b State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China;
c University of Chinese Academy of Sciences, Beijing 100049, China;
d College of Materials Science andEngineering, Jilin University, Changchun 130024, China
Photodynamic therapy (PDT), as a non-invasive and safe cancer treatment, has attracted much attention [1-8]. The photosensitizer, light and oxygen are three-primary elements for PDT. A lot of photosensitizers have been synthesized to obtain an ideal anticancer effect, such as methylene blue, porphyrin, borondipyrrolmethene (BODIPY) and TiO2 [9-11]. Among these photosensitizers, BODIPY is one of most noticeable compounds because of high light-dark toxicity ratios, resistance to harsh environment and high extinction coefficients [12-15]. However, most of them are hydrophobic, which limit the formation of nanostructures in aqueous solution for biological applications.
Multicomponent Passerini reaction is an emerging and powerful tool for synthesis of diverse monomers and polymers in mild reaction conditions [16-18]. Meier's group and Li's group have made a great contribution to synthesis of polymers via Passerini reaction [19-22]. However, little attention is drawn on the synthesis of functional compounds and their applications. Recently, we have reported several compounds synthesized via Passerini reaction for wide varieties of applications [23-26]. For example, we synthesized hydrophobic cyanine-containing polymers for near-infrared fluorescence imaging, photoacoustic imaging and photothermal therapy [27]. If the formed molecules are hydrophobic, the nanoparticle formulations have to be made in the presence of amphiphilic polymers. It is desirable to synthesize new compounds via Passerini reaction, which can directly selfassemble into nanoparticles (NPs) in aqueous solution for bioimaging and cancer therapy.
In this work, we synthesized PEGylated fluorescent compounds (PNB and PCIB) from PEG and BODIPY via Passerini reaction (Scheme 1A and B). Stable NPs could be formed in water directly from obtained compounds and be used for cellular imaging and PDT (Scheme 1C).

|
Download:
|
| Scheme 1. Synthesis of A) PNB and B) PCIB via multicomponent Passerini reaction. C) A schematic illustration of self-assembly and internalization of NPs. | |
The experimental section was shown in Supporting information. 5, 5-Difluoro-2, 8-diiodo-10-(4-isocyanophenyl)-1, 3, 7, 9-tetramethyl-5H-dipyrrolo[1, 2-c:2', 1'-f][1, 3, 2]diazabori-nin-4-ium-5-uide (IBDP) was firstly synthesized as shown in Scheme S1 (Supporting information). The obtained IDBP was characterized by proton nuclear magnetic resonance (1H NMR) and electrospray ionization-mass spectrometry (ESI-MS) as shown in Fig. S1 (Supporting information). All the protons could be clearly resolved in 1H NMR spectra, and the peak at m/z 599.96 is same to [M-H]- of IBDP. Then PEG-750 succinic acid ester (PEG-COOH), o-nitrobenzaldehyde and 4, 4-difluoro-8-(4-isocyanophenyl)-3, 5-dimethyl-l-4-bora-3a, 4a-diaza-indacene (BDP) were mixed to obtain the compound PNB. Similarly, PEG-COOH and 7-diethylamino-4-formylcoumarin (Cou) and iodinated BDP (IBDP) were mixed at room temperature for 4 days to obtain the compound PCIB. The structures of PNB and PCIB were validated by 1H NMR in Figs. S2 and S3 (Supporting information).
PNB and PCIB could self-assemble into NPs (PNB NPs and PCIB NPs) with diameters of 200 nm in aqueous solution, as shown in Fig. S4A and B (Supporting information). The average diameter of two NPs measured by dynamic light scattering (DLS) shown in Fig. S4C and D (Supporting information) were consistent with the diameter observed by TEM. More importantly, the diameter and the polydispersity index (PDI) measured by DLS almost remained unchanged over a week. The stability of NPs in physiological environments is important for biological applications. Moreover, two NPs exhibited favorable structural stability in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) as evidenced in Fig. S5 (Supporting information) at 37 ℃ for 24 h.
The photophysical properties were recorded by UV-vis absorption and photoluminescence emission. As shown in Fig. S6 (Supporting information), the maximum absorption of PNB and corresponding NPs peaked at 499 and 501 nm, respectively. The maximum emission wavelength of PNB centered at 520 nm, but PNB NPs in water showed no fluorescence due to the aggregation-caused quenching (ACQ) [28]. The maximum for PCIB NPs was red-shifted several nanometers (3 nm for Cou and 4 nm for IBDP). The bathochromic shift of absorption confirmed formation of NPs [23, 26]. Interestingly, there was fluorescence resonance energy transfer (FRET) in PCIB (Fig. 1B) [29, 30]. Cou and IBDP in methanol showed blue (λem = 482 nm) and no fluorescence under 370 nm light excitation, but the absorption of maximum emission wavelength of IBDP peaked 557 nm with PCIB peaked at 388 nm for Cou and 537 nm for IBDP (Fig. 1A), respectively. However, the maximum absorption 510 nm light excitation. Furthermore the maximum emission wavelength of PCIB peaked at 482 and 557 nm under 370 nm light excitation, which meant FRET existed in PCIB.

|
Download:
|
| Fig. 1. A) UV-vis absorption and B) fluorescence spectra of PCIB in MeOH and PCIB NPs in water. | |
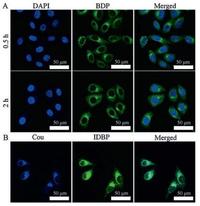
The cytocompatibility of PNB NPs towards human cervical carcinoma (HeLa) cells was evaluated via methyl thiazolyltetrazolium (MTT) assays. Cell viability was more than 80% when cells were incubated with both NPs (≥10 μg/mL) for 24 h as shown in Fig. S7 (Supporting information), indicating the good biocompatibility of both NPs. The cellular uptake was evaluated in HeLa cells by confocal laser scanning microscopy (CLSM). As shown in Fig. 2A, the cell nuclei were stained with 4', 6-diamidino-2-phenylindole (DAPI, blue) and the green fluorescence (BDP) in the cytoplasm could been clearly observed with time from 0.5 to 2 h, which confirmed that PNB NPs could be internalized by HeLa cells to emit fluorescence. To show the internalization of PCIB NPs, HeLa cells were incubated with NPs for 0.5 h. The blue fluorescence from Cou and the green fluorescence from IBDP were match well in cytoplasm (Fig. 2B). Fluorescence distributed in the cytoplasm in evenly for both PNB NPs and PCIB NPs, which showed well the outlines of cells. These results showed the great potential of bioimaging for both NPs.
|
Download:
|
| Fig. 2. CLSM images of HeLa cells incubated with A) PNB for 0.5 h and 2 h and B) PCIB NPs for 0.5 h. In A) cell nuclei stained by DAPI showed blue fluorescence and BDP showed green fluorescence in cells. The overlays of both images were shown in the rightmost. In B) Cou showed blue fluorescence and IBDP showed green fluorescence in cells. The overlays of both images were shown in the rightmost. | |
We used PCIB NPs as potential photosensitizers for PDT. First, active oxygen species (ROS) generation was demonstrated by using indocyanine green (ICG) as scavenger [31]. The absorbance intensity of ICG (λex = 790 nm) was unchanged under green light (560 nm, 10 mW/cm2) irradiation for 5 min as shown in Fig. S8 (Supporting information). However, the intensity of absorbance reduced continually in presence of PCIB as time went by (Fig. 3A), which confirmed that PCIB possessed ability to generate ROS. What's more, the absorption intensity of PCIB had hardly changed but almost 75% of ICG was degraded after irradiation for 4 min. Then the phototoxicities of PCIB NPs tawards HeLa cells were studied by MTT assays as showed in Fig. 3B. HeLa cells under irradiation (concentration of NPs = 0) grew normally and the cell viability did not decrease compared with the negative control in Fig. 3B. Furthermore, the cell viability of HeLa cells incubated with NPs but without irradiation did not decrease, either. However, under green light irradiation for 30 min, more than 85% of cells incubated with P4 NPs (0.2 μg/mL) were killed (Fig. 3B). These results revealed the effective PDT activity of PCIB upon irradiation.

|
Download:
|
| Fig. 3. A) The absorption intensity of ICG under green light irradiation with PCIB in DMF for 4 min. B) Cell viability of HeLa cells treated with PCIB NPs under green light irradiation for 0.5 h.*** P < 0.001. | |
We used 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) as a ROS sensor to assess the intracellular ROS generation ability of PCIB NPs [32, 33]. Oxidized DCFA-DA would emit green fluorescence. The experimental group showed stronger green fluorescence compared to the control groups (Fig. S9 in Supporting information). Flow cytometry (FCM) was used to examine quantitatively the fluorescence intensity in HeLa cells (Fig. S10 in Supporting information), which validated that the fluorescence intensity was higher in the experimental group where cells were incubated with PCIB NPs under irradiation than the control groups. At last, live/dead staining was used to distinguish the live and dead cells. Live cells were stained with calcein-AM to emit green fluorescence and dead/late apoptotic (red) cells were stained with propidium iodide (PI) to emit red fluorescence. As shown in Fig. S11 (Supporting information), only green fluorescence was observed in all control groups and red fluorescence was only found in the experimental group (cells incubated with PCIB NPs under irradiation), which showed PCIB NPs could kill cells under irradiation.
In summary, two PEGylated BODIPY (PNB and PCIB) which could self-assemble into nanoparticles were synthesized via multicomponent Passerini reaction for cellular imaging and photodynamic therapy. The formed PCIB NPs possessed potent photodynamic activity towards HeLa cells. The o-nitrobenzaldehyde and 7-diethylamino-4-formylcoumarin are light-sensitive [34, 35], which lay the root for smart drug delivery systems. This work highlights the potential of multicomponent reaction for functional molecules and corresponding nanoparticles.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 51522307 and 81673396).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.06.017.
| [1] |
L.L. Rui, H.L. Cao, Y.D. Xue, et al., Chin. Chem. Lett. 27(2016) 1412-1420. DOI:10.1016/j.cclet.2016.07.011 |
| [2] |
Z. Meng, B. Shan, L. Zhang, et al., Chin. Chem. Lett. 27(2016) 623-626. DOI:10.1016/j.cclet.2016.03.019 |
| [3] |
T.F. Cui, J. Zhang, X.D. Jiang, et al., Chin. Chem. Lett. 27(2016) 190-194. DOI:10.1016/j.cclet.2015.11.010 |
| [4] |
S.S. Lucky, K.C. Soo, Y. Zhang, Chem. Rev. 115(2015) 1990-2042. DOI:10.1021/cr5004198 |
| [5] |
L. Yang, J. He, Y. Wen, et al., J. Biomed. Nanotechnol. 12(2016) 1348-1373. DOI:10.1166/jbn.2016.2284 |
| [6] |
S. Mallidi, S. Anbil, A.L. Bulin, et al., Theranostics 6(2016) 2458-2487. DOI:10.7150/thno.16183 |
| [7] |
A. Zhou, Y. Wei, Q. Chen, D. Xing, J. Biomed. Nanotechnol. 11(2015) 2003-2010. DOI:10.1166/jbn.2015.2150 |
| [8] |
S.H. Voon, S.X. Tiew, C.S. Kue, et al., J. Biomed. Nanotechnol. 12(2016) 1431-1452. DOI:10.1166/jbn.2016.2263 |
| [9] |
S. Singh, A. Aggarwal, S.D.K. N.V.Bhupathiraju, et al., Chem. Rev 115(2015) 10261-10306. DOI:10.1021/acs.chemrev.5b00244 |
| [10] |
C.Cheng L. Wang, Feng L. K., Chem. Rev 114(2014) 10869-10939. DOI:10.1021/cr400532z |
| [11] |
Z. Hou, Y. Zhang, K. Deng, et al., ACS Nano 9(2015) 2584-2599. DOI:10.1021/nn506107c |
| [12] |
P.Z. Chen, H.R. Zheng, L.Y. Niu, et al., Chin. Chem. Lett. 26(2015) 631-635. DOI:10.1016/j.cclet.2015.04.018 |
| [13] |
Y. Zhang, Y.G. Gao, Y.D. Shi, et al., Chin. Chem. Lett. 26(2015) 894-898. DOI:10.1016/j.cclet.2015.05.032 |
| [14] |
P.C. Shi, X.D. Jiang, R.N. Gao, Y.Y. Dou, W.L. Zhao, Chin. Chem. Lett. 26(2015) 834-838. DOI:10.1016/j.cclet.2014.11.010 |
| [15] |
A. Kamkaew, S.H. Lim, H.B. Lee, et al., Chem. Soc. Rev. 42(2013) 77-88. DOI:10.1039/C2CS35216H |
| [16] |
S. Brauch, van Berkel S.S., B.Westermann, Chem. Soc. Rev 42(2013) 4948-4962. DOI:10.1039/c3cs35505e |
| [17] |
R. Kakuchi, Angew. Chem. Int. Ed. 53(2014) 46-48. DOI:10.1002/anie.v53.1 |
| [18] |
R.K. Li, Q.L. Yang, et al., Chin. Chem. Lett. 27(2016) 345-348. DOI:10.1016/j.cclet.2015.11.008 |
| [19] |
O. Kreye, T. Toth, M.A. Meier, J. Am. Chem. Soc. 133(2011) 1790-1792. DOI:10.1021/ja1113003 |
| [20] |
S.C. Solleder, M.A.R. Meier, Angew. Chem. Int. Ed. 53(2014) 711-714. DOI:10.1002/anie.201308960 |
| [21] |
L. Li, A. Lv, X.X. Deng, F.S. Du, Z.C. Li, Chem. Commun. 49(2013) 8549-8551. DOI:10.1039/c3cc44557g |
| [22] |
J. Zhang, M. Zhang, F.S. Du, Z.C. Li, Macromolecules 49(2016) 2592-2600. DOI:10.1021/acs.macromol.6b00096 |
| [23] |
W. Lin, T. Sun, Z. Xie, J. Gu, X. Jing, Chem. Sci. 7(2016) 1846-1852. DOI:10.1039/C5SC03707G |
| [24] |
W. Lin, T. Sun, M. Zheng, et al., RSC Adv. 4(2014) 25114-25117. DOI:10.1039/C4RA02666G |
| [25] |
W. Lin, X. Guan, T. Sun, et al., Colloids Surf. B 126(2015) 217-223. DOI:10.1016/j.colsurfb.2014.12.030 |
| [26] |
W. Lin, W. Zhang, T. Sun, et al., Langmuir 32(2016) 9575-9581. DOI:10.1021/acs.langmuir.6b02118 |
| [27] |
W. Lin, Y. Li, W. Zhang, et al., ACS Appl. Mater. Interfaces 8(2016) 24426-24432. DOI:10.1021/acsami.6b07103 |
| [28] |
C. Ren, H. Wang, D. Mao, et al., Angew. Chem. Int. Ed. 54(2015) 4823-4827. DOI:10.1002/anie.201411833 |
| [29] |
T.T. Meng, Y.X. Liu, M.T. Liu, et al., Chin. Chem. Lett. 26(2015) 1179-1182. DOI:10.1016/j.cclet.2015.05.039 |
| [30] |
L.X. Yu, Y. Liu, S.C. Chen, Y. Guan, Y.Z. Wang, Chin. Chem. Lett. 25(2014) 389-396. DOI:10.1016/j.cclet.2013.12.014 |
| [31] |
C.Y. Tang, F.Y. Wu, M.K. Yang, et al., Int. J. Mol. Sci. 17(2016) 219-226. DOI:10.3390/ijms17020219 |
| [32] |
W. Zhang, W. Lin, X. Zheng, S. He, Z. Xie, Chem. Mater. 29(2017) 1856-1863. DOI:10.1021/acs.chemmater.7b00207 |
| [33] |
W. Zhang, W. Lin, Q. Pei, et al., Chem. Mater. 28(2016) 4440-4446. DOI:10.1021/acs.chemmater.6b01641 |
| [34] |
W. Szymanski, W.A. Velema, B.L. Feringa, Angew. Chem. Int. Ed. 53(2014) 8682-8686. DOI:10.1002/anie.201402665 |
| [35] |
S. Mura, J. Nicolas, P. Couvreur, Nat. Mater. 12(2013) 100-991. DOI:10.1038/nmat3556 |
 2017, Vol. 28
2017, Vol. 28 


