b Department of Urology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
Recently, a precise and quantitative strategy for the recognition and detection of proteins, such as widely used disease-related biomarkers has been taken seriously in the fields of biomedical clinical diagnosis and screening. Considerable efforts have been made to the research on developing more sensitive detection techniques of biomarkers [1-6]. Enzyme-linked immunosorbent assay (ELISA) is one of the most popular methods for the detection of biomarkers affording good satisfactory sensitivity and specificity by virtue of enzyme-based signal amplification and highly specific identification between antibody and antigen [7-9]. Nevertheless, conventional ELISA has inevitable limitations for point-of-care testing (POCT) applications. With regard to accurate detection of low concentration of analytes in biological vectors, the large-scale, expensive plate reader and skilled trained personnel are indispensable, whereas it against the principle of use-friendliness, portability and POCT. Therefore, it is highly desired to develop modified ELISA to circumvent the disadvantages of conventional ELISA. Many trials have been done in this area. For example, Fu et al. have reported an innovative analytical method based on Fe3O4 nanoparticles (NPs) as labels for converting to a near-infrared laser-driven photothermal agent, Prussian blue NPs [10]. As a result, the detection signal can be transformed into heat, which can be easily monitored by a common used thermometer. However, the results of this assay are unsatisfactory for the detection of prostatespecific antigen with an insufficient sensitivity (limit of detection, LOD: 1 ng/mL). Other improvement trials are paper-based ELISA using affordable photo scanners or camera for a signal output [11-13], and cotton fabric-based ELISA with image captured by camera transform into grey scale and further analyzed through image processing software [14]. Despite the above mentioned techniques make use of low-cost, handy and miniaturized analytical devices, unfortunately, the sensitivity for model analytes is also worse than the conventional ELISA. Therefore, it is essential to actualize a sensitive POCT technique, especially for those resource-limited regions.
Very recently, the portable pH meter with many advantages including miniaturization, low-cost and high detection sensitivity as an analytical device have been applied to biological detection. Kwon et al. have used the ubiquitous pH meter to detect cardiac biomarkers [15]. The principle of this study was based on the decrease of pH value, which was due to acetylcholine was hydrolyzed to choline and acetic acid in the present of acetylcholinesterase. It is well known that the acidity coefficient (pKa) of acetic acid is 4.76 at 25 ℃, and the dissociated ability of hydrion is less than that of glucose acid (pKa = 3.86), which may limit the sensitivity of this analytical technique. Zhang et al. have also employed handheld pH meter for the detection of human oncogenic protein. The immunoassay basically realized the analytical requirements of portability and sensitivity [16]. But in that study, microparticles used as labels have some concerns on the sensitivity by steric hindrance originated from their larger size. Our group has developed a kind of organic-inorganic hybrid nanoflowers as labels for the immobilization of recognition unit (concanavalin A) and signal amplification unit (glucose oxidase, GOx), realizing the determination of food pathogen, E.coli O157:H7 via a handheld pH meter [17]. The hybrid nanocomposites were defined as nanoflowers while the corresponding size actually lies in the scale of micron meter. When the sandwich type immunoassay occurs, the resulted steric hindrance still exists.
With the careful reviewing of previous reported work, we report an improved ELISA used bioinspired synthetic melanin nanoparticles (SMNPs) as a nanocarrier for the simultaneous immobilization of recognition unit (second antibody, Ab2) and signal amplification unit (GOx). SMNPs can anchor a large amount of GOx by virtue of their larger specific surface area. Moreover, low-cost and effortless synthesized SMNPs have excellent biocompatibility and biodegradability. It is worth mentioning that the conjugation process of SMNPs with proteins (e.g. antibody, enzyme) is simple without requiring any activation procedure. In view of those features, SMNPs have drawn extensively attention in the fields of biosensing, drug delivery, theranostics and so on [18-20]. Meanwhile, we chose GOx as signal amplification unit which can catalyze the oxidation of glucose into gluconic acid and H2O2 [21], accordingly leading to decrease of pH value, and portable pH meter as signal readout device to detect the cardiac marker, cardiac troponin I (cTnI). cTnI is one of the most ideally diagnostic markers for acute myocardial infarction (AMI). The boundary of the cTnI concentration between normal people and patients is 0.5 to 2.0 ng/mL. When people suffer from myocardial injury, an increasing cTnI level can be discovered in serum within 3-6 h and stick to the circulation for days, consequently its sensitivity and tissue-specificity are higher than that of creatine kinase MB (CK-MB), the gold standard previously in the diagnosis of AMI [22-24]. On account of the normal level of cTnI generated from the myocardium is very low, thus, although the significant advancements of cTnI biosensor have been reported, it is still a great challenge to explore a sensitive and POCT analytical method to assist the clinical diagnosis of AMI at ultralow concentration of cTnI (less than 0.5 ng/mL) in resource-limited area.
Herein, a new pH ELISA was developed for ultrasensitive determination of cTnI. SMNPs were synthesized (the detailed information see the Supporting information) and used for the co-immobilization of GOx and second antibody as nanotags to enhance the decrease degree of pH value in detection system, which can be simply revealed through a handheld pH meter. The proposed method ensures that it has satisfactory sensitivity and excellent portability simultaneously, which provides a novel, feasible, user-friendly analytical strategy for POCT of cTnI.
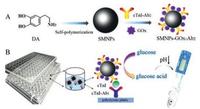
As a proof of concept, a pH ELISA based on SMNPs-GOx-Ab2 as nanotags for the detection of cTnI using a low-cost, portable and precise pH meter was constructed. As illustrated in Scheme 1, SMNPs were synthesized through the self-polymerization of dopamine, which plays an important role in the co-immobilization of GOx and Ab2 via the Michael addition between SMNPs and amino or thiol groups of proteins [25]. Following ELISA type, the nanotags of SMNPs-GOx-Ab2 was immobilized for signal amplification. Thus, according to the varying amount of SMNPs-GOx-Ab2 were captured by different concentration of cTnI resulting in the corresponding decrease of pH value, the amount of cTnI can be quantified by the decreasing level of pH using a portable pH meter, which is beneficial for the early diagnosis and screening of patients with AMI.
|
Download:
|
| Scheme 1. Diagram of the preparation process for SMNPs-GOx-Ab2 (A) and the portable pH meter-based ELISA for cTnI detection in 96-microwell plates (B). | |
The morphology of SMNPs was shown through the SEM image (Fig. 1A). It is clear to see that SMNPs exhibited uniform sphere structure with an average diameter of approximately 175 nm in Fig. 1B. The FTIR spectra further revealed the synthesis of SMNPs was successful. As shown in Fig. 1C, after the polymerization process, the characteristic peak ranges from 1800 to 500 cm-1 have disappeared, which corresponds to the previous report [26, 27]. The following characterizations can be used to demonstrate that GOx and Ab2 were loaded onto the surface of SMNPs successfully. Firstly, the UV-vis spectra of SMNPs, GOx and SMNPs-GOx conjugate were investigated and shown in Fig. 1D. For the pure GOx, the absorbance peak at 277 nm was observed. No absorbance peak was discovered for the SMNPs. After the immobilization of GOx on SMNPs, the obvious absorbance peak appeared at 277 nm, indicated that GOx was successfully covalently bond on SMNPs surface. Secondly, Fig. 2B showed the synthesized SMNPs-GOx was used for the detection of cTnI, no distinct pH values change was observed. In contrast, there was the considerable decrease of pH values via utilizing the 0.5 mg/mL SMNPs-GOx-Ab2 as labels for detecting 1 ng/mL cTnI. The consequence revealed indirectly that the Ab2 as identification unit was successfully immobilized onto SMNPs.

|
Download:
|
| Fig. 1. (A) SEM image of SMNPs. (B) Size distribution of SMNPs. (C) FTIR spectra of dopamine hydrochloride (a) and SMNPs (b). (D) UV-vis spectra of SMNPs, GOx and SMNPsGOx conjugate. | |

|
Download:
|
| Fig. 2. (A) Calibration curve for the DpH about log values of different cTnI concentrations in pH 7.0 PBS (black) and human serum (red). Error bar = RSD (n = 5). (B) DpH values of the proposed immunosensor to 0.5 mg/mL SMNPs-GOx, blank, 1 ng/mL CEA, 1 ng/mL AFP, 1 ng/mL cTnI, mixture including 1 ng/mL cTnI, 100 ng/mL CEA and 100 ng/mL AFP. | |
Under the optimized conditions (Fig. S1 in Supporting information), 35 ℃ as the operating temperature, 75 min as the incubation time of glucose aqueous solution, 0.5 mg/mL SMNPsGOx-Ab2 with the mass ratio of 12:1 for GOx:Ab2 were taken for the detection of cTnI. As shown in Fig. 2A (black curve), the linear calibration curve, ΔpH = 2.2106 + 0.4617*X, R = 0.9915 in the range from 0.5 pg/mL to 10 ng/mL with the LOD of 0.15 pg/mL (S/N = 3) was acquired. Compared with other strategies for the determination of cTnI as shown in Table S1 in Supporting information, this improved ELISA relied on the portable, precise pH meter manifested a more outstanding performance by the compared lower LOD. Fig. 2B demonstrated the selectivity of the proposed pH ELISA. It can be seen that considerable ΔpH values were observed for cTnI and the corresponding mixture, whereas the response signals of other protein biomarkers were almost negligible. Therefore, this improved ELISA for detecting cTnI has satisfactory specificity and selectivity. Furthermore, in order to evaluate the reliability and practical value of the fabricated pH ELISA, we did the detection in real human serum with the parallel operation steps. Consequently, the excellent linear relationship of this immunosensor for the detection of cTnI in serum was obtained ranging from 0.5 pg/mL to 10 ng/mL with the low LOD of 0.18 pg/mL (S/N = 3), the regression equation was ΔpH = 1.9804 + 0.4196*X, R = 0.9940 (Fig. 2A, red curve). The same linear concentration range and the similar sensitivity respectively in PBS and human serum displayed the acceptable reliability for the cTnI determination. Table S2 in Supporting information listed out the RSDs were 6.3%, 2.5%, 3.4%, 2.4% and 5.5% at the addition of 0.05, 0.1, 0.2, 0.5, 1 ng/mL, respectively, and the recoveries were in the range of 96.4% -102.7%. The results further verified the reliability and feasibility of this strategy which can be applied in the clinical analysis.
In conclusion, an improved, low-cost, sensitive pH ELISA has been constructed and served to determine the concentration of cTnI in human serum. On account of the portability and sensitivity of the constructed immunoassay, as well as the acceptable selectivity and reliability, it can be considered to apply to the actual clinical analysis for patients with myocardial injury, especially in the developing or remote areas without ample resources
AcknowledgmentThe authors would like to thank the National Natural Science Foundation of China (Nos. 21245007 and 81000976) for the financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.018.
| [1] |
L.C. Chen, X.T. Zeng, P. Si, et al., Anal. Chem. 86(2014) 4188-4195. DOI:10.1021/ac403635f |
| [2] |
X.M. Fu, Z.J. Liu, S.X. Cai, et al., Chin. Chem. Lett. 86(2016) 920-926. |
| [3] |
T.T. Song, W. Wang, L.L. Meng, et al., Chin. Chem. Lett. 28(2017) 226-230. DOI:10.1016/j.cclet.2016.07.021 |
| [4] |
Q.L. Li, D.L. Liu, L. Xu, et al., ACS Appl. Mater. Inter 7(2015) 22719-22726. DOI:10.1021/acsami.5b07895 |
| [5] |
H.N. Li, Y.W. Mu, J.R. Yan, et al., Anal. Chem. 87(2015) 2007-2015. DOI:10.1021/ac504589d |
| [6] |
W. Li, Y. Li, Y. Wu, et al., J. Biomed. Nanotech. 11(2015) 2050-2056. DOI:10.1166/jbn.2015.2104 |
| [7] |
Z. Li, J. He, Y. Zhang, et al., Chin. Chem. Lett. 27(2016) 1771-1775. DOI:10.1016/j.cclet.2016.06.052 |
| [8] |
C. Li, Y.C. Yang, D. Wu, et al., Chem. Science 7(2016) 3011-3016. DOI:10.1039/C5SC04256A |
| [9] |
W.Y. Sun, W.Y. Liu, L.B. Qu, Chin Chem, Lett. 18(2016) 1107-1110. |
| [10] |
G.L. Fu, S.T. Sanjay, M.W. Dou, et al., Nanoscale 8(2016) 5422-5427. DOI:10.1039/C5NR09051B |
| [11] |
S.M. Wang, L. Ge, X.R. Song, et al., Biosens. Bioelectron. 31(2012) 212-218. DOI:10.1016/j.bios.2011.10.019 |
| [12] |
P. Bai, Y. Luo, Y. Li, et al., Chin J. Anal, Chem 41(2013) 20-24. DOI:10.1016/S1872-2040(13)60620-9 |
| [13] |
S.T. Sanjay, M.W. Dou, J.J. Sun, et al., Sci. Rep. 6(2016) 30474. DOI:10.1038/srep30474 |
| [14] |
S. Bagherbaigi, E.P. Corcoles, D.H.B. Wicaksono, Anal. Methods 18(2014) 7175-7180. |
| [15] |
D. Kwon, J. Joo, S. Lee, et al., Anal. Chem. 85(2013) 12134-12137. DOI:10.1021/ac403329w |
| [16] |
Y. Zhang, J.N. Yang, J.F. Nie, et al., Chem. Commun. 52(2015) 3474-3477. |
| [17] |
R.F. Ye, C.Z. Zhu, Y. Song, et al., Small 12(2016) 3094-3100. DOI:10.1002/smll.v12.23 |
| [18] |
W.B. Qiang, W. Li, X.Q. Li, et al., Chem. Sci. 5(2014) 3018-3024. DOI:10.1039/C4SC00085D |
| [19] |
D.Q. Fan, C.T. Wu, K. Wang, et al., Chem. Commun. 52(2016) 406-409. DOI:10.1039/C5CC06754E |
| [20] |
D.F. Chang, Y.F. Gao, L.J. Wang, et al., Colloid & J.Interface Science 463(2016) 279-287. |
| [21] |
S.N. Sarangi, S. Nozaki, S.N. Sahu, J. Biomed, Nanotech. 11(2015) 988-996. |
| [22] |
X. Han, S.H. Li, Z.L. Peng, et al., Physiologia Plantarum. 49(2016) 141-144. |
| [23] |
A. Chiu, W.K. Chan, S.H. Cheng, et al., Qjm Monthly Journal of the Association of Physicians. 92(1999) 711-718. DOI:10.1093/qjmed/92.12.711 |
| [24] |
S.S. Wong, Ann. Clin. Lab. Sci. 26(1996) 301-312. |
| [25] |
W.H. Hu, G.L. He, H.H. Zhang, et al., Anal. Chem. 86(2014) 4488-4493. DOI:10.1021/ac5003905 |
| [26] |
Y.L. Liu, K.L. Ai, J.H. Liu, et al., Adv. Mater. 25(2013) 1353-1359. DOI:10.1002/adma.v25.9 |
| [27] |
S.A. Centeno, J. Shamir, J. Mol. Structure 873(2008) 149-159. DOI:10.1016/j.molstruc.2007.03.026 |
 2017, Vol. 28
2017, Vol. 28 


