed. As shown in Fig. 2d, XRD analysis proved the calcium-phosphate nanoflowers mainly contained of tricalcium phosphate (Ca3(PO4)2 PDF#29-0359), calcium pyrophosphate and its hydrate (Ca2P2O7 PDF#45-1061, Ca2P2O7·2H2O PDF#22-0536, Ca2P2O7·4H2O PDF#44-0762). The relative contents were 59%, 21%, 13.4%, 6.6%, respectively. Tricalcium phosphate (Ca3(PO4)2) and calcium pyrophosphate (Ca2P2O7) are members of calcium-phosphate ceramics which have been widely studied and used in biomedicine. Comparing with hydroxyapatite, a commonly studied ceramic, their absorption rate were balanced to new bone formation, which lead to better biocompatibility and osteoinductivity [31].
We used CCK-8 assay to estimate the cytotoxicity of these nanoflowers 1 day after culturing OB cells with the materials (Fig. 3). The result indicated that under the concentration of 10 μg/mL, the calcium-phosphate nanoflowers did not affect the OB cells' activity. However, once the concentration reached 20 μg/mL, a cytotoxicity could be noticed.

|
Download:
|
| Fig. 3. CCK-8 assay of osteoblasts cultured with calcium-phosphate nanoflowers for 1 day: (a) and (b). | |
Briefly, the calcium-phosphate nanoflowers mainly contained two kinds of well-studied calcium-phosphate ceramics, tricalcium phosphate and calcium pyrophosphate, whose osteoinductivity and biocompatibility were affirmative. Besides, their high specific surface area and low cytotoxicity endowed them with diverse applications in biomedicine.
The surface morphology of the calcium-phosphate nanoflowers coated Ti discs were also observed through SEM (Fig. 4a and b). In Fig. 4a and b, it was proved that a flower-like coating could be seen seated on the titanium surface. As shown in the SEM electron micrographs, the Ti surface did not affect the growth of nanoflowers. Same as calcium-phosphate nanoflowers, nanosheets under 100 nm had assembled into a flower-like morphology.

|
Download:
|
| Fig. 4. SEM electron micrographs and contact angle of calcium-phosphate flowerlike nanocoating on the titanium surface: (a) and (b) SEM of the coated titanium surface, (c) (d) and (e) contact angle of the surface, (f) SEM electron micrographs of the fixed osteoblasts seeded samples (black arrow: nanoflower coating, white arrow: osteoblasts). | |
Moreover, the contact angle was tested to measure the surface wettability of the novel coating. Surface wettability of biomaterials, as an important role in surface modification, determines the biological cascade of the biomaterial/host interface. In other words, it influences the cell spreading and adhesion in a great way [32, 33]. As shown in Fig. 4c-e, the contact angle of calciumphosphate nanoflowers coated titanium surface (c) and contact angle of titanium surface after alkali-treatment (d) were significantly different (P < 0.0001). The contact angles for coated group were 25.32 ± 1.781º (Fig. 4c), while in the alkali-treatment group, the contact angles were 54.37 ± 1.275º. This result supports that the flower-like coating consisting of calcium-phosphate nanosheets may provide a hydrophilic surface thus enhance cell affinity.
Cell adhesion proprieties were observed after 3 days culturing with OB cells. Fig. 4f shows representative SEM images of OB cells on the coated titanium surface. The cell (see white arrow) was observed flat besides the flower coating, and a decomposednanoflower could be noticed (see black arrow).
According to literatures, nanocoating and nanostructures had played an important role in titanium surface modification in recent years [34]. Based on the current statement, comparing to microstructured surfaces and smooth surfaces, nanostructured surfaces, especially nanostructures under 300 nm, have been reported a positive effect on osteoblast proliferation and differentiation. As for our calcium-phosphate nanoflower coating, the flower-like morphology gives the coating a rough surface and a high surfaceto-volume ratio, which may offer an increased surface area and improve cell migration at the same time. Moreover, the hierarchical and porous structure endows the coating a facile contact with drugs or growth factors. Hence, this novel biocoating can be seen as a carrier to bioactive factors and contributes to further modification of the titanium surface.
In summary, a potential hybrid nanoflower coating consisting of calcium-phosphate nanosheets was performed as a novel modification method on the titanium surface in our study. The calciumphosphate nanoflowers consist of two types of calcium-phosphate ceramic that were widely studied in bone regeneration: tricalcium phosphate and calcium pyrophosphate. The nanoflower coating which was formed by nanosheets under 100 nm appeared to have high surface-to-volume ratios and low cytotoxicity to osteoblast. What's more, the novel calcium-phosphate coating has proved to have promising wettability, thus may enhance the cell spreading and adhesion. It was therefore concluded, that the novel calciumphosphate flower-like biocoating consisting of nanosheets could be seen as a potential alternative modification method for titanium surface. It is easy-prepared and has proved to have low cytotoxicity and desirable cell affinity. Moreover, the flower-like morphology gives the coating a high specific surface area, which make the coating a potential carrier of bioactive factors in future studies.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (No. 81471803) and Sichuan Province Youth Science and Technology Innovation Team (No. 2014TD0001).
| [1] |
E.T. den Braber, J.E. de Ruijter, H.T. Smits, et al., J. Biomed. Mater. Res. 29(1995) 511-518. DOI:10.1002/(ISSN)1097-4636 |
| [2] |
J. Xie, Y. Hou, Y. Yao, et al., J. Biomed. Nanotechnol. 11(2015) 1826-1835. DOI:10.1166/jbn.2015.2119 |
| [3] |
W.L. Lu, N. Wang, P. Gao, et al., Cell Prolif. 48(2015) 95-104. DOI:10.1111/cpr.2015.48.issue-1 |
| [4] |
Y. Ma, Z. Zhang, Y. Liu, et al., J. Biomed. Nanotechnol. 11(2015) 236-244. DOI:10.1166/jbn.2015.2006 |
| [5] |
D.G. Olmedo, G. Nalli, S. Verdu, et al., J. Periodontol. 84(2013) 78-83. DOI:10.1902/jop.2012.110757 |
| [6] |
Y. Yang, H. Ao, Y. Wang, et al., Bone Res. 4(2016) 16027. DOI:10.1038/boneres.2016.27 |
| [7] |
G. Li, N. Fu, J. Xie, et al., J. Biomed. Nanotechnol. 11(2015) 105-116. DOI:10.1166/jbn.2015.2053 |
| [8] |
Y.D. Kwon, D.H. Yang, D.W. Lee, J. Biomed. Nanotechnol. 11(2015) 1007-1015. DOI:10.1166/jbn.2015.2039 |
| [9] |
J. Zhong, B. Guo, J. Xie, et al., Bone Res. 4(2016) 15036. DOI:10.1038/boneres.2015.36 |
| [10] |
M. Roy, A. Bandyopadhyay, S. Bose, Surf. Coat. Technol. 205(2011) 2785-2792. DOI:10.1016/j.surfcoat.2010.10.042 |
| [11] |
B. Xue, A.A. Farghaly, Z. Guo, et al., J. Nanosci. Nanotechnol. 16(2016) 2254-2263. DOI:10.1166/jnn.2016.10919 |
| [12] |
X. Shao, S. Lin, Q. Peng, et al., Small 13(2017) 1602770. DOI:10.1002/smll.v13.12 |
| [13] |
S. Shi, Q. Peng, X. Shao, et al., ACS Appl. Mater. Interfaces 8(2016) 19353-19363. DOI:10.1021/acsami.6b06528 |
| [14] |
G.A. Knoll, S.M. Romanelli, A.M. Brown, et al., J. Nanosci. Nanotechnol. 16(2016) 2464-2473. DOI:10.1166/jnn.2016.12039 |
| [15] |
T. Gong, J. Xie, J. Liao, et al., Bone Res. 3(2015) 15029. DOI:10.1038/boneres.2015.29 |
| [16] |
H. Yi, F. Ur Rehman, C. Zhao, et al., Bone Res. 4(2016) 16050. DOI:10.1038/boneres.2016.50 |
| [17] |
J. Wen, I.Y. Kim, K. Kikuta, C. Ohtsuki, J. Nanosci. Nanotechnol. 16(2016) 3077-3083. DOI:10.1166/jnn.2016.12463 |
| [18] |
S.I. Lee, E.S. Lee, A. El-Fiqi, et al., J. Biomed. Nanotechnol. 12(2016) 1048-1062. DOI:10.1166/jbn.2016.2209 |
| [19] |
S. Zhang, K. Ba, L. Wu, et al., J. Biomed. Nanotechnol. 11(2015) 1799-1807. DOI:10.1166/jbn.2015.2112 |
| [20] |
S.W. Lee, S.A. Cheon, M.I. Kim, T.J. Park, J. Nanobiotechnol. 13(2015) 54. DOI:10.1186/s12951-015-0118-0 |
| [21] |
C. Altinkaynak, S. Tavlasoglu, N. Ozdemir, I. Ocsoy, Enzyme Microb. Technol. 93- 94(2016) 105-112. |
| [22] |
J. Yoo, S.J. Park, S.W. Lee, J. Nanosci. Nanotechnol. 16(2016) 6289-6293. DOI:10.1166/jnn.2016.12119 |
| [23] |
X. Wang, J. Shi, Z. Li, et al., ACS Appl. Mater. Interfaces 6(2014) 14522-14532. DOI:10.1021/am503787h |
| [24] |
J. Liao, X. Cai, T. Tian, et al., Bone Res. 5(2017) 17018. DOI:10.1038/boneres.2017.18 |
| [25] |
J.M. Zhao, W.U. Park, K.H. Hwang, et al., J. Nanosci. Nanotechnol. 15(2015) 2552-2555. DOI:10.1166/jnn.2015.10266 |
| [26] |
Q. Huang, L. Hao, J. Xie, et al., ACS Appl. Mater. Interfaces 7(2015) 20893-20901. DOI:10.1021/acsami.5b06300 |
| [27] |
N. Fu, J. Liao, S. Lin, et al., Cell Prolif. 49(2016) 729-739. DOI:10.1111/cpr.2016.49.issue-6 |
| [28] |
T. Zhang, S. Lin, X. Shao, et al., Cell Prolif. 50(2017) e12338. DOI:10.1111/cpr.2017.50.issue-3 |
| [29] |
Y. Bai, W. Wang, G. Sun, et al., Cell Prolif. 49(2016) 751-762. DOI:10.1111/cpr.2016.49.issue-6 |
| [30] |
T. Zhang, T. Gong, J. Xie, et al., ACS Appl. Mater. Interfaces 8(2016) 22884-22891. DOI:10.1021/acsami.6b07097 |
| [31] |
E.R. Urquia Edreira, J.G. Wolke, A.A. Aldosari, et al., J. Biomed. Mater. Res. A 103(2015) 300-310. DOI:10.1002/jbm.a.v103.1 |
| [32] |
F. Rupp, R.A. Gittens, L. Scheideler, et al., Acta Biomater. 10(2014) 2894-2906. DOI:10.1016/j.actbio.2014.02.040 |
| [33] |
S.H. Jeon, K.H. Hwang, W.S. Jung, et al., J. Biomed. Nanotechnol. 11(2015) 319-324. DOI:10.1166/jbn.2015.2032 |
| [34] |
S. Shi, J. Xie, J. Zhong, et al., Cell Prolif. 49(2016) 341-351. DOI:10.1111/cpr.2016.49.issue-3 |
 2017, Vol. 28
2017, Vol. 28 

