2 Chinese PLA Air Force General Hospital, Beijing 100142, China;
3 Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China
Ubiquitous environmental stress factors for the human skin, such as UV radiation, atmospheric dust from wind erosion and other air pollutants, could lead to the excess generation of reactive oxygen species (ROS), which induces various forms of skin damage. ROS is considered as an important initial step associated with cell damage, photoaging, immune system changes and skin cancer [1]. ROS may directly be correlated to the protein amino acid sidechain reactions, DNA oxidative damage, lipid peroxidation and reactive endogenous electrophile generation [2, 3].
The balance between ROS production and antioxidant defense system determines the degree of oxidative stress, which has a significant role in ageing processes [4, 5]. As a physical barrier, skin protects the body against pathogens, chemicals and solar UVR throughout life [6]. The antioxidant defense of the skin is a major determinant of its response to oxidative conditions. Antioxidants eliminating ROS have become an important strategy to protect the skin against photo-oxidative damage [7]. Many studies have been conducted by using various antioxidants to protect the skin from the deleterious effects [8, 9]. Some safe antioxidants were obtained from dietary or exogenous sources, such as vitamins A, E and C. But most antioxidants, such as tertbutylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are chemical compounds and potentially toxic to humans.
Fullerenes, a class of sphere-shaped molecules made of carbon atoms, were discovered by Kroto et al. in 1985. Both in vitro and in vivo studies have shown that polyhydroxylated C60 fullerene derivative, fullerol (C60(OH)n, n = 3-26), is a potential antioxidative agent and free radical scavenger in biological systems. C60(OH)n could scavenge and modulate ROS such as superoxide radical anion, hydrogen peroxide, singlet oxygen, and hydroxyl radical [10-12]. The antiproliferative effect of C60(OH)n and enzyme inhibition activity can be attributed to its antioxidative property [13, 14]. Most importantly, C60(OH)n possesses good biocompatibility according to the considerable investigations. Until now, C60(OH)n, acting as an effective agent for prevention of skin aging, is widely used in the lotion and sunscreens in the field of cosmetics, such as DHC® Fullerene Essence, Missha time revolution and Romasin series, but rarely used in the masks.
In this study, we prepared C60(OH)n-loaded nanofibrous membranes to protect human keratinocytes cells from ROS-associated damage. The nanofibrous membrane made by electrospinning method has high specific surface area and nano space structure formed between fibers. Electrospinning is a simple and convenient method for preparing nanometer fibers as well as loading biomacromolecules or drugs. The notable applications include tissue engineering [15-17], biosensors, filtration, wound dressings [18-20], drug delivery [21], battery devices [22], and enzyme immobilization. Two FDA-approved biodegradable polymers, poly(lactide-co-glycolide) (PLGA) and polycaprolactone (PCL), were used for making the electrospun nanofibers. Polyhydroxylated fullerene derivative C60(OH)n was added to the polymers as an antioxidant. We found that C60(OH)n-loaded nanofibrous membranes could scavenge ROS and regulate intracellular Ca2+ to protect HaCaT cells from ROS-associated damage induced by H2O2 injury. The nanofibrous membranes with the good biocompatibility might be potentially applied in clinical practice to reduce the skin aging.
All the experimental details are in Supporting information.
As shown in Fig. 1A, non-woven sheets of randomly aligned fibers with nanometer scale diameters were fabricated via electrospinning. Both blank and C60(OH)n-loaded nanofibrous membranes displayed smooth surfaces, and the average diameter of fibers was 200 nm. With the increased concentration of C60(OH)n, nanofibrous membranes become more hydrophilic. The contact angle of blank membranes was 103.9θ2θ, while that of C60(OH)n-loaded nanofibrous membranes (100 μmol/L) was 50θ2θ. C60(OH)n in fibers could increase the hydrophilicity of the nanofibrous membranes. Both C60(OH)n-loaded nanofibrous membranes and C60(OH)n had peaks at 3408 cm-1, which is the characteristic absorption peak of -OH. The results of infrared spectroscopy indicated that C60(OH)n had been wrapped in the fibers during the electrospinning process.

|
Download:
|
| Fig. 1. Characterization of electrospun nanofibrous membranes. (A) SEM images of nanofibrous membranes (Scale bar = 1 μm). a, Blank nanofibrous membrane; b–e, C60(OH)n-loaded nanofibrous membranes. (B) and (C) Contact angle of different nanofibrous membranes. (D) Infrared spectra of C60(OH)n and different nanofibrous membranes. ** represent P < 0.01 as indicated. | |
We evaluated the cell viability of C60(OH)n nanoparticles and C60(OH)n-loaded nanofibrous membranes. After cultured for 12 h, 24 h and 48 h with 75 μmol/L, 150 μmol/L, 300 μmol/L, and 600 μmol/L C60(OH)n, HaCaT cells showed no significantly cytotoxicity, all the survival rates maintained above 90% (Fig. 2A). As Fig. 2B showed, 5-100 μmol/L C60(OH)n used in the nanofibrous membranes was safe and had no apparent cytotoxicity to the keratinocytes. The cell viability had no significant difference between HaCaT cells on the C60(OH)n-loaded nanofibrous membranes and those on the blank membranes.

|
Download:
|
| Fig. 2. In vitro cytotoxicity and antioxidative activity of C60(OH)n-loaded nanofibrous membranes. (A) Cell viability of HaCaT cells after cultured with different concentrations of C60(OH)n nanoparticles. (B) Cell viability of HaCaT cells cultured on different nanofibrous membranes. (C) Fluorescent images of HaCaT cells upon H2O2 were examined to determine the antioxidant of C60(OH)n nanoparticles. Cells were stained by DCFH-DA and Hoechst 33342. a–d represent cells treated with H2O2 and C60(OH)n (5 μmol/L, 10 μmol/L. 50 μmol/L, or 100 μmol/L), e stands for cells upon H2O2 (1 mmol/L) and f stands for normal cells (not treated with C60(OH)n and H2O2). (D) ROS level in HaCaTcells upon H2O2 was examined to assess the antioxidative activity of different nanofibrous membranes. ** represent P < 0.01 as indicated. | |
To determine the antioxidant properties of C60(OH)n, ROS of HaCaT cells were detected using the fluorescent dye DCFH-DA and cell nuclei were labeled using Hoechst 33342. The blue fluorescent intensity of after H2O2 exposure was found to be significantly enhanced compared to cells not treated with C60(OH)n (Fig. 2C). The results suggested that C60(OH)n had cytoprotective effect at the examined concentration range. In addition, a series of nanofibrous membranes loaded with various concentration C60(OH)n to examine the antioxidant effects, including 5 μmol/L (0.12 wt%), 10 μmol/L (0.25 wt%), 50 μmol/L (1.25 wt%) and 100 μmol/L (2.5 wt%). Flow cytometry was used to detect the ROS levels of cells on different nanofibrous membranes before and after H2O2 treatment. ROS is a direct indicator to reflect intracellular oxidative stress level. Increased ROS can lead to lipid peroxidation, protein aggregation and DNA fragmentation [23]. As shown in Fig. 2D, the fluorescence intensity of the cells in H2O2-treated group (without membranes) was significantly higher than that in the blank membranes group (P < 0.01). After H2O2 treatment, cells on C60(OH)n-loaded nanofibrous membranes had lower fluorescence intensity than those on blank membrane. After incubated with H2O2, the fluorescence intensity of cells on the blank nanofibrous membrane was 500, and that on the 100 μmol/L C60(OH)n-loaded nanofibrous membrane was 200. The protective effect on cells was positively related to the increased concentration of C60(OH)n. The blank nanofibrous membrane did not cause increased intracellular ROS. Thus, C60(OH)n-loaded nanofibrous membranes can protect HaCaT cells from ROS-associated damage induced by H2O2 treatment.
To further find the underlying mechanism of protective effects of C60(OH)n-loaded nanofibrous membranes, the cellular enzymatic activities including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) were investigated. As shown in Fig. 3A-C, we found that the intracellular enzyme activities of CAT, SOD and GSH-Px of cells on the C60(OH)n-loaded nanofibrous membranes were 56.77, 22.60, 7.81 μmol/mg protein, while those were 12.26, 6.16 and 1.68 μmol/mg protein in H2O2-treated group (without membranes), respectively. After cells were cultured on the C60(OH)n-loaded nanofibrous membranes, the activities of these antioxidant enzymes were significantly higher than that of H2O2-treated group. The enzyme activities of CAT, SOD and GSH-Px of cells on the blank membrane were 55.98, 20.11 and 7.87 μmol/mg protein, which were similar to those on the C60(OH)n-loaded nanofibrous membranes. These results suggest that nanofibrous membranes loaded with different concentrations of C60(OH)n can prevent oxidative damage by regulating the oxidative defense system in HaCaT cells.

|
Download:
|
| Fig. 3. In vitro free radical scavenging effect of C60(OH)n-loaded nanofibrous membranes under oxidative stress. (A), (B) and (C) Nanofibrous membranes loaded with C60(OH)n attenuate H2O2-induced decrease of antioxidant enzyme activities. (D) Fibers with C60(OH)n attenuate H2O2-induced elevation of intracellular calcium level. (E) Protective effects of C60(OH)n against H2O2-induced loss of MMP in HaCaT cells. ** and ## represent P < 0.01 as indicated. | |
ROS and Ca2+ levels are closely related to the process of apoptosis. Ca2+ in cytoplasm is activated by excessive ROS in cells. ROS and Ca2+ are involved in the pathogenesis of many diseases [24-26]. To test whether C60(OH)n-loaded nanofibrous membranes could regulate intracellular Ca2+ concentration in HaCaT cells, we measured the concentration of Ca2+ in cells. As shown in Fig. 3D, in H2O2 treated group, the fluorescence intensity of Ca2+ was significantly increased compared with the control group. With the increased concentration of C60(OH)n in nanofibrous membranes, the fluorescence intensity of Ca2+ was decreased, indicating that C60(OH)n-loaded nanofibrous membranes can finely tune intracellular Ca2+ concentration.
C60(OH)n is able to cross the cell membrane and localizes preferentially to mitochondria which could generate a great mass of cellular oxygen-free radicals [27]. The mitochondria-derived ROS (mtROS) play a critical role in the process of apoptosis [28]. C60(OH)n might be a kind of mitochondria-targeted ROS scavenger to have potential clinical utility in apoptosis-associated disorders involving mitochondrial oxidative damage. In our study, mitochondrial membrane potential (MMP) was detected using JC-1. In Fig. 3E, red fluorescence reflected normal cells and green fluorescence reflected cells with mitochondria damage. Under H2O2 conditions, the red/green ratio (% of control) was about 39.4%. After cells were cultured on the 100 μmol/L C60(OH)n-loaded nanofibrous membranes, the red/green ratio was 89.1%, showing the obvious protective effect of C60(OH)n-loaded nanofibrous membranes on human keratinocyte HaCaT cells injury induced by hydrogen peroxide.
ROS could result in the production of lipid peroxides, affect the function of membrane proteins, and modify cell membrane structure [28]. In this study, environmental scanning electron microscope (ESEM) is employed to image the morphological response of the cells under oxidative damage. After treated with H2O2, the cells on nanofibrous membranes were fixed using 2.5% (v/v) glutaraldehyde and prepared for ESEM. Because it is not necessary to make a nonconductive sample conductive under the ESEM, cells are not coated with gold palladium. The original characteristics of cells could be preserved for observation. As shown in Fig. 4A, more granules and holes on the cell surface are shown in H2O2-treated group (Fig. 4A-b) compared to the blank membranes (Fig. 4A-a) and 100 μmol/L C60(OH)n-loaded nanofibrous membranes (Fig. 4A-c). Thus, C60(OH)n-loaded nanofibrous membranes could protect HaCaT cells from ROS-associated damage.
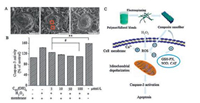
|
Download:
|
| Fig. 4. Regulating the apoptosis in HaCaT cells under oxidative stress. (A) ESEM images of HaCaT cells on fibers. The red box claimed granules and holes on the cell surface (Scale bar = 5 μm). a, A cell on blank fibers; b, A cell on blank fibers was treated with H2O2; c, A cell on fibers preloaded with fullerol was treated with H2O2. (B) Fibers with fullerol derivative can attenuate H2O2-induced decrease of caspase-3 activity. (C) Schematic diagram. ** represents P < 0.01 and # represents P < 0.05 as indicated. | |
Caspase-3 is a key enzyme responsible for apoptosis in animal cells [29]. We further considered whether C60(OH)n-loaded nanofibrous membranes inhibit caspase pathway or not. In this experiment, nanofibrous membranes loaded with different concentration of C60(OH)n were observed in H2O2-induced HaCaT apoptosis model. As shown in Fig. 4B, H2O2 treatment group significantly increased caspase-3 activity (158.25% of control), while the activity of caspase-3 was reversed after pretreatment with C60(OH)n-loaded nanofibrous membranes (100 μm, 109.71% of control). The results showed that C60(OH)n-loaded nanofibrous membranes could inhibit H2O2-induced apoptosis of HaCaT cells by regulating the activity of caspase-3.
In summary, we have proposed a novel protective nanofibrous membrane in which C60(OH)n act as an antioxidant. C60(OH)n in the nanofibrous membrane can fight against oxidative damage by removing ROS through cellular enzymatic mechanisms and protecting mitochondria from damage (Fig. 4A). Moreover, pretreatment with C60(OH)n-loaded nanofibrous membranes resulted in a decrease in Ca2+ and caspase-3 activity. C60(OH)n as the antioxidant has showed some pharmacodynamic advantages owing to its oxidatively decomposed in the aqueous solution. Thus, higher stability than vitamin, which is so labile to be C60(OH)n-loaded nanofibrous membranes would be a new therapeutic material in the definite concentration range for the prevention of both H2O2 damage, UV skin-injuries and skin aging without cytotoxicity. The use of low-cost electrospinning method and FDA-approved biodegradable polymers increased the probability of clinical application.
AcknowledgmentsThis work was financially supported by the project ElectroMed (No. 11-115313) from the Danish Council for Strategic Research, the National Science Fund for Excellent Young Scholars (No. 31622026), the National Natural Science Foundation of China (Nos. U1532122, 11435002, 21471044), the National Key Research and Development Plan (Nos. 2016YFA0201600, 2016YFA0203204) and CAS Youth Innovation Promotion Association (No. 2014031).
Appendix C. Supplementary dataSupplementary data associated with this article can be found, in the online version, at 10.1016/j.cclet.2017.07.021.
| [1] |
Y.R. Helfrich, D.L. Sachs, J.J. Voorhees, Dermato. Nurs. 20(2008) 177-184. |
| [2] |
E.D. Lephart, Ageing Res. Rev. 31(2016) 36-54. DOI:10.1016/j.arr.2016.08.001 |
| [3] |
A. Pal, S. Alam, S. Mittal, et al., Mutat. Res-Gen. Tox. En. 807(2016) 15-24. DOI:10.1016/j.mrgentox.2016.06.005 |
| [4] |
T. Finkel, N.J. Holbrook, Nature 408(2000) 239-247. DOI:10.1038/35041687 |
| [5] |
G. Buonocore, S. Perrone, M.L. Tataranno, Semin. Fetal. Neonatal Med. 15(2010) 186-190. DOI:10.1016/j.siny.2010.04.003 |
| [6] |
Y.C. Chang, W.M. Lin, S.T. Hsieh, Neuroreport 15(2004) 149-153. DOI:10.1097/00001756-200401190-00029 |
| [7] |
V. Gasperi, E. Dainese, S. Oddi, et al., Curr. Med. Chem. 20(2013) 64-78. |
| [8] |
M.R. McCall, B. Frei, Free Radical Biol. Med. 26(1999) 1034-1053. DOI:10.1016/S0891-5849(98)00302-5 |
| [9] |
B.P. Yu, Mech. Ageing Dev. 111(1999) 73-87. DOI:10.1016/S0047-6374(99)00072-X |
| [10] |
G. Bogdanovic, V. Kojic, A. Dordevic, et al., Toxicol. In Vitro 18(2004) 629-637. DOI:10.1016/j.tiv.2004.02.010 |
| [11] |
L. Brunet, D.Y. Lyon, E.M. Hotze, et al., Environ. Sci. Technol. 43(2009) 4355-4360. DOI:10.1021/es803093t |
| [12] |
B. Vileno, P.R. Marcoux, M. Lekka, et al., Adv. Funct. Mater. 16(2006) 120-128. DOI:10.1002/(ISSN)1616-3028 |
| [13] |
X. Cai, H. Jia, Z. Liu, et al., J. Neurosci. Res. 86(2008) 3622-3634. DOI:10.1002/jnr.v86:16 |
| [14] |
P. Witte, F. Beuerle, U. Hartnagel, et al., Org. Biomol. Chem. 5(2007) 3599-3613. DOI:10.1039/b711912g |
| [15] |
R.D. Nascimento Arifa, T.P. de Paula, M.F. Moreira Madeira, et al., Pharmacological Res. 107(2016) 102-110. DOI:10.1016/j.phrs.2016.03.004 |
| [16] |
B. Srdjenovic, V. Milic-Torres, N. Grujic, et al., Toxico. Mech. Methods 20(2010) 298-305. DOI:10.3109/15376516.2010.485622 |
| [17] |
T. Xia, M. Kovochich, J. Brant, et al., Nano Letters 6(2006) 1794-1807. DOI:10.1021/nl061025k |
| [18] |
E. Biazar, Polym. Advan. Technol. 27(2016) 1404-1412. DOI:10.1002/pat.v27.11 |
| [19] |
H.J. Jin, J.S. Chen, V. Karageorgiou, et al., Biomaterials 25(2004) 1039-1047. DOI:10.1016/S0142-9612(03)00609-4 |
| [20] |
C.M. Li, C. Vepari, H.J. Jin, et al., Biomaterials 27(2006) 3115-3124. DOI:10.1016/j.biomaterials.2006.01.022 |
| [21] |
K.S. Rho, L. Jeong, G. Lee, et al., Biomaterials, 1452-1461. |
| [22] |
S. Zhou, J. Chen, L. Gan, et al., Sci. Bull. 61(2016) 227-235. DOI:10.1007/s11434-015-0992-8 |
| [23] |
S.S. Saravanabhavan, S. Dharmalingam, Chem. Eng. J. 234(2013) 380-388. DOI:10.1016/j.cej.2013.08.076 |
| [24] |
G.M. Xing, J. Zhang, Y.L. Zhao, et al., J. Phys. Chem. B 108(2004) 11473-11479. DOI:10.1021/jp0487962 |
| [25] |
J. Tang, G.M. Xing, H. Yuan, et al., J. Phys. Chemi. B 109(2005) 8779-8785. DOI:10.1021/jp050374k |
| [26] |
J. Li, W. Xie, X. Chen, et al., Sci. Bull. 62(2017) 5-8. DOI:10.1016/j.scib.2016.12.001 |
| [27] |
S. Foley, C. Crowley, M. Smaihi, et al., Biochem. Bioph. Res. Commun. 294(2002) 116-119. DOI:10.1016/S0006-291X(02)00445-X |
| [28] |
S.A. Susin, N. Zamzami, G. Kroemer, BBA-Bioenergetics 1366(1998) 151-165. DOI:10.1016/S0005-2728(98)00110-8 |
| [29] |
S.J. Riedl, Y.G. Shi, Nat. Rev. Mol. Cell Biol. 5(2004) 897-907. |
 2017, Vol. 28
2017, Vol. 28 


