b University of Chinese Academy of Sciences, Beijing 100049, China
In the past decades, nanomedicine has been extensively developed for versatile medical applications ranging from diagnostic imaging [1-8] to therapy [9-11]. These nanosystems can improve the therapeutic efficiency by altering their in vivo biodistribution [12] and prolonging the circulation within the body [13, 14]. Especially, researchers have explored abundant stimuliresponsive nanoplatforms for targeted imaging and therapeutic treatments with high disease specificity [15]. These stimuliresponsive nanosystems change their physiochemical or biological performance, and release agents for diagnosis and therapy when exposed to corresponding stimuli [16, 17]. These stimuli include extrinsic triggers (e.g., magnetic field [18, 19], ultrasound [20, 21] and X-ray irradiation [22]) and internal triggers [23-25] (e.g., pH [26-28], reducing condition [28-31], enzyme [32, 33] and H2O2 [7]). Among these stimuli-responsive modalities, pH-responsive nanoplatforms have been extensively explored because of the mild acidic microenvironment of tumor [5, 7, 34, 35]. This review focuses on the H2O2-sensive nanomedicine which can be endowed with more specific theranostic applications since H2O2 is one kind of important reactive oxygen species (ROS) in human body [36].
Free radicals and other ROS [37-44], are present in the human body, which are also persistently generated. There are various kinds of free radicals in physiological environment, such as the superoxide radical (O2•-) [39-41], hydroxyl (OH•) [39-41], and nitric oxide (NO•) [38-42]. Hydrogen peroxide (H2O2) [45, 46], singlet oxygen (1O2) [39-41, 47] and peroxynitrite (ONOO-) [38-42] are not the typical free radicals but they can be easily transformed into free radicals in physiological conditions. ROS is the generic term of free radicals and nonradicals. Under normal circumstances, the generation of ROS and the antioxidant defenses of organism maintain a dynamical balance. However, this balance can be broken when it is triggered by external stimulation (such as injury). The imbalance of ROS and antioxidant defenses will induce oxidative stress which can cause cell damage and death. It has been verified that numerous human diseases are associated with severe oxidative stress [37, 38, 48-51], such as Parkinson's disease [51], neurotoxins [48] atherosclerosis, etc.
Hydrogen peroxide (H2O2) [39-41] is generally regarded as the most abundant and stable ROS. On one hand, it can be converted to hydroxyl radical (OH•), which is more toxic than other ROS with a short half-time of only 10-9 s. On the other hand, H2O2 can be generated in vivo when catalyzed by O2•-dismutase. Therefore, H2O2 plays a vital function in balancing the free radicals. Based on the close relationship of ROS with some diseases, the ROS detection, especially H2O2, is believe to provide important physiological and biological information for diagnosis of these diseases.
To defense against ROS-induced damage in physiological conditions, the enzymes of glutathione peroxidase and catalase play a key role [36]. Both of these two enzymes can remove H2O2. Additionally, superoxide dismutase (SOD) [43], which can convert O2•-into H2O2 and O2, exert the specific function in weakening inflammation induced by excessive ROS. All of the abovementioned enzymes can be used to regulate the ROS balance (especially H2O2) and further relieve the physiological damages. It offers a new strategy for the treatment of H2O2-relevant diseases, such as tumor, Alzheimer's disease [51-56] and inflammation [57-60], etc.
Especially, the malignant tumor cells can produce excessive H2O2, therefore the tumor microenvironment presents a significantly high H2O2 concentration as compared to normal tissues. Catalase, which can catalyze H2O2 to generate oxygen, provides a feasible method to modulate the tumor hypoxia [61-64] and improves the efficiency of photodynamic therapy (PDT) [65-67] and chemo/radiotherapy [68, 69]. The tumor hypoxia [70] (the imbalance between the intake oxygen and the consumption of oxygen) is one of the specific features of solid tumors, which is induced by the rapid proliferation of cancer cells and the abnormal angiogenesis [71-77]. Photodynamic therapy (PDT) and radiotherapy (RT) [68], as non-invasive treatments of tumor, have shown the high therapeutic efficiency. However, the therapeutic species as produced by PDT/RT is the different kinds of ROS [78]. Therefore, the therapeutic efficiency of PDT/RT would be significantly lowered due to the inadequate oxygen supply in hypoxic solid tumors. As a result, using catalase or catalase-mimic metallic oxide (e.g., MnO2 and CeO2) to catalyze H2O2 and generate oxygen can be developed to improve the therapeutic efficiency/outcome of PDT/ RT [68].
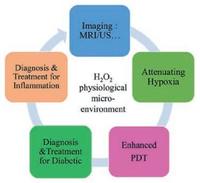
Because of relatively high H2O2 concentration of some diseases, H2O2-responsive nanosystems can be developed for some specific biomedical applications, including diagnostic imaging [79, 80], relieving tumor hypoxia [71], enhanced PDT [79], controllable drug release [81] and therapy of certain diseases [58-60] (Scheme 1). Furthermore, since the oxidation process of glucose into gluconic acid by glucose oxidase (GOx) can generate H2O2 as the main byproduct, the detection of glucose concentration of diabetes [44, 82] can be translated into detecting H2O2, which provides a new method for the diagnosis and treatment of diabetes by using GOxcoated nanosystems. Based on the specific functions of H2O2 for theranostic applications and the fast development of this field, this review will focus on the design and application of H2O2-responisive theranostic nanoplatforms for versatile theranostic applications. Following the discussion on several representative paradigms of H2O2-responsive nanosystems, the facing challenges and future developments of this research field are also briefly discussed, aiming to further promote the clinical translation of this unique stimuli-responsive theranostic modality.
|
Download:
|
| Scheme1. The summative scheme of H2O2-responsive theranostic nanomedicine, including diagnostic imaging, attenuating hypoxia, enhanced PDT and diagnosis/ therapy for diabetic and inflammation. | |
2. H2O2-responsive diagnostic imaging
Nanoparticles-based contrast agents have been extensively explored for various diagnostic-imaging modalities (e.g., MRI, ultrasound imaging, computed tomography, positron emission tomography) because they can significantly improve the imaging precision or monitor the therapeutic process [1-8, 83]. Especially, the imaging modality with specific responses to the diseases can achieve the imaging specificity and efficiency. In this respect, H2O2-responsive nanosystems can be used to achieve the specific imaging effect in the lesion regions with high H2O2 concentration locally as compared to normal tissues [84-88].
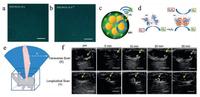
Although various pH-responsive imaging agents have been developed for tumor imaging [89-91], very few reports have been found to focus on the detection of reactive oxygen species (ROS). Wang et al. proposed one kind of hybrid nanogel probe (SGC), which was constituted by superparamagnetic iron oxide (SPIO) particles, two kinds of enzymes (catalase and superoxide dismutase), and glycol chitosan gel [85]. SGC could generate bubbles (oxygen gas) continuously when triggered by 50 μmol/L H2O2 (Fig. 1a and b), making it a desirable US contrast agent in physiological microenvironment (Fig. 1c). The generation of oxygen microbubbles included two steps: (ⅰ) superoxide (O2⋅) in the tissues was converted into H2O2 and O2 by superoxide dismutase; (ⅱ) the generated H2O2 was then converted to water and O2 by catalase (Fig. 1d). The rapid generation of abundant O2 induced the formation of bubbles, which then served as USimaging contrast agent. The enhanced US-imaging performance was further verified on VX2-tumor-bearing rabbits on both transverse and longitudinal scanning orientations to demonstrate the high US-imaging performance (Fig. 1e and f). This result demonstrates the possibility of the decomposition of H2O2 to generate O2 bubbles for US imaging.
|
Download:
|
| Fig. 1. Dual enzyme-loaded hybrid nanogel probe (SGC) for H2O2-responsive ultrasound and MR imaging. (a, b) Representative optical microscopic images of in vitro bubble generation from SGC triggered by 50 μmol/L H2O2 at various durations. (c) Schematic diagram of SGC for enhanced US and MR imaging. (d) The scheme of the mechanism of H2O2-responsive bubble generation for US imaging. (e) The scheme and (f) US images of VX2-tumor-bearing rabbits before and after intratumoral injection of SGC at different times. Yellow arrows indicate the injection sites in the VX2 tumors. Copied with permission [85]. Copyright 2015, American Chemical Society. | |
As H2O2 is an important indicator in diagnosis of some diseases such as lung inflammation, cystic fibrosis, etc. Precise non-invasive detection of H2O2 is particularly important. Christopher J. Chang et al. have developed a reaction-based MRI contrast agent for detecting H2O2 [92]. As shown in Fig. 2a, hyperpolarized 13C was used as the MRI contrast agent and the reaction of α-ketoacids oxidized by hydrogen peroxide to carboxylic acids was used as the H2O2-detecting mechanism. 13C NMR spectra showed that hyperpolarized 13C-BFA could be rapidly oxidized to 13C-BA in the presence of H2O2 (the peak position of 13C-BA appeared at 176 ppm, and 13C-BFA as a dualpeaks cantered at 173.5 ppm) (Fig. 2b). From the result of 13C NMR spectra, the dependence of the ratio of 13C-BA and 13C-BFA versus the concentration of H2O2 was acquired, indicating the feasibility of using 13C-BFA as the H2O2-responsive MRI contrast agent (Fig. 2c). In fact, the MRI images verified that 13C-BFA presented an enhanced T2-weighted MR imaging with the increase of H2O2 concentration (Fig. 2d-h).

|
Download:
|
| Fig. 2. H2O2-responsive MR imaging. (a) The scheme of a MRI contrast agent-hyperpolarized 13C-BFA and its reaction with H2O2. (b) 13C NMR spectra of hyperpolarized 13CBFA reacted with 10, 100, 250, 500, 750, and 1000 μmol/L H2O2 for 21 s respectively. (c) Linear dependence of the percentage of integrated peak intensities in the 13C NMR spectra of the C1 (carboxylate carbon) of 13C-BA to the one of 13C-BFA versus the concentration of H2O2. (d) 1H T2-weighted MR imaging images of different samples including 5 mol/L thermally polarized 13C-BA in dimethyl acetamide (DMA), 5 mol/L thermally polarized 13C-BFA in water and 20 μmol/L 13C-BFA in phosphate buffer (pH.7.8, with 0.3 μmol/L EDTA) with 0, 25, 50, 100, and 200 μmol/L H2O2 respectively. (e-h) The 13C MRI images of 13C-BA in DMA (e), 13C-BFA in water (f), 13C-BA produced from the H2O2-triggered conversion of 13C-BFA in phosphate buffer (pH.7.8, with 0.3 μmol/L EDTA) (g) and hyperpolarized 13C-BFA in phosphate buffer (pH.7.8, with 0.3 μmol/L EDTA) (h). Copied with permission [92]. Copyright 2011, American Chemical Society. | |
3. Attenuating tumor hypoxia
Hypoxia [61, 62, 70-77] is a general feature of various burgeoning solid tumors, which is usually caused by the proliferation of tumor cells but the intake oxygen can't balance the oxygen consumption [63, 64]. Unfortunately, hypoxia plays a significant role in tumor proliferation and resistance to radio-/chemo-and photodynamic therapy [93]. As a result, attenuating tumor hypoxia is a feasible strategy to improve the efficacy of tumor treatments. A variety of methods have been developed to relief tumor hypoxia, including reducing the consumption of oxygen [94], increasing intratumoral blood flow [95] and using oxygen reservoirs for more intake oxygen [68, 96], Especially, the decomposition of the intratumoral hydrogen peroxide (H2O2) to generate oxygen has been utilized to alleviate hypoxia [88, 97].
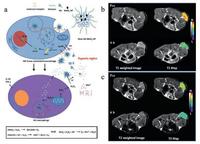
Based on the high catalase-like performance of MnO2 for the decomposition of H2O2, MnO2 nanoparticles (NPs) have been used to catalyze H2O2 decomposition and generate O2 for relieving the tumor hypoxia. Moreover, hyaluronic acid (HA) was adopted for reprogramming M2-skewed tumor-associated macrophages (TAMs) of pro-tumor to anti-tumor M1 macrophages. Therefore, HA-MnO2 NPs had the ability to relief tumor hypoxia and enhance chemotherapy efficiency simultaneously. Furthermore, mannan was modified on the surface of HA-MnO2 NPs to target the tumor [98]. As a result, Man-HA-MnO2 NPs can alleviate tumor hypoxia and change the M2-TAMs into M1 macrophages, which could modulate the tumor chemo-resistance (Fig. 3a). From the immunofluorescence images of tumor sections, the increase of M1 macrophages and the decrease of M2-TAMs in tumors were observed (Fig. 3b). Especially, the influence of Man-HA-MnO2 NPs on tumor oxygen levels can be obviously observed by using blood oxygen level-dependent MRI (BOLD MRI) (Fig. 3c). With the increase of observing duration, the T2* values of tumor treated with Man-HA-MnO2 NPs increased significantly. This paradigm provides the direct evidence that the decomposition of H2O2 could improve the tumor oxygen level and enhance the chemotherapeutic efficiency subsequently.
|
Download:
|
| Fig. 3. MnO2 NPs catalyze the decomposition of H2O2 to generate O2 for overcoming tumor hypoxia. (a) The acting mechanism illustration of multifunctional Man-HA-MnO2 (hyaluronic acid modified, mannan-conjugated MnO2 nanoparticles). (b) The immunofluorescence images of 4T1 tumor sections obtained from tumor-bearing mice treated by Man-HA-MnO2 NPs. The sections were stained with antitumor M1 macrophages and M2-skewed tumor-associated macrophages maker (green). The scale bar in the picture represent was 100 μm. (c) T2* values' increase of BOLD MRI in tumor site after 3 and 7 days with intravenous injection of Man-HA-MnO2 NPs. Copied with permission [98]. Copyright 2015, American Chemical Society. | |
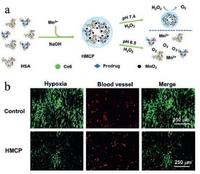
An intelligent albumin-MnO2 NPs were proposed as H2O2-responsive nanocarriers for modulating tumor hypoxia [99]. Human serum albumin (HAS) went through a biomineralization process with the addition of manganese ions in NaOH solutions and finally turned into HAS-MnO2-Ce6&Pt (HMCP) NPs. These HMCP NPs could catalyze the decomposition of H2O2 to generate oxygen and modulate the hypoxia of tumor tissue (Fig. 4a). This assumption has been verified from the immunofluorescence images of tumor sections. The hypoxia areas decreased obviously when the 4T1 tumor-bearing mice were treated with HMCP NPs as compared to the control group without the treatment (Fig. 4b).
|
Download:
|
| Fig. 4. Schematic diagram for the synthetic procedure of HMCP NPs. (b) Representative immunofluorescence images of tumor sections with hypoxia staining after different treatments. Copied with permission [99]. Copyright 2016, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | |
For attenuating tumor hypoxia and improving the efficiency of radiotherapy, a novel type of catalase-encapsulated TaOx nanoshells (TaOx@Cat) were explored [69]. Furthermore, to improve the biocompatibility, PEG-modified TaOx@Cat (PEG-TaOx@Catalase) were further synthesized (Fig. 5a). The as-synthesized TaOx@Cat nanoshells exhibited high mono-dispersity with uniform sizes (Fig. 5b). Moreover, the encapsulation of catalase could generate oxygen sequentially in H2O2 solutions (Fig. 5c and d). The in vivo experiments further verified the capability of PEG-TaOx@Catalase to alleviate tumor hypoxia (Fig. 5e-g) and enhance the radiotherapy efficiency subsequently.

|
Download:
|
| Fig. 5. H2O2-responsive TaOx@Cat-PEG for hypoxia relief and enhanced RT. (a) The scheme showing the synthetic process of PEG-TaOx@Catalase. (b) Representative TEM images of as-prepared PEG-TaOx@Catalase NPs. (c) The generation of oxygen in H2O2 solutions with TaOx@BSA and TaOx@Cat addition respectively. (d) The oxygen concentration changes in H2O2 solutions with the addition of various concentrations of TaOx@Cat-PEG. (e) Representative fluorescent micrographs of tumor sections with cell nuclei (blue), blood vessels (red), and hypoxia areas (green) stained with DAPI, anti-CD31 antibody, and HIF-1a antibody, respectively. (f) Quantification of tumor hypoxia for mice with various treatments. (g) Quantification of angiogenesis area of tumor-bearing mice with different treatments. Copied with permission [69]. Copyright 2016, WILEYVCH Verlag GmbH & Co. KGaA, Weinheim. | |
4. Enhancing photodynamic therapy
Photodynamic therapy (PDT) [65-67], as a non-invasive therapeutic modality, has attracted extensive attention and been approved by United States Food and Drug Administration (FDA). However, the generation of ROS, which plays a key role in PDT treatment, highly depends on the oxygen level in tumor tissues. Therefore, tumor hypoxia is deemed to weaken the therapeutic efficiency of PDT. Fortunately, H2O2 overexpressed in tumor can be utilized to generate oxygen for mitigating hypoxia, so it is also anticipated to have the capability for improving the PDT efficiency [86]. On this ground, several kinds of H2O2-triggered nanoplatforms have been developed for enhancing PDT efficiency [78, 79, 100-103].
As aforementioned, MnO2 can react with H2O2 to generate oxygen. Since oxygen is a necessarily required in the PDT process, MnO2 is also expected to play a significant role in enhancing the PDT efficiency [104]. As a typical paradigm, MnO2 and Ce6 (a typical photosensitizer) were co-coated in poly(allylamine hydrochloride) (PAH) and then packaged in the polymer polyacrylic acid (PAA) shell [104]. To further improve the stability of NPs in physiological condition, PEG was anchored onto the surface of the pre-formed NPs to form Ce6@MnO2-PEG composite nanosystems (Fig. 6a). These Ce6@MnO2-PEG NPs significantly alleviated the tumor hypoxia (Fig. 6b), endowing the cancer cells with high sensitivity to PDT treatment. Importantly, the substantially suppressed tumor growth (Fig. 6c) and the H&E staining results (Fig. 6d) of the tumor-bearing mice treated after the treatment with Ce6@MnO2-PEG and laser further verified the high therapeutic efficiency of Ce6@MnO2-PEG for hypoxia tumor.

|
Download:
|
| Fig. 6. Ce6@MnO2-PEG for H2O2-enhanced PDT against cancer. (a) Schematic illustration of the synthetic procedure and the composition of Ce6@MnO2-PEG. (b) Modulating effect of Ce6@MnO2-PEG on tumor hypoxia: representative immunofluorescence images of tumor slices. (c) Relative tumor-volume changing curves of tumor-bearing mice with different treatments. (d) H&E staining images of tumor sections collected from mice with different treatments on 24 h after light irradiation. Copied with permission [104]. Copyright 2016, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | |
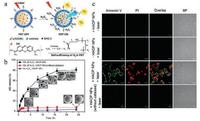
In addition to inorganic MnO2 NPs, the organic enzyme catalase could also be utilized to catalyze the decomposition of hydrogen peroxide and generate oxygen afterwards. For instance, the H2O2-activatable HAOP NPs were elaborately constructed with the loaded catalase, black hole quencher-3 (BHQ-3), methylene blue (MB) and c(RGDfk) modified poly(D, L-lactic-co-glycolic acid) (PLGA) shell [105]. Each component of this nanosystem has it's unique function: (ⅰ) catalase was loaded in PLGA shell and used to catalyze the decomposition of H2O2 and generate O2; (ⅱ) BHQ-3 was applied to quench the excited photosensitizer; (ⅲ) MB as a typical photosensitizer was loaded for tumor PDT; (ⅳ) c(RGDfk) as a classical tumor-targeting ligand was anchored for targeted DPT; (ⅴ) PLGA shell served as nanocarriers for catalase, MB and BHQ-3 (Fig. 7a). In the presence of H2O2, the catalase component of HAOP NPs catalyzed H2O2 to generate O2, making the tumor cells sensitive to PDT treatment. In addition, the generation of oxygen also caused the PLGA shell collapse and controllable release of MB (Fig. 7b). Furthermore, the effect of H2O2-activatable HAOP NPs on improving phototoxicity of tumor cells was evaluated by confocal fluorescence assay (Fig. 7c) and the results verified the capability of HAOP NPs for enhanced PDT treatment.
|
Download:
|
| Fig. 7. H2O2-activatable HAOP NPs for improving the PDT efficiency. (a) Schematic illustration of H2O2-activatable oxygen generation and controllable release of photosensitizer. (b) In vitro H2O2-activatable controllable-release of MB from HAOP NPs with/without the loaded catalase. The inset SEM image showed the time-dependent morphological changes of HAOP NPs in medium with 100 μmol/L H2O2. (c) Confocal fluorescence images of U87-MG cells after various treatments. The cells were stained with Annexin V-FITC/PI. Copied with permission [105]. Copyright 2015, American Chemical Society. | |
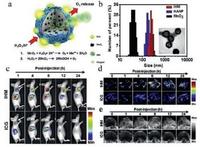
Based on the high reactivity of MnO2 with H2O2, an O2-generating hybrid nanoparticle (IHM, ICG-HANP/MnO2) with three-modality imaging capability were designed for enhanced PDT of tumor [106]. As shown in Fig. 8a, MnO2 NPs were encapsulated into hyaluronic acid nanoparticle (HANP), which could react with H2O2. During this reaction, the generated O2 could suppress the tumor hypoxia so that PDT efficiency could be improved. Furthermore, ICG were modified on the surface of HANP which acted as contrast agent for the fluorescent/PA imaging. Compared with free-ICG, IHM presented a tumor-targeting behaviour, causing significantly enhanced diagnostic-imaging contrast (Fig. 8c and d). The results of DLS and TEM (Fig. 8b) verified that MnO2 NPs and ICG were loaded into IHM simultaneously with nanoscale size and monodispersity. Interestingly, O2 bubbles generated from the reaction of MnO2 and H2O2 induced a high US imaging signal (Fig. 8e). Therefore, the fluorescent, PA and ultrasound imaging could be simultaneously achieved to monitor the PDT process in real time. Importantly, the tumors of tumorbearing mice treated with IHM were almost completely eliminated, suggesting that O2-generating IHM could bring with the high PDT efficiency (Fig. 8f and g).
|
Download:
|
| Fig. 8. H2O2-responsive fluorescent/photoacoustic/ultrasound imaging and enhanced PDT. (a) Schematic diagram of design principle of ICG-HANP/MnO2 (IHM). (ⅰ) MnO2 NPs were encapsulated into the hyaluronic acid nanoparticle (HANP) and reacted with H2O2 inducing oxygen generation for enhanced US imaging and PDT efficiency. (ⅱ) ICG (indocyanine green) was modified on the surface of HANP, acting as the contrast agent for the fluorescent/PA imaging. (b) TEM image and dynamic light scattering analysis of MnO2 (35 ± 2.5 nm), HANP (180 ± 4.5 nm), and IHM (239 ± 5.8 nm) NPs. (c) In vivo monitoring of the IHM accumulation in tumor location (as arrows indicated in the image) via NIR fluorescent imaging of SCC7 tumor-bearing mice treated with IHM via intravenous injection. (d) IHM injected intravenously for PA imaging on tumor-bearing mice. (e) IHM injected intravenously for ultrasound imaging and monitoring the MnO2-induced generation of O2 in tumors and enhanced the PDT efficiency subsequently. (f) Digital pictures of tumor-bearing mice treated with IHM at different time points. (g) Tumor-volume changes of mice treated after different treatments. Copied with permission [106]. Copyright 2016, Elsevier ltd. | |
5. H2O2-responsive drug-release
As discussed above, the designed H2O2-activatable HAOP NPs behaved a sequential and responsive release of MB in presence of H2O2. [105] Therefore, similar to mostly explored pH-responsive drug-releasing pattern, H2O2-responsive release of loaded agent could also be achieved within the specific microenvironment with overexpressed H2O2 amount [81].
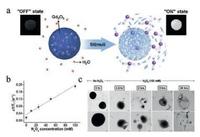
Because of the diversity of organic polymers, a variety of bioresponsive degradable polymer capsules have been explored for biomedical applications [17, 102]. On the basis of H2O2-degradable polymer matrix, T1-weighted MRI contrast agent Gd2O3 NPs were encapsulated and protected from the aqueous environment [87]. As a result, Gd2O3 NPs behaved selective imaging in targeting tissue with higher H2O2 concentration as compared to normal tissues (Fig. 9a). As a matter of fact, D1/T1 values of Gd2O3 encapsulated in H2O2-responsive polymer NPs exhibited an obvious increase with the elevation of H2O2 concentrations (Fig. 9b). TEM images of the morphology of the composite NPs after the incubation with/without 100 μmol/L H2O2 at varied durations were observed. It has been found that these NPs behaved a H2O2-responsive degradation which would further facilitate the release and MR imaging of Gd2O3 contrast agents (Fig. 9c).
|
Download:
|
| Fig. 9. H2O2-triggered release of Gd2O3 and T1-weighted MR imaging. (a) Schematic illustration of H2O2-degradable polymer matrix controlling the release of Gd2O3 and enhanced MR imaging. (b) Δ1/T1 values of Gd2O3 encapsulated in H2O2-responsive polymer capsule in versus H2O2 concentrations. (c) TEM images of the morphology of H2O2-responsive polymer nanoparticles with Gd2O3 encapsulation incubated with/without 100 μmol/L H2O2 at varying times. Copied with permission [87]. Copyright 2013, American Chemical Society. | |
6. Diagnosis and treatment of inflammation
It has been demonstrated that excessive H2O2 generation is one of the specific features of inflammation tissues [58-60]. Therefore, the detection of H2O2 might be effective in inflammation diagnosis. In addition, H2O2 molecules could be potentially converted to hydroxyl radical, which might induce severe tissue damage and apoptosis. Therefore, the consumption of H2O2 for some H2O2-relevant diseases is expected to achieve the enhanced efficiency [107].
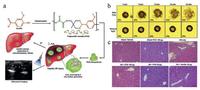
Hepatic ischemia/reperfusion (I/R) injury is the most common problem in a variety of liver clinical surgeries, which further caused tissues damages and inflammatory diseases [108]. Especially, I/R injury could also cause the generation of abundant ROS (hydroxyl radical, H2O2, etc.). Therefore, the consumption of H2O2 and triggering the release of antiphlogistic are considered as a feasible method to relieve hepatic I/R injury. On this ground, H2O2-responsive bubble-generating PVO microparticles were designed to conquer hepatic I/R injury (Fig. 10a) [108]. The PVO microparticles could decompose responsive to H2O2, which further released vanillin (as antioxidant and anti-inflammatory drug) and generated CO2 bubbles to serve as contrast agents of ultrasound imaging. The ability of generating CO2 bubbles of PVO microparticles were directly observed by TEM. The images also showed the collapse of the PVO microparticles to release vanillin simultaneously (Fig. 10b). H&E staining images of liver tissues revealed that PVO microparticles tremendously attenuated the tissue damage induced by hepatic I/R injury.
|
Download:
|
| Fig. 10. H2O2-responsive bubble-generating nanotheranostics. (a) Schematic illustration of the synthesis of poly(vanillin oxalate) (PVO), the consumption of H2O2 inducing CO2 generation for US imaging and vanillin release for anti-inflammation. (b) Representative optical microscopic pictures of CO2 bubbles generated from PVO microparticles when incubated with 10 μmol/L H2O2. (c) H&E staining images of liver slices obtained from mice bearing hepatic ischemia/reperfusion (I/R) injury after different treatments. Copied with permission [108]. Copyright 2016, Elsevier Ltd. | |
7. Diagnosis and treatment for diabetic
It has been well demonstrated that glucose can be oxidized by glucose oxidase (GOx) to generate H2O2 simultaneously, which provide possibility and foundation for the detection of H2O2 to monitor and regulate the glucose concentration [82, 109, 110].
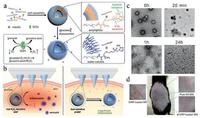
The painless and real-time glucose-monitoring therapy of diabetic remains a great challenge. Recently, a painless microneedle-array patch was developed for achieve this goal [110]. In this work, researchers developed hypoxia and H2O2 dualsensitive diblock copolymer self-assembly vesicles (d-GRPs), and then used d-GRPs loaded with insulin and GOx [110]. These dual-sensitive vesicles could disassemble to release insulin as triggered by higher glucose concentration (Fig. 11a). GOx oxidized glucose to generate H2O2 and consume the oxygen. Simultaneously, hypoxia and H2O2 dual-sensitive diblock copolymer could consume H2O2 to avoid possible inflammation (Fig. 11b). TEM test verified the vesicles disassembly after the incubation with glucose (Fig. 11c). Furthermore, the in vivo experiments on mouse verified that the d-GRP(E)-loaded microneedles wouldn't induce the significant damage. This work provides a feasible method to potentially improve the quality of diabetic patients' life.
|
Download:
|
| Fig. 11. H2O2/hypoxia dual-sensitive and glucose-responsive d-GRPs loading painless microneedle-array patch. (a) Schematic illustration of self-assembly process of d-GRPs and the mechanism of its dual-sensitive insulin releasing. (b) The scheme of d-GRPs/GRPs loading microneedle-array for in vivo delivery of insulin. d-GRPs: H2O2 and glucose dual-responsive polymersomes; GRP: non-H2O2-responsive polymersomes. (c) TEM images of insulin encapsulated d-GRPs incubated with glucose for 0 min, 20 min, 1 h and 24 h. (d) Digital photograph of a mouse treated with hyaluronic acid, GRP-and d-GRP-loaded microneedle patches at different sites. Copied with permission [110]. Copyright 2017 the, American Chemical Society. | |
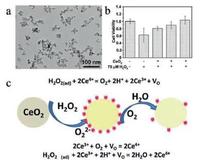
In addition to MnO2 NPs, other inorganic metallic oxides can also react with H2O2 for biomedical applications. CeO2 NPs are one of the mostly explored nanosystems for versatile biomedical purposes, including the reaction with H2O2. [109] For instance, CeO2 NPs with uniform spherical shape were synthesized via a simple hydrothermal method (Fig. 12a). In vitro experiment proved its anti-oxidation performance when the cells were incubated with CeO2 NPs and 75 μmol/L H2O2 (Fig. 12b). Raman spectra were used to explore the mechanism of the reaction of CeO2 NPs with H2O2 (Fig. 12c). It was proved that with the addition of H2O2, Ce4+ was reduced into Ce3+ and H2O2 was oxidized to generate O2⋅. Furthermore, in the mild acidic microenvironment, the H2O2 could further oxidize Ce3+ into Ce4+, which guaranteed the continuous anti-oxidation process of ceria NPs.
|
Download:
|
| Fig. 12. The anti-oxidation performance of CeO2 NPs. (a) TEM images of synthesized CeO2 NPs. (b) In vitro assessment verifying the anti-oxidation performance of ceria NPs at cell level. (c) Schematic illustration of the mechanism of anti-oxidation process of ceria with H2O2. Copied with permission [109]. Copyright 2015, Royal Society of Chemistry. | |
8. Conclusions and outlook
The rapid development of nanotechnology and materials chemistry has spurred the fast progress of theranostic nanomedicine very recently. Over the past decades, various nanosystems with specific functionalities for efficient diagnosis and therapy of diseases have been explored to satisfy the strict clinicalapplication requirements. Since stimuli-sensitive nanomedicine can induce site-/time-specific theranostic performance, the microenvironment-triggered theranostic nanoplatform have aroused the great attention of scientific community recently. On this ground, H2O2-responsive nanoplatforms have been extensively explored to exert the specific theranostic performance in biomedical applications because of the high level of overexpressed H2O2 as a hallmark of some diseases, such as malignant tumor, inflammation, neurodegenerative diseases (Alzheimer and Parkinson), etc. The very-recent specific biomedical applications of H2O2-responsive nanoplatforms, including diagnostic imaging, attenuating tumor hypoxia, enhancing the therapeutic efficiency of photodynamic therapy/radiation therapy/chemotherapy and theranostic of inflammation/diabetic, have been summarized and discussed in this review.
In addition to aforementioned biomedical applications of H2O2-responsive theranostic nanomedicine, there are also other application paradigms, such as neurodegenerative diseases (Alzheimer [53-56], Parkinson [51], etc.). However, the development of H2O2-responsive nanomedicine is much slower as compared to other stimuli-responsive modalities, such as pH responsibility. There are still some unresolved critical issues those restrict their extensive biomedical applications and clinical translations.
First, the fabrication of H2O2-responsive nanosystems is still challenging. Very few organic (e.g., enzyme-loaded organic NPs) or inorganic (e.g., MnO2, CeO2, Fe3O4) nanoplatforms have been successfully developed for this specific responsibility. The fast development of nano-synthetic chemistry makes the exploring of new theranostic nanoplatforms with H2O2 responsibility possible. Especially, the following researches should focus on the large-scale fabrication of these nanosystems to guarantee their further potential industrial translation.
Second, H2O2-responsive theranostic modalities are still very rare. The tumor tissues overexpresses H2O2, but the H2O2 level is still low, which cannot guarantee the following theranostic process with high efficiency. Therefore, the theranostic efficiency of these developed nanoplatforms should be further enhanced. In addition, other H2O2-responsive theranostic modalities should be further developed to take the specific advantage of this responsive modality for theranostic applications. For instance, the Fe3O4-based Fenton reaction has been designed for chemo-dynamic therapy against tumor [111].
Third, the biosafety of these H2O2-responsive nanoplatforms should be fully revealed to further guarantee their clinical translation. The current researches mainly focus on the exploring of new H2O2-responsive nanoplatforms and improving their theranostic performances, ignoring their biological effect and biosafety. Therefore, the following researches should focus on their systematic in vivo biosafety evaluations of these H2O2-responsive nanoplatforms for guaranteeing their further clinical translation.
Last but not least, more extensive collaborations should be established based on the multidisciplinary nature of this research field. The progress of H2O2-responsive theranostic nanomedicine should be devoted by the researcher with varied research background, including material science, chemistry, biology and biomedicine. The close collaborations among different researches provide the bases for the potential clinical translation of this theranostic modality.
As a new but highly versatile emerging responsive theranostic modality, H2O2-responsive theranostic nanomedicine has shown its high specificity and theranostic performance for various biomedical applications, which has paves a new way for the theranostic of diseases with overexpressed H2O2. As far as the aforementioned critical issues are adequately solved, this unique H2O2-responsive theranostic nanomedicine is highly expected to enter the clinical stage to benefit the personalized biomedicine in the near future.
AcknowledgmentsWe greatly acknowledge the financial support from the National Key Research and Development Program of China (No. 2016YFA0203700), National Natural Science Foundation of China (No. 51672303), Shanghai Excellent Academic Leaders Program (No. 14XD1403800), Young Elite Scientist Sponsorship Program by CAST (No. 2015QNRC001), Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2013169) and Development Fund for Shanghai Talents (2015).
| [1] |
F. Yang, L. Li, Y. Li, et al., Phys. Med. Biol. 53(2008) 6129-6141. DOI:10.1088/0031-9155/53/21/016 |
| [2] |
E. Terreno, D.D. Castelli, A. Viale, et al., Chem. Rev. 110(2010) 3019-3042. DOI:10.1021/cr100025t |
| [3] |
A. Louie, Chem. Rev. 110(2010) 3146-3195. DOI:10.1021/cr9003538 |
| [4] |
Y. Chen, H. Chen, Y. Sun, et al., Angew. Chem. 50(2011) 12505-12509. DOI:10.1002/anie.201106180 |
| [5] |
J.L. Major, T.J. Meade, Acc. Chem. Res. 42(2009) 893-903. DOI:10.1021/ar800245h |
| [6] |
M. Ao, Z. Wang, H. Ran, et al., J. Miomed. Mater. Res. B:Appl. Biomater 93(2010) 551-556. |
| [7] |
S. Kummar, R. Kinders, L. Rubinstein, et al., Nat. Rev. Cancer. 7(2007) 131-139. DOI:10.1038/nrc2066 |
| [8] |
Y. Sun, Y. Zheng, H. Ran, et al., Biomaterials 53(2012) 5854-5864. |
| [9] |
Y. Ma, J. Huang, S. Song, et al., Small 12(2016) 4936-4954. DOI:10.1002/smll.v12.36 |
| [10] |
W.J. Stark, Angew. Chem. 50(2011) 1242-1258. DOI:10.1002/anie.v50.6 |
| [11] |
T.M. Allen, P.R. Cullis, Science 303(2004) 1818-1822. DOI:10.1126/science.1095833 |
| [12] |
Q. He, Z. Zhang, F. Gao, et al., Small 7(2011) 271-280. DOI:10.1002/smll.v7.2 |
| [13] |
S.P. Low, N.H. Voelcker, L.T. Canham, et al., Biomaterials 30(2009) 2873-2880. DOI:10.1016/j.biomaterials.2009.02.008 |
| [14] |
J.Rocca Della, D. Liu, W. Lin, Acc. Chem. Res. 44(2011) 957-968. DOI:10.1021/ar200028a |
| [15] |
Y. Zhu, J. Shi, W. Shen, et al., Angew.Chem. 117(2005) 5213-5217. DOI:10.1002/(ISSN)1521-3757 |
| [16] |
R. Deng, X. Xie, M. Vendrell, et al., J. Am. Chem. Soc. 133(2011) 20168-20171. DOI:10.1021/ja2100774 |
| [17] |
Z. Ge, S. Liu, Chemi. Soc. Rev. 42(2013) 7289. DOI:10.1039/c3cs60048c |
| [18] |
L. Zhang, T. Wang, L. Yang, et al., Chemistry 18(2012) 12512-12521. DOI:10.1002/chem.201200030 |
| [19] |
M.Y. Hua, H.L. Liu, H.W. Yang, et al., Biomaterials 32(2011) 516-527. DOI:10.1016/j.biomaterials.2010.09.065 |
| [20] |
N.Y. Rapoport, A.M. Kennedy, J.E. Shea, et al., Control J.Release 138(2009) 268-276. DOI:10.1016/j.jconrel.2009.05.026 |
| [21] |
A. Schroeder, R. Honen, K. Turjeman, et al., Control J.Release 137(2009) 63-68. DOI:10.1016/j.jconrel.2009.03.007 |
| [22] |
J. Liu, W. Bu, L. Pan, et al., Angew. Chem. 52(2013) 4375-4379. DOI:10.1002/anie.201300183 |
| [23] |
F.R. Balkwill, M. Capasso, T. Hagemann, Cell J.Sci. 125(2012) 5591-5596. DOI:10.1242/jcs.116392 |
| [24] |
M.R. Junttila, F.J. de Sauvage, Nature 501(2013) 346-354. DOI:10.1038/nature12626 |
| [25] |
S. Mura, J. Nicolas, P. Couvreur, Nat. Mater. 12(2013) 991-1003. DOI:10.1038/nmat3776 |
| [26] |
D. Ling, W. Park, S.J. Park, et al., J. Am. Chem. Soc. 136(2014) 5647-5655. DOI:10.1021/ja4108287 |
| [27] |
R. Liu, Y. Zhang, X. Zhao, et al., J. Am. Chem. Soc. 132(2010) 1500-1501. DOI:10.1021/ja907838s |
| [28] |
A. Jhaveri, P. Deshpande, V. Torchilin, Control J.Release 190(2014) 352-370. DOI:10.1016/j.jconrel.2014.05.002 |
| [29] |
M.A. Swartz, N. Iida, E.W. Roberts, et al., Cancer. Res. 72(2012) 2473-2480. DOI:10.1158/0008-5472.CAN-12-0122 |
| [30] |
R. Cheng, F. Feng, F. Meng, et al., Control J.Release 152(2011) 2-12. DOI:10.1016/j.jconrel.2011.01.030 |
| [31] |
G.S. Loving, S. Mukherjee, P. Caravan, J. Am. Chem. Soc. 135(2013) 4620-4623. DOI:10.1021/ja312610j |
| [32] |
P.D. Thornton, R.J. Mart, S.J. Webb, et al., Soft Matter 4(2008) 821. DOI:10.1039/b714750c |
| [33] |
P.D. Thornton, R.J. Mart, R.V. Ulijn, Adv. Mater. 19(2007) 1252-1256. DOI:10.1002/(ISSN)1521-4095 |
| [34] |
S.H. Bhang, J. Han, H.K. Jang, et al., Biomaterials 55(2015) 33-43. DOI:10.1016/j.biomaterials.2015.03.025 |
| [35] |
F. Wei, W. Zhuyuan, Z. Shenfei, et al., Bioelectron. 57(2014) 10-15. DOI:10.1016/j.bios.2014.01.042 |
| [36] |
S. Mena, A. Ortega, J.M. Estrela, Mutat. Res. 674(2009) 36-44. DOI:10.1016/j.mrgentox.2008.09.017 |
| [37] |
T. Finkel, N.J. Holbrook, Nature 408(2000) 239-247. DOI:10.1038/35041687 |
| [38] |
A.J. Shuhendler, K. Pu, L. Cui, et al., Nat. Biotechnol. 32(2014) 373-380. DOI:10.1038/nbt.2838 |
| [39] |
M. Valko, C.J. Rhodes, J. Moncol, et al., Chem. Biol. Interact 160(2006) 1-40. DOI:10.1016/j.cbi.2005.12.009 |
| [40] |
M. Valko, D. Leibfritz, J. Moncol, et al., Int. J. Biochem. Cell. Biol. 39(2007) 44-84. DOI:10.1016/j.biocel.2006.07.001 |
| [41] |
J.F. Woolley, J. Stanicka, T.G. Cotter, Trends. Biochem. Sci. 38(2013) 556-565. DOI:10.1016/j.tibs.2013.08.009 |
| [42] |
X. Chen, X. Tian, I. Shin, et al., Chem. Soc. Rev. 40(2011) 4783-4804. DOI:10.1039/c1cs15037e |
| [43] |
W. Chen, Q.Q. Ren, Q. Yang, et al., Anal. Lett. 45(2012) 156-167. DOI:10.1080/00032719.2011.633185 |
| [44] |
N. Houstis, E.D. Rosen, E.S. Lander, Nature 440(2006) 944-948. DOI:10.1038/nature04634 |
| [45] |
S.G. Rhee, T.S. Chang, W. Jeong, et al., Mol. Cells 29(2010) 539-549. DOI:10.1007/s10059-010-0082-3 |
| [46] |
E.W. Miller, O. Tulyathan, E.Y. Isacoff, et al., Nat. Chem. Biol. 3(2007) 263-267. DOI:10.1038/nchembio871 |
| [47] |
M.C. DeRosa, R.J. Crutchley, Coord. Chem. Rev. 233(2002) 351-371. |
| [48] |
M.K. Reddy, L. Wu, W. Kou, et al., Appl. Biochem. Biotechnol. 151(2008) 565-577. DOI:10.1007/s12010-008-8232-1 |
| [49] |
H.P. Indo, H.C. Yen, I. Nakanishi, et al., J. Clin. Biochem. Nutr. 56(2015) 1-7. DOI:10.3164/jcbn.14-42 |
| [50] |
Y. Wei, Y. Zhang, Z. Liu, et al., Chem. Commun. 46(2010) 4472-4474. DOI:10.1039/c000254b |
| [51] |
M.T. Lin, M.F. Beal, Nature 443(2006) 787-795. DOI:10.1038/nature05292 |
| [52] |
B. Hu, F. Dai, Z. Fan, et al., Adv. Mater. 27(2015) 5499-5505. DOI:10.1002/adma.201502227 |
| [53] |
P. Shi, M. Li, J. Ren, et al., Adv. Funct. Mater. 23(2013) 5412-5419. DOI:10.1002/adfm.v23.43 |
| [54] |
M. Rosini, E. Simoni, A. Milelli, et al., J. Med. Chem. 57(2014) 2821-2831. DOI:10.1021/jm400970m |
| [55] |
J. Geng, M. Li, L. Wu, et al., Adv. Healthc. Mater. 1(2012) 332-336. DOI:10.1002/adhm.201200067 |
| [56] |
G. Eskici, P.H. Axelsen, Biochemistry 51(2012) 6289-6311. DOI:10.1021/bi3006169 |
| [57] |
K. Zhang, R.J. Kaufman, Nature 454(2008) 455-462. DOI:10.1038/nature07203 |
| [58] |
R. Medzhitov, Nature 454(2008) 428-435. DOI:10.1038/nature07201 |
| [59] |
de Oliveira-Marques V, L. Cyrne, H.S. Marinho, et al., Immunol J 178(2007) 3893-3902. DOI:10.4049/jimmunol.178.6.3893 |
| [60] |
J. Fang, T. Seki, H. Maeda, Adv. Drug Deliv. Rev. 61(2009) 290-302. DOI:10.1016/j.addr.2009.02.005 |
| [61] |
M. Hockel, P. Vaupel, J. Natl. Cancer Inst. 93(2001) 266-276. DOI:10.1093/jnci/93.4.266 |
| [62] |
P. Vaupel, A. Mayer, Cancer Metastasis Rev. 26(2007) 225-239. DOI:10.1007/s10555-007-9055-1 |
| [63] |
F. Danhier, O. Feron, V. Preat, Control J.Release 148(2010) 135-146. DOI:10.1016/j.jconrel.2010.08.027 |
| [64] |
S. Thomas, M.A. Harding, S.C. Smith, et al., Cancer Res. 72(2012) 5600-5612. DOI:10.1158/0008-5472.CAN-11-3666 |
| [65] |
N.L. Oleinick, R.L. Morris, T. Belichenko, Photochem. Photobiol. Sci. 1(2002) 1-21. DOI:10.1039/b108586g |
| [66] |
J.P. Celli, B.Q. Spring, I. Rizvi, et al., Chem. Rev. 110(2010) 2795-2838. DOI:10.1021/cr900300p |
| [67] |
H. Fan, G. Yan, Z. Zhao, et al., Angew. Chem. Int. Ed. 55(2016) 5477-5482. DOI:10.1002/anie.201510748 |
| [68] |
G. Song, C. Ji, C. Liang, et al., Biomaterials 112(2017) 257-263. DOI:10.1016/j.biomaterials.2016.10.020 |
| [69] |
G. Song, Y. Chen, C. Liang, et al., Adv. Mater. 28(2016) 7143-7148. DOI:10.1002/adma.201602111 |
| [70] |
J.M. Brown, W.R. Wilson, Nat. Rev. Cancer 4(2004) 437-447. DOI:10.1038/nrc1367 |
| [71] |
W.R. Wilson, M.P. Hay, Nat. Rev. Cancer 11(2011) 393-410. DOI:10.1038/nrc3064 |
| [72] |
A.L. Harris, Nat. Rev. Cancer 2(2002) 38-47. DOI:10.1038/nrc704 |
| [73] |
D.J. Manalo, A. Rowan, T. Lavoie, et al., Blood 105(2005) 659-669. DOI:10.1182/blood-2004-07-2958 |
| [74] |
Y. Lou, P.C. McDonald, A. Oloumi, et al., Cancer Res. 71(2011) 3364-3376. DOI:10.1158/0008-5472.CAN-10-4261 |
| [75] |
W. Zeng, P. Liu, W. Pan, et al., Cancer Lett. 356(2015) 263-267. DOI:10.1016/j.canlet.2014.01.032 |
| [76] |
G.L. Semenza, Trends. Pharmacol. Sci. 33(2012) 207-214. DOI:10.1016/j.tips.2012.01.005 |
| [77] |
Y. Liu, Y. Liu, W. Bu, et al., Biomaterials 49(2015) 1-8. DOI:10.1016/j.biomaterials.2015.01.028 |
| [78] |
C. Zhang, K. Zhao, W. Bu, et al., Angew. Chem. 54(2015) 1770-1774. DOI:10.1002/anie.201408472 |
| [79] |
J. Wang, L. Zhang, M. Chen, et al., ACS Appl. Mater. Interfaces 7(2015) 23248-23256. DOI:10.1021/acsami.5b07316 |
| [80] |
M. Yu, S.L. Ambrose, Z.L. Whaley, et al., J. Am. Chem. Soc. 136(2014) 12836-12839. DOI:10.1021/ja507034d |
| [81] |
S.E. Bae, J.S. Son, K. Park, et al., Control J.Release 133(2009) 37-43. DOI:10.1016/j.jconrel.2008.09.006 |
| [82] |
H. Zhu, L. Li, W. Zhou, et al., J. Mater. Chem. B 4(2016) 7333-7349. DOI:10.1039/C6TB02037B |
| [83] |
S. Gross, A. Gilead, A. Scherz, et al., Nat. Med. 9(2003) 1327-1331. DOI:10.1038/nm940 |
| [84] |
F. Yang, S. Hu, Y. Zhang, et al., Adv. Mater. 24(2012) 5205-5211. DOI:10.1002/adma.201202367 |
| [85] |
X. Wang, D. Niu, P. Li, et al., Acs. Nano 9(2015) 5646-5656. DOI:10.1021/nn5068094 |
| [86] |
Z. Ma, M. Zhang, X. Jia, et al., Small 12(2016) 5477-5487. DOI:10.1002/smll.v12.39 |
| [87] |
M.L. Viger, J.C. de Gracia Lux Sankaranarayanan, et al., J. Am. Chem. Soc. 135(2013) 7847-7850. DOI:10.1021/ja403167p |
| [88] |
W. Fan, W. Bu, B. Shen, et al., Adv. Mater. 27(2015) 4155-4161. DOI:10.1002/adma.v27.28 |
| [89] |
X.R. Song, S.H. Li, J. Dai, et al., Small(2017). DOI:10.1002/smll.201603997 |
| [90] |
W. Chao, C. Liang, L. Yumeng, et al., Adv. Funct. Mater. 23(2013) 3077-3086. DOI:10.1002/adfm.v23.24 |
| [91] |
E. Toth, R.D. Bolskar, A. Borel, et al., J. Am. Chem. Soc. 127(2005) 799-805. DOI:10.1021/ja044688h |
| [92] |
A.R. Lippert, K.R. Keshari, J. Kurhanewicz, et al., J. Am. Chem. Soc. 133(2011) 3776-3779. DOI:10.1021/ja111589a |
| [93] |
B.W. Henderson, V.H. Fingar, Cancer Res. 47(1987) 3110-3114. |
| [94] |
V.E. Zannella, A.Pra Dal, H. Muaddi, et al., Clin. Cancer Res. 19(2013) 6741-6750. DOI:10.1158/1078-0432.CCR-13-1787 |
| [95] |
M. Milosevic, I. Quirt, W. Levin, et al., Gynecol. Oncol. 83(2001) 428-431. DOI:10.1006/gyno.2001.6426 |
| [96] |
G. Song, C. Liang, X. Yi, et al., Adv. Mater. 28(2016) 2716-2723. DOI:10.1002/adma.201504617 |
| [97] |
C.R. Gordijo, A.Z. Abbasi, M.A. Amini, et al., Adv. Funct. Mater. 25(2015) 1858-1872. DOI:10.1002/adfm.201404511 |
| [98] |
M. Song, T. Liu, C. Shi, et al., Acs Nano 10(2016) 633-647. DOI:10.1021/acsnano.5b06779 |
| [99] |
Q. Chen, L. Feng, J. Liu, et al., Adv. Mater. 28(2016) 7129-7136. DOI:10.1002/adma.201601902 |
| [100] |
J.F. Lovell, T.W. Liu, J. Chen, et al., Chem. Rev. 110(2010) 2839-2857. DOI:10.1021/cr900236h |
| [101] |
Y. Cheng, J.D. Meyers, A.M. Broome, et al., J. Am. Chem. Soc. 133(2011) 2583-2591. DOI:10.1021/ja108846h |
| [102] |
H. Chen, L. Xiao, Y. Anraku, et al., J. Am. Chem. Soc. 136(2014) 157-163. DOI:10.1021/ja406992w |
| [103] |
C. Zhang, W. Bu, D. Ni, et al., Angew. Chem. Int. Ed. 55(2016) 2101-2106. DOI:10.1002/anie.201510031 |
| [104] |
W. Zhu, Z. Dong, T. Fu, et al., Adv. Funct. Mater. 26(2016) 5490-5498. DOI:10.1002/adfm.201600676 |
| [105] |
H. Chen, J. Tian, W. He, et al., J. Am. Chem. Soc. 137(2015) 1539-1547. DOI:10.1021/ja511420n |
| [106] |
S. Gao, G. Wang, Z. Qin, et al., Biomaterials 112(2017) 324-335. DOI:10.1016/j.biomaterials.2016.10.030 |
| [107] |
L. Rong, C. Zhang, Q. Lei, et al., Reg. Biol. 3(2016) 217-222. |
| [108] |
C. Kang, W. Cho, M. Park, et al., Biomaterials 85(2016) 195-203. DOI:10.1016/j.biomaterials.2016.01.070 |
| [109] |
Y.J. Wang, H. Dong, M. G-Lyu, et al., Nanoscale 7(2015) 13981-13990. DOI:10.1039/C5NR02588E |
| [110] |
J. Yu, C. Qian, Y. Zhang, et al., Nano Lett. 17(2017) 733-739. DOI:10.1021/acs.nanolett.6b03848 |
| [111] |
W.P. Li, C.H. Su, Y.C. Chang, et al., ACNano 10(2016) 2017-2027. |
 2017, Vol. 28
2017, Vol. 28 


