Small molecular weight hydrogel, as an important class of soft materials, formed primarily by the self-assembly of small molecular compounds (molecular weight less than 2000) via noncovalent interactions, such as π-π stacking, hydrogen bonds, ionic bridge and so on [1-6]. Over the past three decades, significant progress have been achieved for application of various small molecular weight hydrogels in the field of tissue engineering, wound healing, drug delivery, biosensor and etc. [7-12]. Regarding of a novel drug delivery system, the small molecular weight hydrogel possess several favorable characteristics over conventional drug delivery system [2, 3, 5, 13-15]. First, different from the polymeric hydrogelator, the small molecular weight hydrogelator could be purified precisely in terms of molecular weight and architecture via simple high performance liquid chromatography (HPLC) method [2, 3]. Secondly, the critical gelation concentration (CGC) for small molecular weight hydrogel normally is very low ( < 1 wt%), which significantly alleviates concerns over carrier accompanied adverse effects [3, 9]. Third, the drug payload for small molecular weight hydrogel usually is higher than that of conventional drug delivery systems (>10%) [2, 16]. More importantly, the drug release behavior and degradation behavior of small molecular weight hydrogel could be fine-tuned by the rational design of hydrogelator [8, 11].
Apart from the physical encapsulation of drugs into small molecular weight hydrogels via noncovalent interaction, covalent attachment of drug molecules with small molecules (peptides and etc.) conferring the self-assembly of prodrug to form small molecular weight hydrogel represents the next logical step in the evolution of self-drug delivery system [3, 9, 11, 17]. Distinction from encapsulation approach, small molecular weight hydrogels of prodrugs formed its own drug delivery vehicle, which not only conquers its water solubility problem, but also offers the great opportunity for enhanced biological activity accompanying with reduced side-effects. Up to now, there are two FDA approved small molecular weight hydrogel formed by therapeutic agents (Lanreotide and Degarelix), which greatly extended the in-vivo half-time of drugs and significantly enhanced the therapeutic efficacy with reference to its solution formulation [3, 11, 17]. Inspiring by successful clinical application of these two drugs, pharmaceutical scientist attempted to exploit other small molecular weight hydrogels based on clinically used drugs. Unfortunately, the majority of clinically used drugs could not self-assemble into hydrogel without additional modification because it is either too hydrophobic or too hydrophilic. To address this problem, rational molecular architecture design of the prodrug with counterpart moiety provides a great opportunity to fine-tune over the structural features and physicochemical properties, which potentially further influence the self-assembly behavior in aqueous solution. Theoretically, the optimal amphiphilic prodrug conjugates possesses the great ability to self-assemble into nanostructure, further forming small molecular weight hydrogel under various conditions. In this review, we focus our discussion primarily on the recent advancement of small molecular weight hydrogels of prodrugs. In particular, we will emphasize the rational design of prodrug hydrogelator and the strategies for the construction of hydrogel (Table 1) [18-32].
|
|
Table 1 Stimuli sensitive prodrugs hydrogelators. |
2. Small molecular weight hydrogel of prodrugs 2.1. Heating-cooling induced hydrogel
Since the temperature changes in the stimuli can induce the self-assembly and dissociation of molecules, it is advantageous to take this strategy to explore a small molecular weight hydrogel of prodrugs [33, 34]. Suffering from the poor water solubility of hydrophobic drugs, the hydrophobic drugs without additional modification could not self-assemble into hydrogel under various conditions. By conjugation of hydrophilic moieties to the hydrophobic agents, not only affords the amphiphilicity of drug molecule, but also confers the self-assembly of prodrugs into supramolecular hydrogel under heating-cooling process (Fig. 1). For instance, Kim and co-workers designed and synthesized a series of ibuprofen based hydrogelators with a variety of dipeptide residues [18]. Under heating-cooling cycle, the supramolecular hydrogel formed spontaneously in aqueous solution with minimal critical gelation concentration (CGC) of 0.9 wt%. Most importantly, they demonstrated that the proposed supramolecular hydrogel was susceptible to enzyme in vitro, yet resulting in the possible sustained release of its native drugs. More recently, similar results were also reported by Xu and co-workers [19], they combines Damino acid with non-steroidal anti-inflammatory drugs (NSAID) for the development of functional hydrogelator, which offers a class of molecular hydrogels of therapeutic agents. Incorporation of D-amino acids renders naproxen (Npx) a highly selective NSAID for inhibition of COX-2 accompanying with reduced side-effects. Another successful examples of self-assemble therapeutic agent hydrogels was the generation of prodrugs based on acetaminophen (Ace) via covalent conjugation of a biocompatible fatty acid [20]. With the induction by heating-cooling strategy, the prodrugs hydrogelator self-assemble into a small molecular weight hydrogel in aqueous solution at a very low concentration. It is noteworthy that the small molecular weight hydrogel of prodrugs allowed the payload of a second drug through traditional encapsulation approach and the encapsulated both drugs were sustained released from hydrogels via an enzymatically controlled manner at physiological conditions.

|
Download:
|
| Fig. 1. Small molecular weight hydrogel constructed by amphiphilic prodrug platform with heating-cooling strategy. | |
2.2. Enzymatically instructed hydrogel
Enzymatic biocatalysis of biomacromolecuar self-assembly such as the formation of collagen fibrils is a prominent dynamic feature of life [35, 36]. In past several decades, by mimicking in vivo enzymatic biocatalysis, the integration of enzymatic reactions with self-assembly of prodrugs have gained considerable attention and achieved some progress in the generation and application of small molecular weight hydrogel [4, 5]. Despite the huge diversity of enzymes in vivo, only a few of them have been explored for instructing prodrugs hydrogelation. Alkaline phosphatases are a group of enzymes with low substrate selectivity that catalyze the hydrolysis of phosphate esters, which widely existed in many human tissues including serum, bone, intestine, liver and so on [37]. Based on the alkaline phosphatase catalysis, Xu and coworkers have developed a body of small molecular weight hydrogels of therapeutic agents and its derivatives [1, 9, 21]. The first case of enzymatic catalysis to develop a taxol-bearing small molecular weight hydrogel by using a phosphorylated peptide precursor with conjugated taxol (Fig. 2) [22]. Upon the dephosphorylation by alkaline phosphatase (ALP), the precursor successfully transformed into hydrogelator to initiate the self-assembly of a derivative of taxol in aqueous solution to afford a small molecular weight hydrogel. Contrast with its native taxol, the proposed taxol small molecular weight hydrogel exhibited a comparable biological activity in HeLa cancer cell lines, suggesting that the covalent conjugation of taxol to peptide sequence would not result in the decrease or loss of drug activity. This work demonstrated a fantastic strategy to create small molecular weight hydrogel of clinically used therapeutic agent without compromising its bioactivities. Taking advantage of this strategy, Xu and co-workers further designed and synthesized a new class of hydrogelator as a self-delivery system composing of non-steriod anti-inflammatory drug (NSAID) and anti-HIV agents [21]. The supramolecular hydrogel was formed spontaneously by initiating with prostate acid phosphatase (PAP) and possessed the both anti-inflammatory activity and anti-HIV efficacy. Inspired by the pioneer study of the Xu group, Yang and co-workers rationally designed and synthesized a hydrogelator of folic acid (FA)-peptide-Taxol conjugate, which could self-assemble into nanosphere to afford a small molecular weight hydrogel as injectable materials for the longterm delivery of Taxol after the initiation with alkaline phosphatase [23]. Although the considerable advance in the development of prodrug small molecular weight hydrogels by using alkaline phosphatase catalysis strategy has been achieved, the promises of other enzymatic hydrogelation are far from fully realized.

|
Download:
|
| Fig. 2. Schematic illustration of small molecular weight hydrogel initiated by phosphatase. Reused with permission [22]. Copyright 2009, American Chemical Society. | |
2.3. Redox responsive hydrogel
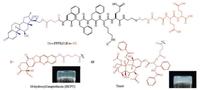
Redox reactions including reduction and oxidation reaction are basic and complementary chemical reactions in biological process. In past two decades, using the redox reactions to tailor materials properties have gained considerable attention and numerous redox responsive polymeric hydrogel have been explored and demonstrated for various biomedical applications [38-41]. In this review, we only focus on redox responsive small molecular weight hydrogel of prodrugs. To design a redox responsive prodrug hydrogelator, the selection of promoiety that enables intermolecular interactions and co-assembly is very critical. An initial report of redox responsive prodrug hydrogelator was the generation of prodrug based on taxol and dexamethasone with a cleavable disulfide bond linkage [24]. Through covalent transformations, the hydrophobic drugs (Taxol and Dex) were converted into precursor with great water solubility in aqueous solution. By the treatment with reductants such as glutathione (GSH) and dithiothreitol (DTT), the disulfide bond cleaves to induce the self-assembly of hydrogelator to form small molecular weight hydrogel (Fig. 3). They demonstrated that the stability of drugs was significantly improved in hydrogel and the native drugs could be sustained release from hydrogel via ester bond hydrolysis. Using the same concept, Yang and co-workers also constructed a number of prodrug hydrogelators (curcumin, taxol, folic acid and etc.) and evaluated its therapeutic efficacy towards different cancer cell lines and in vivo tumor model [25, 26]. Similarly, encouraging by the result of Yang, Li and co-workers adopted redox strategy to develop a small molecular weight hydrogel composed of triamcinolone acetonide (TA) and rapamycin, which exhibited excellent ocular biocompatibility after sub-Tenon's injection, might be a promising alternative therapy for posterior disease [27].
|
Download:
|
| Fig. 3. Chemical structures of precursors of hydrogelators (blue: Dexamethasone (Dex), black: peptide of FFFKE, red: hydrophilic part of EE derivative, and brown: 10-hydroxyCamptothecin (HCPT) or Taxol) and its optical image of hydrogel. Reproduced with permission [24]. Copyright 2012, Royal Society of Chemistry. | |
2.4. Ionic bridge triggered hydrogel
Although the temperature and biocatalysis are the most prevalent stimulus for hydrogelation, specific ionic bridge also have dramatic effects on molecular self-assembly and hydrogelation [17]. Gelation of macromolecules, including gelatin, polysaccharides and so on by triggering with ionic bridge strategy has been extensively investigated due to its vital role in food processing and pharmaceutical applications [42-44]. One of the most common case is using divalent cation (Ca2+, Zn2+ and etc.) to coordinately bind with carboxyl groups of polymers to create ionic bridge between molecules, thus leading to the hydrogelation [45-47]. Inspired by this strategy, several attempts were achieved to rational design and synthesize the sequences of oligo-and polypeptides bearing single or multiple carboxylic acids, which could bind with different cations via an ionic bridge to induce the self-assembly of molecules. For instance, Schneider and coworkers explored a number of peptide supramolecular hydrogels by raising the ionic strength of the solution and triggering by Zn2+ coordination [48-50]. In another example, Stupp and co-workers explored a peptide amphiphile (PA) molecules, which could selfassemble into supramolecular hydrogel of nanofibers after saltmediated screening of the charged residues in their peptide segment [28, 29]. Extending by this strategy, they successfully conjugate an anti-inflammatory drugs (dexamethasone) with peptide amphiphile (PA) to generate a hydrogelator, which could self-assemble into nanofibers to form supramolecular hydrogel with the addition of calcium salts to screen electrostatic repulsion between nanofibers [30]. They demonstrated that the pharmacological activity of drugs was well preserved in supramolecular hydrogel and encapsulated drugs sustained release from hydrogel over 1 month. More recently, Kohane and co-workers presented a shear-thinning and self-healing supramolecular hydrogels based on clinically used steroidal drugs (dexamethasone, betamethasone, and hydrocortisone) via a simple metal ionic coordination strategy [16]. By simple mixing drugs with divalent cationic aqueous solution (Ca2+, Mg2+ and Ba2+), the supramolecular hydrogels was formed spontaneously with the ionic bridge between divalent cation and phosphate group (Fig. 4). The drug release behavior could be well tailored by adjusting ionic concentrations and the proposed supramolecular hydrogel exhibited a great in vivo therapeutic efficacy with minimizing the inflammation by bupivacaine and prolonging the duration of nerve blockade. Inspired by these results, it is reasonable to believe that the ionic coordination strategy might be able to extend to other kinds of clinical drug molecules bearing carboxylic acid, phosphate group and so on.

|
Download:
|
| Fig. 4. Characterization of the hydrogels with TEM. (a-c) Top panels: chemical structure of the steroid drugs. Bottom panels: TEM images of their associated hydrogels. The insets show the HR-TEM images. The concentrations of the steroid drugs and Ca2+ were both 2.5×10-2 mol/L. Reproduced with permission [16]. Copyright 2016, the WILEY-VCH Verlag GmbH & Co. KGaA. | |
2.5. pH hydrolyzed hydrogel
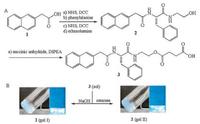
A change of pH value to result in the hydrolysis of precursor might be a promising and simplest method to boost the hydrogelation due to its easy manipulation, low cost and so on. Zhao et al. pioneer reported a small molecular weight hydrogel by triggering with pH hydrolysis strategy [51]. A simple chemical modification of a small molecule affords the precursor to have excellent water solubility at physiological condition. With slight changing pH value to weak base condition or upon incubation with esterase, the hydrolysis of the carboxylic ester bond occurred, yet resulting in the self-assembly of hydrogelator to form small molecular weight hydrogel (Fig. 5). This result hints that the drug molecule could also be rationally designed to produce a pH sensitive hydrogelator. Following this concept, Yang and co-workers rationally designed and synthesized a hydrogelator, namely taxol-succinic acidoxidized glutathione (taxol-SA-GSSG) [31]. Upon incubation at phosphate buffer solution (pH 7.4) for 6 h, the supramolecular hydrogel was formed with triggering by hydrolysis of ester bond. After topical administration, the supramolecular hydrogel could significantly inhibit the growth of tumor and prevent their metastasis, which suggested great possibility for the practical applications. Except the hydrophilic peptide used in this paper, they also believed that other hydrophilic molecules such as PEG could be adopted for construction of precursors of taxol. More recently, Li and co-workers successfully designed and synthesized a supramolecular hydrogelator of succinated triamcinolone acetonide [32]. Upon changing pH value of system to about 7.4, the supramolecular hydrogel formed immediately due to the hydrolysis of ester bonds (Fig. 6). Compared with complex design of hydrogelator, the chemical structure of succinated triamcinolone acetonide is very simple with only one-step synthesis. Furthermore, the developed supramolecular hydrogel possessed excellent ocular biocompatibility and exhibited great therapeutic efficacy in the suppression of uveitis without causing complications such as high intraocular pressure and cataracts.
|
Download:
|
| Fig. 5. (A) Synthesis route to 2 and 3. (B) Reactions to convert the precursor (3) to the hydrogelator (2) under basic conditions (pH 9) to afford gel Ⅰ or via hydrolysis catalyzed by esterase (1 U/μL, 10 μL) at pH 7.5 to give gel Ⅱ (optical images: left, normal; right, through a pair of crossed polarizers). Reused with permission [51]. Copyright 2011, American Chemical Society. | |

|
Download:
|
| Fig. 6. Chemical structures of succinated triamcinolone acetonide (STA) and triamcinolone acetonide (TA) and the hydrogelation process via an ester bond hydrolysis process. Hydrolysis percentage of succinated triamcinolone acetonide (STA) in the hydrogel formed from 2.0 wt% STA (inset: optical image of the gel) and rheological measurement of the gel at the 48 h time point from 2.0 wt% STA Reproduced with permission [32]. Copyright 2014, Royal Society of Chemistry. | |
2.6. Other strategies for hydrogelation
Besides the strategies mentioned above, other approaches including photo-responsive, ultrasound and etc. were also exploited for the hydrogelation of small molecular weight compounds [52-55]. For example, Zhang and coworker explored a small molecular weight hydrogelator by integration of photosensitive spiropyran with dipeptide D-Ala-D-Ala, which could selfassemble into supramolecular hydrogel with response to both light and ligand-receptor interactions (Fig. 7) [56]. Yao and coworkers demonstrated a supramolecular hydrogel based on (R)-N-Fmococtylglycine (Fmoc-OG), which exhibited a sol-gel transition upon triggering by ultrasonication [57]. The alteration in the morphologies and properties of the obtained nanomaterials induced by the ultrasound wave demonstrates a potential method for smart controlling of the functions of nanomaterials from the molecular level.

|
Download:
|
| Fig. 7. Chemical structure of the spiropyran-linked dipeptide in SP form (1-SP) and MC form (1-MC); Optical image of the hydrogel formed by 1-MC and its response to photo-irradiation. Reproduced with permission [57]. Copyright 2008, Royal Society of Chemistry. | |
3. Conclusion and perspective
In this review, we have summarized several kinds of prodrug hydrogelators, which confers the self-assembly into nanostructure under various stimulus (pH, temperature, ionic strength and enzymatic catalysis) to form small molecular weight hydrogel for potential drug delivery application. With the rational molecular design, a number of hydrophobic drugs have been successfully conjugated with hydrophilic moieties such as peptide to convert into an amphiphilic prodrugs, which exhibited great water solubility. Upon triggering by various stimuli, the amphiphilic prodrugs could self-assemble into small molecular weight hydrogels for drug itself delivery. Although favorable characteristics such as precise drug payload, lack of drug carries and so on have been achieved by small molecular weight hydrogels of prodrugs, some of the issues remains the challenges. (1) The strict requirement of the drug molecular structure for building up of prodrug hydrogelator and only limited number of drug molecules were tested so far. (2) Suffering from the weak mechanical properties, how to extend its half-life time after in vivo application is another important issue. (3) If the prodrug hydrogel was triggered by particular chemical or biological agents, the long-term stability of drugs in hydrogel should be seriously considered. Until to now, self-assembled prodrug hydrogels are currently in the early development stage and its clinical application has not be fully realized, but at least presents a promising therapeutic alterative approach.
AcknowledgementsThis work was financially supported by grants from the National Natural Science Foundation of China (No. 31671022), the Key Program for International S&T Cooperation Projects of China (No. 2015DFA50310) and the National Science and Technology Major Project (No. 2014ZX09303301).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.033.
| [1] |
X. Du, J. Zhou, J. Shi, B. Xu, Chem. Rev. 115(2015) 13165-13307. DOI:10.1021/acs.chemrev.5b00299 |
| [2] |
R. Lin, H. Cui, Curr. Opin. Chem. Eng. 7(2015) 75-83. DOI:10.1016/j.coche.2014.11.005 |
| [3] |
W. Ma, A.G. Cheetham, H. Cui, Nano Today 11(2016) 13-30. DOI:10.1016/j.nantod.2015.11.003 |
| [4] |
B. Mei, Q. Miao, A. Tang, G. Liang, Nanoscale 7(2015) 15605-15608. DOI:10.1039/C5NR04563K |
| [5] |
H. Wang, Z. Feng, D. Wu, et al., J. Am. Chem. Soc. 138(2016) 10758-10761. DOI:10.1021/jacs.6b06075 |
| [6] |
J. Raeburn, A.Z. Cardoso, D.J. Adams, Chem. Soc. Rev. 42(2013) 5143-5156. DOI:10.1039/c3cs60030k |
| [7] |
Z. Hai, J. Li, J. Wu, J. Xu, G. Liang, J. Am. Chem. Soc. 139(2017) 1041-1044. DOI:10.1021/jacs.6b11041 |
| [8] |
L. An, H.S. Gilani, M. Rehan, G. Liang, Curr. Med. Chem. 21(2014) 2453-2466. DOI:10.2174/0929867321666140205140600 |
| [9] |
Y. Wang, A.G. Cheetham, G. Angacian, et al., Adv. Drug Deliv. Rev.(2016). DOI:10.1016/j.addr.2016.1006.1015 |
| [10] |
E.F. Banwell, E.S. Abelardo, D.J. Adams, et al., Nat. Mater. 8(2009) 596-600. DOI:10.1038/nmat2479 |
| [11] |
P.K. Vemula, N. Wiradharma, J.A. Ankrum, et al., Curr. Opin. Biotechnol. 24(2013) 1174-1182. DOI:10.1016/j.copbio.2013.02.006 |
| [12] |
B.O. Okesola, D.K. Smith, Chem. Soc. Rev. 45(2016) 4226-4251. DOI:10.1039/C6CS00124F |
| [13] |
I.C. Li, A.N. Moore, J.D. Hartgerink, Biomacromolecules 17(2016) 2087-2095. DOI:10.1021/acs.biomac.6b00309 |
| [14] |
S. Sundar, Y. Chen, Y.W. Tong, Curr. Med. Chem. 21(2014) 2469-2479. DOI:10.2174/0929867321666131212152637 |
| [15] |
M. Bibian, J. Mangelschots, J. Gardiner, et al., J. Mater. Chem. B 3(2015) 759-765. DOI:10.1039/C4TB01294A |
| [16] |
Q. Liu, C. Zhan, A. Barhoumi, et al., Adv. Mater. 28(2016) 6680-6686. DOI:10.1002/adma.201601147 |
| [17] |
S. Roy, N. Javid, J. Sefcik, P.J. Halling, R.V. Ulijn, Langmuir 28(2012) 16664-16670. DOI:10.1021/la303388s |
| [18] |
S. Bhuniya, Y.J. Seo, B.H. Kim, Tetrahedron Lett. 47(2006) 7153-7156. DOI:10.1016/j.tetlet.2006.08.002 |
| [19] |
J. Li, Y. Kuang, Y. Gao, et al., J. Am. Chem. Soc. 135(2012) 542-545. |
| [20] |
P.K. Vemula, G.A. Cruikshank, J.M. Karp, G. John, Biomaterials 30(2009) 383-393. DOI:10.1016/j.biomaterials.2008.09.045 |
| [21] |
J. Li, X. Li, Y. Kuang, et al., Adv. Healthc. Mater. 2(2013) 1586-1590. DOI:10.1002/adhm.v2.12 |
| [22] |
Y. Gao, Y. Kuang, Z.F. Guo, et al., J. Am. Chem. Soc. 131(2009) 13576-13577. DOI:10.1021/ja904411z |
| [23] |
H. Wang, C. Yang, L. Wang, et al., Chem. Commun. 47(2011) 4439-4441. DOI:10.1039/c1cc10506j |
| [24] |
L. Mao, H. Wang, M. Tan, et al., Chem. Commun. 48(2012) 395-397. DOI:10.1039/C1CC16250K |
| [25] |
C. Yang, D. Li, Feng Q.Zhao, et al., Org. Biomol. Chem. 11(2013) 6946-6951. DOI:10.1039/c3ob40969d |
| [26] |
C. Yang, Z. Wang, C. Ou, et al., Chem. Commun. 50(2014) 9413-9415. DOI:10.1039/C4CC03139C |
| [27] |
X. Li, C. Yang, Z. Zhang, et al., J. Mater. Chem. 22(2012) 21838-21840. DOI:10.1039/c2jm35329f |
| [28] |
M.A. Greenfield, J.R. Hoffman, de la M.Cruz Olvera, S.I. Stupp, Langmuir 26(2009) 3641-3647. |
| [29] |
S. Zhang, M.A. Greenfield, A. Mata, et al., Nat. Mater. 9(2010) 594-601. DOI:10.1038/nmat2778 |
| [30] |
M.J. Webber, J.B. Matson, V.K. Tamboli, S.I. Stupp, Biomaterials 33(2012) 6823-6832. DOI:10.1016/j.biomaterials.2012.06.003 |
| [31] |
H. Wang, J. Wei, C. Yang, et al., Biomaterials 33(2012) 5848-5853. DOI:10.1016/j.biomaterials.2012.04.047 |
| [32] |
X. Li, Y. Wang, C. Yang, et al., Nanoscale 6(2014) 14488-14494. DOI:10.1039/C4NR04761C |
| [33] |
C. Tomasini, N. Castellucci, Chem. Soc. Rev. 42(2013) 156-172. DOI:10.1039/C2CS35284B |
| [34] |
G. Fichman, E. Gazit, Acta Biomater. 10(2014) 1671-1682. DOI:10.1016/j.actbio.2013.08.013 |
| [35] |
E.L. Bakota, L. Aulisa, K.M. Galler, J.D. Hartgerink, Biomacromolecules 12(2010) 82-87. |
| [36] |
P. Yin, H.M.T. Choi, C.R. Calvert, N.A. Pierce, Nature 451(2008) 318-322. DOI:10.1038/nature06451 |
| [37] |
M.M. Kaplan, New Engl. J. Med. 286(1972) 200-202. DOI:10.1056/NEJM197201272860407 |
| [38] |
X. Yan, F. Wang, B. Zheng, F. Huang, Chem. Soc. Rev. 41(2012) 6042-6065. DOI:10.1039/c2cs35091b |
| [39] |
M. Nakahata, Y. Takashima, H. Yamaguchi, Nat. Commun. 2(2011) 511. DOI:10.1038/ncomms1521 |
| [40] |
A. Gasnier, G. Royal, P. Terech, Langmuir 25(2009) 8751-8762. DOI:10.1021/la900174e |
| [41] |
A. Gasnier, C. Bucher, J.C. Moutet, et al., Macromol. Symp. 304(2011) 87-92. DOI:10.1002/masy.v304.1 |
| [42] |
J. Berger, M. Reist, J.M. Mayer, et al., Eur. J. Pharm. Biopharm. 57(2004) 19-34. DOI:10.1016/S0939-6411(03)00161-9 |
| [43] |
Y.H. Lin, H.F. Liang, C.K. Chung, M.C. Chen, H.W. Sung, Biomaterials 26(2005) 2105-2113. DOI:10.1016/j.biomaterials.2004.06.011 |
| [44] |
M.K. Nguyen, E. Alsberg, Prog. Polym. Sci. 39(2014) 1235-1265. DOI:10.1016/j.progpolymsci.2013.12.001 |
| [45] |
K. Möbus, J. Siepmann, R. Bodmeier, Eur. J. Pharm. Biopharm. 81(2012) 121-130. DOI:10.1016/j.ejpb.2012.01.018 |
| [46] |
Y. Murata, E. Miyamoto, S. Kawashima, J. Control. Release. 38(1996) 101-108. DOI:10.1016/0168-3659(95)00098-4 |
| [47] |
H. Dong, J.F. Snyder, K.S. Williams, J.W. Andzelm, Biomacromolecules 14(2013) 3338-3345. DOI:10.1021/bm400993f |
| [48] |
B. Ozbas, J. Kretsinger, K. Rajagopal, J.P. Schneider, D.J. Pochan, Macromolecules 37(2004) 7331-7337. DOI:10.1021/ma0491762 |
| [49] |
C.M. Micklitsch, P.J. Knerr, M.C. Branco, et al., Angew. Chem. Int. Ed. 123(2011) 1615-1617. DOI:10.1002/ange.201006652 |
| [50] |
B. Ozbas, K. Rajagopal, L.Butterick Haines-, J.P. Schneider, D.J. Pochan, J. Phys. Chem. B 111(2007) 13901-13908. DOI:10.1021/jp075117p |
| [51] |
F. Zhao, Y. Gao, J. Shi, H.M. Browdy, B. Xu, Langmuir 27(2010) 1510-1512. |
| [52] |
H. Svobodová, V. Noponen, E. Kolehmainen, E. Sievänen, RSC Adv. 2(2012) 4985-5007. DOI:10.1039/c2ra01343f |
| [53] |
S. Matsumoto, S. Yamaguchi, S. Ueno, et al., Chem.-Eur. J. 14(2008) 3977-3986. DOI:10.1002/chem.v14:13 |
| [54] |
I. Tomatsu, K. Peng, A. Kros, Adv. Drug Deliv. Rev. 63(2011) 1257-1266. DOI:10.1016/j.addr.2011.06.009 |
| [55] |
J. Wu, T. Yi, T. Shu, et al., Angew. Chem. Int. Ed. 120(2008) 1079-1083. DOI:10.1002/(ISSN)1521-3757 |
| [56] |
Z. Qiu, H. Yu, J. Li, Y. Wang, Y. Zhang, Chem. Commun.(2009), 3342-3344. |
| [57] |
Y. Wang, C. Zhan, H. Fu, et al., Langmuir 24(2008) 7635-7638. DOI:10.1021/la801499y |
 2017, Vol. 28
2017, Vol. 28