2 School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 200233, China;
3 Shanghai Research Institute of Fragrance & Flavor Industry, Shanghai 200232, China;
4 Department of Pediatrics, Capital Medical University Affiliated Beijing Anzhen Hospital, Beijing 100029, China
Since the last century, the prevalence of neurodegenerative diseases has increased worldwide, including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD) [1]. Common features of these diseases are the progressive death and loss of neurons in the brain, accompanied by motor disorders, loss of language skills and mental decline and other symptoms [1]. Etiology and pathogenesis of these diseases have not been yet fully revealed, and the existing treatment is not entirely effective. The most common treatment is drug treatment, with side effects and drug resistance and other issues. It is very necessary to find new and effective treatments.
Flavors are special products closely related to the human life. Not only are they used in food, cosmetic, tobacco, but also in medicine. For example, curcumin has shown significant effect on wound repair, cancer, and inflammatory diseases, particularly in terms of neurodegenerative diseases [2]. In Asia, especially in India, curcumin is widely used as a kind of food additive, and it is the main yellow pigment in curry, mustard. And the disease rate is relatively low in India compared with other countries [3]. Coumarin compounds are found to be antioxidants and antiinflammatory agents, neuroprotective agents, antidepressants, anticonvulsants, antibacterial agents, antiviral agents, anticancer agents, and have great effect on neurodegenerative diseases, such as AD, PD [4]. Caffeine is widely found in coffee and tea. Some studies have investigated the long-term impact of caffeine on AD or cognitive decline. In the Finland, Italy and the Netherlands Elderly (FINE) Study among elderly men, drinking three cups of coffee per day was associated with the least 10-year cognitive decline [5]. Additionally, results from the Three City Study among 65 years old persons indicated that over three cups of caffeine (from coffee and tea) per day was associated with less decline in verbal cognitive functioning [5]. Also, caffeine has been reported to reduce the risk of Parkinson's disease [6-8]. Honolulu Heart Program-A prospective study of aging and neurodegenerative STDs in the United States shows that coffee drinkers and some people who took caffeine from non-coffee sources had significantly lower incidence of PD than nondrinkers [6], but the decaf coffee did not have this protective effect [7]. Rutin (quercetin-3-O-rutinoside) is a multifunctional natural flavonoid glycoside with profound effects on the various cellular functions under pathological conditions. It has also been shown to modify the cognitive and various behavioral symptoms of neurodegenerative diseases [9].
The biggest challenge for the treatment of neurodegenerative diseases is the blood-brain barrier (BBB), which is a natural barrier to brain drug delivery and absorption [10]. BBB makes the treatment of brain diseases such as brain tissue malignancies and neurodegenerative diseases difficult. In order to solve the above problems, using brain-targeted nano-drug delivery system can significantly improve the low utilization of drugs and be more effective on treatment of related diseases. Common nano-carriers include liposomes, polymer nanoparticles, polymer micelles, magnetic nanoparticles and nanoemulsion [11, 12].
2. Neurodegenerative disease 2.1. Alzheimer's diseaseAlzheimer's disease (AD) is one of the most common neurodegenerative dementia. Clinical manifestations of AD patients include the decline of memory and cognitive abilities [13]. The pathology of this disease is characterized by the deposition of senile plaques (SPs) in the extracellular space, neurofibrillary tangles (NFT) in the intracellular space, and the loss of synapses and neurons [13]. The senile plaques are composed of the core of amyloid beta peptide (Aβ), and neurofibrillary tangles are composed of paired helix filaments (PHF) consisting of hyperphosphorylated tau proteins [13]. Ab is one of the proteolytic productsof amyloid precursor protein (APP) existingon thesurface of neurons [14]. Normally, the substance is usually cleared by the body, but the balance is broken in AD patients. The Aβ is no longer decomposed by the corresponding protease, but rather aggregates to form insoluble fibers and further form SPs. Aβ peptides contain 39-43 amino acid residues, and two C-terminal variants Aβ1-40 and Aβ1-42 are thought to be common in SPs, especially Aβ1-40 is one of the most abundance [14]. Aβ-induced excitotoxicity is considered as a key factor of the neurodegeneration [15]. And tau protein as a kind of microtubule-associated protein, plays an important role in the structure and function of microtubules. Excess phosphorylated tau proteins lose the biological activity of microtubule assembly and have increased resistance to proteolytic enzymes, resulting in neurotoxicity [16]. In addition, the reduction of neurotransmitter acetylcholine, oxidative stress, the imbalance of metal ion in body may also be important factors in the production of AD, so the disease may be the result of a variety of factors interacting [17].
There are a wide variety of drugs currently available for AD, but the treatment is limited. Acetylcholine inhibitors, such as Donepezil, Rivastigmine, Galantamine, have a good therapeutic effect on earlier Alzheimer's disease, but have a strong side effect [17]. Antioxidants Selegiline, Vitamin E, and Melatonin, may have the effect of treatment on AD. Immunotherapy for Aβ and a variety of β-secretase and γ-secretase inhibitors are also developing, but side effects are still major problems of treatment. So the need for new effective treatment is urgent [17].
2.2. Parkinson's diseaseTremor, stiffness and vague words are common symptoms of Parkinson's disease [18]. The root cause of these symptoms is the death of dopaminergic neurons. The most significant pathological feature is the formation of Lewy bodies, mainly in the cytoplasm [18]. Abnormal aggregation caused by the mutation of α-synuclein is the key to the formation of Lewy bodies, which promotes the accumulation of dopaminein thecytoplasmand increases thelevel of oxidative stress to produce large amounts of free radicals [19]. In addition, α-synuclein can also activate microglia and astrocytes to release a variety of cytokines such as IL-1β and TNF-α to promote inflammatory response. These all can lead to damage and death of dopaminergic neurons [18]. The current clinical treatment of Parkinson's disease is mainly drug treatment. There are mainly compound levodopa preparations, anticholinergic drugs, amantadine, dopamine receptor agonists, monoamine oxidase B inhibitors, etc. [19]. Although there are many types of drugs, no one can cure Parkinson's disease. The side effects are also very serious. Compound levodopa preparations are generally considered to be more effective drugs. But after using 2-5 years, the drug will produce side-effects such as fluctuations in motion or dysmotility and other complications in patients. Dopamine agonists pergolide withdrew from the market due to its adverse effects on heart valve [20].
3. Application of flavors in neurodegenerative diseasesFlavors are essential oils, natural extracts and aromatic compounds, which areused inpackagedfoods and some consumer products. Some of these flavors get a lot of attention because of the effect of treating diseases, such as curcumin [2], coumarin [4], caffeine [5] and rutin [9].
3.1. CurcuminCurcumin is a kind of polyphenol compound, abundant in turmeric content [2]. Its structure is shown in Fig. 1. Curcumin has attracted much attention because of its use in neurodegenerative disease, particularly AD and PD. For two major pathological changes in AD, curcumin has anti-Aβ properties and anti-highly phosphorylated tau protein properties, and also shows affinity for Aβ and tau proteins [21]. Curcumin also regulates secondary changes that occur during the disease, such as oxidative stress, inflammatorystress, and cholesterol regulation [22]. Curcumin has a protective effect on the PD rat model prepared by 6-OHDA, which may play a protective role by anti-oxidative stress and antiapoptotic mechanisms [23]. Curcumin can effectively antagonize dopaminergic neuronal injury of parkinsonian mice model induced by MPTP. Its mechanism may be related to the effect of curcumin on reducing the content of active oxygen in dopaminergic neurons and inhibiting the inflammatory reaction [24]. The natural fluorescence of curcumin may be useful for monitoring disease progression, especially by imaging retinal Aβ plaques which can mirror brain plaques for early diagnosis and therapy assessment [25].

|
Download:
|
| Fig. 1. Chemical structure of curcumin | |
However, poor pharmacokinetics and pharmacodynamicsof curcumin limit the use, such as poor absorption, short half-life and fast metabolism in the gastrointestinal tract [22]. In addition, curcumin must cross the BBB, and go into the brain tissue to have a therapeutic effect on AD and PD. Although curcumin is a fatsoluble small molecule that can cross the BBB, the presence of the BBB still greatly limits the role of curcumin in brain tissue [22].
3.2. CoumarinStudies have shown that Wnt/β-catenin signaling plays an important role in the development and progression of AD [26]. Once a large number of Aβ accumulate in the nerve cells, Wnt signaling will be weakened, which in turn increases the activity of GSK3β, resulting in excessive degradation of β-catenin, neuronal degeneration and apoptosis [26]. Therefore, AD can be prevented and treated partly by regulating the key molecules of Wnt signaling, and using the specific signal molecules and their downstream effector. Osthole is a kind of natural coumarin extracted from a variety of traditional Chinese medicines such as Cnidium, and now the pharmacological effects identified include antioxidant, anti-inflammatory, modulating immune effects, etc. [22]. Its structure is shown in Fig. 2. Yao et al. transfected APP plasmid associated with AD pathogenic factors into neural stem cells removed from the subventricular zone (SVA) and hippocampus of neonatal rats by lentivirus and cultured. It was found that osthole could promote proliferation and differentiation of neural stem cells. Furthermore Wnt/β-catenin signaling was blocked and activated by inhibitor IWR-1-endo and agonist LiCl of the Wnt pathway, respectively. It was found that osthole and agonist LiCl could similarly reduce the apoptosis of neural stem cells, inhibit GSK3β and stimulate the overexpression of β-catenin mRNA in NSCs [27].

|
Download:
|
| Fig. 2. Chemical structure ofosthole | |
Lactate dehydrogenase (LDH) is normally present in the cytoplasm. Once the cell membrane is damaged, LDH will be released to the cell. So the degree of cell damage can be judged by detecting the activity of LDH in the brain tissue [28]. Liu et al. established a dopaminergic neuronal injury model by using D-methylpiperazine (MPP+) to treat PC12 cells (commonly used nerve cell lines), and observed the protective effect of osthole on MPP+-induced cytotoxicity in PC12 cells. The result showed that osthole could significantly improve PC12 cell activity, reduced lactate dehydrogenase (LDH) release, indicating that osthole has a protective effect on dopaminergic neuronal cells [29]. In addition, coumarin-based fluorescent probes such as coumarin-based monoamine oxidase (MAO), coumarin-based hydrogen peroxide fluorescent probes can be used for the diagnosis and treatment of neurodegenerative diseases [30].
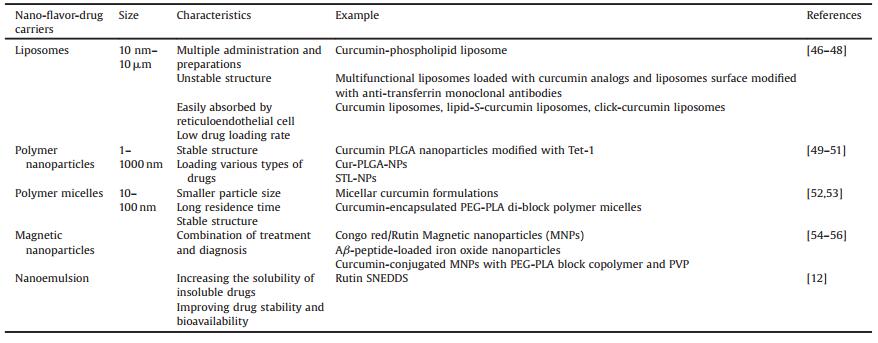
3.3. CaffeineLaurent et al. evaluated effects of chronic caffeine intake (0.3 g/L through drinking water), given at an early pathologic stage, in the THY-Tau22 transgenic mouse model of progressive AD-like tau pathology. They found that chronic caffeine intake prevents from the development of spatial memory deficits in tau mice. Improved memory was associated with reduced hippocampal tau phosphorylation and proteolytic fragments. Moreover, caffeine treatment mitigated several proinflammatory and oxidative stress markers found upregulated in the hippocampus of THY-Tau22 animals [31]. Caffeine reduces Aβ production and increases Aβ clearance [32]. Caffeine's beneficial effects in AD are through its interaction with β-and γ-secretase [33]. Arendash et al. found that caffeine treatment at 1.5 mg/d in APPsw mice reduced Aβ deposition in the hippocampus (40%) and the entorhinal cortex (46%). With caffeine, Aβ1-40 and Aβ1-42 levels were reduced in the cortex (25% and 51%, respectively) and hippocampus (37% and 59%, respectively) [34]. Caffeine is a kind of xanthine methyl derivative that acts as an adenosine receptors antagonist to enhance dopamine neurotransmission [35]. Its structure is shown in Fig. 3. Adenosine A2A receptor is the most concerned. In the striatum, A2A receptors and dopamine receptors coexist in γ-aminobutyric acid (GABA) neurons. The excitability of A2A receptor can reduce the neurotransmission of dopamine and the affinity of the ligand with dopamine receptor [36]. A2A receptor antagonist enhances the neurotransmission of dopamine by enhancing the affinity of dopamine ligands with receptors [36].
|
Download:
|
| Fig. 3. Chemical structure ofcaffeine | |
3.4. Rutin
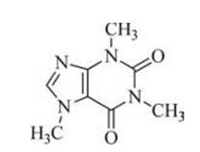
Rutin is a glycone of quercetin with a flavonol structure and is a powerful phenolic antioxidant that has various pharmacological properties, including antitumor, anti-inflammatory, antidiarrheal, antimutagenic, myocardial protecting, and immunemodulator [9]. Its structure is shown in Fig. 4. Wang et al. have demonstrated that rutin could interfere with Aβ aggregation and neurotoxicity, prevent oxidative stress induced by Aβ, reduce Aβ levels in mutant neurons, and decrease senile plaques in the brains of AD transgenic mice [37]. Magalingam et al. studied that the neuroprotective effects of rutin in 6-OHDA-induced rat pheochromocytoma (PC-12) cells. The results showed rutin increased antioxidant enzymes, catalase, superoxide dismutase, glutathione peroxidase, and glutathione level that were attenuated by 6-OHDA in PC-12 cells [38].
|
Download:
|
| Fig. 4. Chemical structure ofrutin | |
4. Brain-targeting drug delivery system 4.1. Blood-brain barrier
The blood-brain barrier (BBB) refers to the barrier between plasma and brain cells formed by the brain capillaries and glial cells, and the barrier between plasma and cerebrospinal fluid formed by choroid plexus [39]. BBB is a unique characteristic of cerebral capillary, which can be a barrier to the substances that pass through the surrounding blood vessels and protect the brain tissue. It plays an important role in maintaining the stability of the environment within the brain tissue [40]. BBB also limits the transport of drugs from the blood to the brain, which is a major obstacle to the treatment of neurological diseases [41]. Unless there are good fat-soluble drug molecules with molecular weight less than 600 Da, other treatment drugs are difficult to penetrate into the brain [42]. However, even some fat-soluble small molecule drugs, are also difficult to enter the brain because of efflux proteins such as P-glycoprotein (Pgp) [42] and multi-drug resistance protein (MRP) [43], which are ATP-dependent efflux pumps and can inversely connect with the drug that enters the cell. And efflux pumps can pump the drug back to the blood by using the energy released by ATP hydrolysis, so they can limit drugs into the brain effectively [44, 45]. The basic requirement for any compound to act on a neurodegenerative process is to enter the brain tissue, that is, pass through BBB. There are some methods of assisting drugs to enter the central nervous system (CNS): (1) Change the structure of the drug, and make lipophilic drugs to improve the permeability. (2) Regulate the tight junction among BBB endothelial cells to increase the permeability of drug to BBB and the brain tissue. Make tight connection among endothelial cells open instantaneously by causing blood hypertonic. (3) Alter the route of administration, for example by nasal administration, via olfactory epithelium and olfactory nerve access. (4) Increased brain delivery of the drug by using the relevant drug carriers. Nano-drug carrier provides a very important way for drug treatment of CNS diseases. Not only can nano-drug carrier carry drugs through BBB and achieve brain targeted drug delivery, but also improve drug stability and efficacy, and reduce side effects. The common nano-drug carriers include liposomes, polymer micelles, polymer nanoparticles, etc. Information on the nano-flavor-drug carriers is listed in Table 1.
|
|
Table 1 Nano-flavor-drug carriers mentioned in this article |
4.2. Nano-drug carriers 4.2.1. Liposomes
The liposome is a spherical lipid bilayer with a cavity, which can load drug molecules, and transports to the target organ. Liposomes can fuse with plasma membranes to release drugs into the cell. Because liposomes are not immunogenic, and can protect drug molecules from degradation by the ubiquitous enzymes in the body, and are simple to prepare, liposomes are often used as drug carriers. Modification of liposome surface with polyethylene glycol (PEG) is a common strategy to increase the half-life of liposomes in circulation [57].
Curcumin as a kind of flavors drug, has a certain therapeutic effect on neurodegenerative diseases. The liposomes with a size of 170nm prepared by the curcumin-phospholipid conjugates showed a very high affinity for Aβ1-42 fibers in vitro [21]. Lazar et al. designed the nano-curcumin liposome because curcumin is a fluorescent molecule with high affinity for the Aβ peptide. The property makes curcumin specifically mark Aβ peptide in brain tissue of Alzheimer's disease. Curcumin-conjugated nanoliposomes not only improves drug solubility, but also has a fluorescence targeting diagnostic and therapeutic effect, and can specifically mediate the drug into the brain tissue [46]. In another work, a comparative study with multifunctional liposomes loaded with curcumin analogs and liposomes surface modified with antitransferrin monoclonal antibodies was performed. The results showed that these deliverysystems havehigh affinity to β-amyloid plaques, slowing their aggregation. From the results of these experiments, authors concluded that the developed liposomes may be used in diagnosis and treatment of Alzheimer's disease [47]. Taylor et al. prepared three forms of curcumin liposomes. Nanoliposomes incorporating curcumin (curcumin liposomes) were prepared by adding curcumin in the lipid phase during liposome preparation, and curcumin surface-decorated liposomes were prepared by using a curcumin-lipid conjugate (lipid-Scurcumin liposomes) or by attaching a curcumin derivative on preformed liposomes by click chemistry (click-curcumin liposomes). All nanoliposomes were able to inhibit the formation of fibrillar and/or oligomeric Aβ in vitro. Of the three forms of curcumin liposomes tested, the click-curcumin type was by far the most effective [48]. Curcumin-based liposomes could be further developed as a novel treatment for Alzheimer's disease. The encapsulation of liposomes with suitable surface properties to increase the half-life of these factors in vivo and to promote targeting the brain, is considered a viable strategy for the treatment of neurodegenerative diseases such as AD and PD [36].
4.2.2. Polymer nanoparticlesPolymeric nanoparticles refer to particles formed by polymers with a size of 1-1000nm generally [58]. In particular degradable nanoparticles have become the main type of neurodegenerative drug carriers, due to their low toxicity, adjustable degradation rates and high drug loading capacity and their ability to pass through BBB and target CNS [58].
The application of curcumin is limited due to hydrophobicity, which can be better overcome by water-soluble curcumin nanoparticles. Mathew et al. prepared the curcumin-loaded PLGA nanoparticles, and tested the abilites of free radical scavenging and handling amyloid aggregates of nanoparticles, and found that nanoparticles have antioxidant and anti-amyloid activities. The nanoparticles were modified with Tet-1 to achieve the purpose of brain targeting. Tet-1 peptides consisting of 12 amino acids have a high affinity with ganglioside GT1b receptors (more content in nerve tissue) [49]. Induction of neurogenesis by targeting endogenous neural stem cells (NSC) could be a promising therapeutic approach to such diseases by influencing the brain self-regenerative capacity [50]. Tiwari et al. reported that curcumin-encapsulated PLGA nanoparticles (Cur-PLGA-NPs) potently induce NSC proliferation and neuronal differentiation in vitro and in the hippocampus and subventricular zone of adult rats, as compared to uncoated bulk curcumin. Cur-PLGA-NPs induce neurogenesis by internalization into the hippocampal NSC and may offer a therapeutic approach to treating neurodegenerative diseases such as AD, by enhancing a brain self-repair mechanism [50]. Lectin is a class of natural proteins that can bind to the oligosaccharide structure of glycoproteins and glycolipids on the surfaces of cells, which can regulates many biological processes. A variety of glycosyl groups such as N-acetylglucosamine, L-fucose are highly expressed in nasal mucosa epithelial cells, which can specifically bind to lectins. The nanoparticles (NPs) can be modified with lectins, which are expected to selectively increase the contact of the delivery system with the olfactory mucosa and prolong its residence time on the olfactory mucosa. And the drug delivery system can be absorbed into the brain via the olfactory mucosa, thereby enhancing drug concentration in the brain [59]. Chen prepared Solanum tuberosum L. modified coumarin-6-loaded PLGA nanoparticles (STL-NPs). The concentration of coumarin-6 in brain tissue samples following intranasal administration of STLficantly higher than that of coumarin-6 incorporated in NPs. The result showed that STL-NPs can promote the transport of drugs from the nose into the brain [51].
4.2.3. Polymer micellesPolymer micelles are stable nuclear-shell structures formed by amphiphilic block copolymers in aqueous solutions. When the concentration of the block polymer reaches the critical micelle concentration, self-assembly takes place to form the polymer micelle. The polymer micelle has a small particle size, usually between 10 nm and 100 nm [60]. Huang et al. prepared two block polymers, polyethylene glycol-b-polycaprolactone (PEG-b-PCL) and poly(N-isopropylacrylamide)-b-polycaprolactone (PNIPAM-b-PCL). Two micelles self-assembled in aqueous solution to form temperature-responsive mixed-shell polymeric micelles (MSPMs). The effect of MSPMs on aggregation of Aβ1-42 was investigated. The results show that the MSPMs can adsorb the Aβ peptide or oligomer to the hydrophobic microdomain of the surface by hydrophobic interaction force, thus inhibiting the aggregation of the peptides and reducing the formation of the fibers. So the MSPMs can provide some useful reference for the treatment of AD [60].
Schiborr et al. developed micellar curcumin formulations, and compared the bioavailability of curcumin from native powder, micronized powder and liquid micelles. The results showed higher plasma concentration with liquid micelles, when compared to micronized powder and free curcuminoids [52]. The therapeutic effects of curcumin in treating AD depend on the ability to penetrate the blood-brain barrier. Cheng et al. developed a stable curcumin nanoparticle formulation. Using a multi-inlet vortex mixer (MIVM) and flash nanoprecipitation, curcumin was encapsulated within polyethyleneglycol-polylactide (PEG-PLA) di-block polymer micelles within milliseconds. Pharmacokinetic studies showed that the nanoparticle formulation significantly improved curcumin bioavailability, with a much greater plasma concentration and sixfold higher AUC and MRT in Tg2576 CE model mice brains [53].
4.2.4. Magnetic nanoparticlesThe ranostics systems need to satisfy the following conditions: (1) There should be diagnostic probes that can image amyloid plaques in vivo and (2) theranostics systems should have the abilities of targeted delivery and controlled release of therapeutic agents [54]. Yang et al. have designed Aβ-peptide-loaded iron oxide nanoparticles to detect amyloid plaques in vivo by magnetic resonance imaging (MRI) [55]. Iron oxide nanoparticles, which have high relaxivity for MRI, a long half-life, and can be covalently conjugated to drugs and antibodies, may emerge as a new paradigm for nanotheranostics applications for AD [61, 62]. Ultrasmall superparamagnetic iron oxide nanoparticles (USPIONs) can penetrate the BBB to some extent and for the imaging of amyloid plaques by MRI and targeted delivery of drugs [63]. Hu et al. successfully developed congo red (specifically detect amyloid plaques)/rutin magnetic nanoparticles (MNPs) nanotheranostics with the diameter of about 13 nm, which could specifically detect amyloid plaques by MRI, realize targeted deliveryof AD therapeutic agents, achieve drug controlled release by H2O2 response prevent oxidative stress [54]. Cheng et al. used MNPs made of superparamagnetic iron oxide (SPIO) conjugated with curcumin, a natural compound that specifically binds to amyloid plaques. Coating of curcumin-conjugated MNPs with polyethylene glycol polylactic acid (PEG-PLA) block copolymer and polyvinylpyrrolidone (PVP) produced stable and biocompatible curcumin magnetic nanoparticles (Cur MNPs) with mean diameter < 100 nm. The particles show low cytotoxicity and exhibit BBB penetration potential in an in vitro monolayer cell permeability test. In vivo, the particles can penetrate the BBB of both Tg2576 CE model and nontransgenic mice. Cur-MNPs bind amyloid plaques in mouse brains which can be detected by MRI. Therefore, Cur-MNPs are novel nanoparticles with potential use for visualizing amyloid plaques and non-invasive diagnosis of AD using MRI [56].
4.2.5. NanoemulsionNanoemulsion (NE), also known as microemulsion (ME), is a transparent or translucent thermodynamically stable system formed by mixing the oil phase, the aqueous phase, the surfactant and the cosurfactant in an appropriate ratio [64]. The averagesize generally ranges from 10 to 100 nm [64]. Rutin suffers from the problem of low oral bioavailability which is due to its poor aqueous solubility [12]. Sharma et al. prepared self-nanoemulsifying drug delivery systems (SNEDDS) of rutin to increase the solubility. Pharmacokinetic study showed a 2.3-fold increase in relative oral bioavailability. Rutin SNEDDS can serve as an effective tool in enhancing the oral bioavailability and efficacy of rutin, thus helping in ameliorating oxidative stress in neurodegenerative disorders like Parkinson's disease [12].
5. SummaryIn recent years, with the increasing incidence and mortality of AD and PD, the relevant research has been paid more and more attention. Flavor-drugs get a lot of attention due to their versatility and beneficial effects on AD and PD. But its own poor water solubility, low bioavailability, poor stability and other issues limit its development and application. In addition, the BBB hinders the bioavailability of drugs, which is the most basic and critical issue in the treatment of AD and PD. So the design of brain-targeted drug carrier is particularly important. Liposomes, polymer nanoparticles and other carriers can enable drugs to reach the brain disease area and play an important role in the treatment of AD and PD. In the future, flavors drugs can be carried by a reasonable nano-drug delivery system, which can effectively pass the BBB and improve the therapeutic effect on neurodegenerative disease
AcknowledgmentsThis work was financially supported by the National High Technology Research and Development Program (No. 2016YFA0200303), the National Natural Science Foundation of China (No. 51373177, 51573188, and 31522023), Beijing Municipal Science & Technology Commission (No. Z161100002616015), the Beijing Natural Science Foundation (No. 2164071), and the "Strategic Priority Research Program" of the Chinese Academy of Sciences (No. XDA09030301-3)
| [1] |
M.T. Lin, M.F. Beal, Nature 443(2006) 787-795. DOI:10.1038/nature05292 |
| [2] |
S. Hu, P. Maiti, Q. Ma, et al., Expert Rev. Neurother. 15(2015) 629-637. DOI:10.1586/14737175.2015.1044981 |
| [3] |
B.M. van Gelder, B. Buijsse, M. Tijhuis, et al., Eur. J. Clin. Nutr. 61(2007) 226-232. DOI:10.1038/sj.ejcn.1602495 |
| [4] |
M.J. Matos, F. Rodriguez-Enriquez, F. Borges, et al., Chem. Med. Chem. 10(2015) 2071-2079. DOI:10.1002/cmdc.201500408 |
| [5] |
K. Ritchie, I. Carrière, M.A. De, et al., Neurology 69(2007) 536-545. DOI:10.1212/01.wnl.0000266670.35219.0c |
| [6] |
G.W. Ross, R.D. Abbott, H. Petrovitch, et al., J. Am. Med. Assoc. 283(2000) 2674-2679. DOI:10.1001/jama.283.20.2674 |
| [7] |
A. Ascherio, S.M. Zhang, M.A. Hernán, et al., Ann. Neurol. 50(2001) 56-63. DOI:10.1002/ana.1052 |
| [8] |
G. Hu, S. Bidel, P. Jousilahti, et al., Mov. Disord. 22(2007) 2242-2248. DOI:10.1002/(ISSN)1531-8257 |
| [9] |
P.X. Xu, S.W. Wang, X.L. Yu, et al., Behav. Brain Res. 264(2014) 173-180. DOI:10.1016/j.bbr.2014.02.002 |
| [10] |
M. Carvey, B. Hendey, A.J. Monahan, J. Neurochem. 111(2009) 291-314. DOI:10.1111/jnc.2009.111.issue-2 |
| [11] |
R.H. Reza, N. Reza, S.S. Alireza, N. Shima, K.O. Reza, Avicenna J. Phytomed. 6(2016) 383-398. |
| [12] |
L.S. Cui, J.U. Kim, H. Nomura, et al., Angew. Chem. Int. Ed. Engl. 55(2016) 6864-6868. DOI:10.1002/anie.201601136 |
| [13] |
H.C. Huang, Z.F. Jiang, J. Alzheimers Dis. 16(2009) 15-27. DOI:10.3233/JAD-2009-0960 |
| [14] |
H.C. Huang, P. Chang, S.Y. Lu, et al., J. Recept. Signal Transduct. Res. 35(2015) 450-457. DOI:10.3109/10799893.2015.1006331 |
| [15] |
W.Y. Ong, K. Tanaka, G.S. Dawe, et al., J. Alzheimers Dis. 35(2013) 643-668. |
| [16] |
V. Rhein, X. Song, A. Wiesner, et al., Proc. Natl. Acad. Sci. U. S. A. 106(2009) 20057-20062. DOI:10.1073/pnas.0905529106 |
| [17] |
M.B. Čolović, D.Z. Krstić, T.D. Lazarević-Pašti, et al., Curr. Neuropharmacol. 11(2013) 315-335. DOI:10.2174/1570159X11311030006 |
| [18] |
J. Lotharius, P. Brundin, Nat. Rev. Neurosci. 3(2002) 932-942. DOI:10.1038/nrn983 |
| [19] |
G. Wang, H.Y. Zhou, R. Zheng, et al., J. Clin. Neurol. 19(2006) 336-338. |
| [20] |
H.B. Chen, J. Adverse Drug React. 7(2005) 161-164. |
| [21] |
S. Mourtas, M. Canovi, C. Zona, et al., Biomaterials 32(2011) 1635-1645. DOI:10.1016/j.biomaterials.2010.10.027 |
| [22] |
X.Y. Fu, M.F. Yang, M.Z. Cao, et al., Mol. Neurobiol. 53(2016) 369-378. DOI:10.1007/s12035-014-9021-1 |
| [23] |
B.L. Xiao, X. Fang, Centr. S. Pharm. 1(2015) 34-37. |
| [24] |
P. Jing, J.Q. Ding, S.D. Chen, Chin. J. Contemp. Neurol. Neurosurg. 7(2007) 447-452. |
| [25] |
Y. Koronyo, B.C. Salumbides, K.L. Black, M. Koronyo-Hamaoui, Neurodegener. Dis. 10(2012) 285-293. DOI:10.1159/000335154 |
| [26] |
Y. Yao, Z. Gao, W. Liang, et al., Toxicol. Appl. Pharmacol. 289(2015) 474-481. DOI:10.1016/j.taap.2015.10.013 |
| [27] |
T. Chen, W. Liu, X. Chao, et al., Neuroscience 183(2011) 203-211. DOI:10.1016/j.neuroscience.2011.03.038 |
| [28] |
A. Cohen, J. Lerneryardeni, D. Meridor, Mol. Med. 21(2015) 505-514. |
| [29] |
W.B. Liu, J. Zhou, Y. Qu, et al., Neurochem. Int. 57(2010) 206-215. DOI:10.1016/j.neuint.2010.05.011 |
| [30] |
G. Chen, D.J. Yee, N.G. Gubernator, D. Sames, J. Am. Chem. Soc. 127(2005) 4544-4545. DOI:10.1021/ja0428457 |
| [31] |
C. Laurent, S. Eddarkaoui, M. Derisbourg, et al., Neurobiol. Aging 35(2014) 2079-2090. DOI:10.1016/j.neurobiolaging.2014.03.027 |
| [32] |
M. Kolahdouzan, M.J. Hamadeh, CNS Neurosci. Ther. 23(2017) 272-290. DOI:10.1111/cns.2017.23.issue-4 |
| [33] |
P. Rajendran, A. Bhatt, S. Manthuruthil, S. Pericherla, Int. J. Biol. Med. Res. 350(2013) S4-S5. |
| [34] |
G.W. Arendash, T. Mori, C. Cao, et al., J. Alzheimers Dis. 17(2009) 661-680. DOI:10.3233/JAD-2009-1087 |
| [35] |
P. Svenningsson, M.C. Le, G. Fisone, B.B. Fredholm, Prog. Neurobiol. 59(1999) 355-396. DOI:10.1016/S0301-0082(99)00011-8 |
| [36] |
M. Cieslak, M. Komoszynski, A. Wojtczak, Purinergic Signal. 4(2008) 305-312. DOI:10.1007/s11302-008-9100-8 |
| [37] |
S.W. Wang, Y.J. Wang, Y.J. Su, et al., Neurotoxicology 33(2012) 482-490. DOI:10.1016/j.neuro.2012.03.003 |
| [38] |
K.B. Magalingam, A. Radhakrishnan, N. Haleagrahara, Int. J. Immunopathol. Pharmacol. 29(2016) 30-39. DOI:10.1177/0394632015613039 |
| [39] |
G. Leyvagómez, H. Cortés, J.J. Magaña, Drug Discov. Today 20(2015) 824-837. DOI:10.1016/j.drudis.2015.02.009 |
| [40] |
A.R. Jones, E.V. Shusta, Pharm. Res. 24(2007) 1759-1771. DOI:10.1007/s11095-007-9379-0 |
| [41] |
W.M. Pardridge, Curr. Opin. Pharmacol. 6(2006) 494-500. DOI:10.1016/j.coph.2006.06.001 |
| [42] |
E.V. Batrakova, S.V. Vingoradov, S.M. Robinson, et al., Bioconjug. Chem. 16(2005) 793-802. DOI:10.1021/bc049730c |
| [43] |
A. Tivnan, Z. Zakaria, C. O'Leary, et al., Front. Neurosci. 9(2015) 218-228. |
| [44] |
D.S. Miller, B. Bauer, A.M. Hartz, Pharmacol. Rev. 60(2008) 196-209. DOI:10.1124/pr.107.07109 |
| [45] |
K. Lingineni, V. Belekar, S.R. Tangadpalliwar, P. Garg, Mol. Divers. 21(2017) 355-365. DOI:10.1007/s11030-016-9715-6 |
| [46] |
A.N. Lazar, S. Mourtas, I. Youssef, et al., Nanomed:NLM 9(2013) 712-721. DOI:10.1016/j.nano.2012.11.004 |
| [47] |
S. Mourtas, A.N. Lazar, E. Markoutsa, et al., Eur. J. Med. Chem. 80(2014) 175-183. DOI:10.1016/j.ejmech.2014.04.050 |
| [48] |
M. Taylor, S. Moore, S. Mourtas, et al., Nanomedicine 7(2011) 541-550. DOI:10.1016/j.nano.2011.06.015 |
| [49] |
A. Mathew, T. Fukuda, Y. Nagaoka, et al., PLoS One 7(2012) e32616. DOI:10.1371/journal.pone.0032616 |
| [50] |
S.K. Tiwari, S. Agarwal, B. Seth, et al., ACS Nano 8(2014) 76. DOI:10.1021/nn405077y |
| [51] |
J. Chen, C. Zhang, Q. Liu, et al., J. Drug Target. 20(2012) 174-184. DOI:10.3109/1061186X.2011.622396 |
| [52] |
C. Schiborr, A. Kocher, D. Behnam, et al., Mol. Nutr. Food Res. 58(2014) 516-527. DOI:10.1002/mnfr.201300724 |
| [53] |
K.K. Cheng, C.F. Yeung, S.W. Ho, et al., AAPS J. 15(2013) 324-336. DOI:10.1208/s12248-012-9444-4 |
| [54] |
B. Hu, F. Dai, Z. Fan, et al., Adv. Mater. 27(2015) 5499-5505. DOI:10.1002/adma.201502227 |
| [55] |
J. Yang, Y.Z. Wadghiri, D.M. Hoang, et al., Neuroimage 55(2011) 1600-1609. DOI:10.1016/j.neuroimage.2011.01.023 |
| [56] |
K.K. Cheng, P.S. Chan, S. Fan, et al., Biomaterials 44(2015) 155-172. DOI:10.1016/j.biomaterials.2014.12.005 |
| [57] |
C. Spuch, C. Navarro, J. Drug Deliv. 2011(2011) 1-12. |
| [58] |
D. Hadavi, A.A. Poot, Front. Bioeng. Biotechnol. 4(2016) 49. |
| [59] |
F. Gabor, E. Bogner, A. Weissenboeck, et al., Adv. Drug Deliv. Rev. 56(2004) 459-480. DOI:10.1016/j.addr.2003.10.015 |
| [60] |
F. Huang, J. Wang, A. Qu, et al., Angew. Chem. Int. Ed. Engl. 53(2014) 8985-8990. DOI:10.1002/anie.201400735 |
| [61] |
J. Zeng, L. Jing, Y. Hou, et al., Adv. Mater. 26(2014) 2694-2698. DOI:10.1002/adma.201304744 |
| [62] |
Y. Zhong, F. Dai, H. Deng, et al., J. Mater. Chem. B 2(2014) 2938-2946. DOI:10.1039/C4TB00085D |
| [63] |
J.S. Weinstein, C.G. Varallyay, E. Dosa, et al., J. Cereb. Blood Flow Metab. 30(2010) 15-35. DOI:10.1038/jcbfm.2009.192 |
| [64] |
M.J. Lawrence, G.D. Rees, Adv. Drug Deliv. Rev. 45(2001) 89-121. |
 2017, Vol. 28
2017, Vol. 28