b Department of Chemical & Life Science Engineering, Virginia Commonwealth University, Richmond VA 23219, United States;
c Department of Pharmaceutics, Virginia Commonwealth University, Richmond VA 23298, United States;
d Massey Cancer Center, Virginia Commonwealth University, Richmond VA 23298, United States
The eye is a major sensory organ that harbors a variety of barriers to drug delivery due to its complex anatomy and physiology [1]. Therefore, ocular interventions are often invasive or require a high degree of patient compliance in order for therapy to be effective. Pediatric ocular growth and development predominantly spans from intrauterine life to early puberty. Though subsequent changes to visual acuity can occur later in life as a function of age, health, and environmental factors, high visual acuity during the first three years of life is imperative for development of the visual cortex [2]. The manifestation of visual impairments or blindness during infancy and early childhood severely undermines the other areas of development and quality of life. The repercussions of oversight, misdiagnosis, or ineffectual treatment of pediatric ocular diseases can be severe in early development stages. There has been a diminished focus on development of novel therapies and delivery systems for children, presumably due to lower rates of ocular diseases and degeneration in comparison to adults. However, the necessity of developing formulations in relation to pediatric ocular diseases is paramount [3]. This review elucidates recent advancements in pediatric ocular nanomedicines and calls attention to continued efforts in optimizing both quality of life and quality of treatment for pediatric eye diseases.
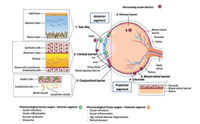
2. Ocular anatomy and vision developmentThe eye derives from all three developmental germ layers (ectoderm, mesoderm, and endoderm) starting at the 3-week embryo stage. Eye morphology and adult visual capacity is established within the first three years of life. The pediatric eye is anatomically similar to the adult eye in structure and function. The human eye is typically considered in two anatomical segments: anterior and posterior (Fig. 1) [4]. The anterior eye consists of the cornea, iris, lens, ciliary body, and sclera (anterior). The posterior eye consists of the vitreous humor, retina, choroid, sclera (posterior), and optic nerve. Aqueous humor pervades both segments [5]. Similarities in structure and function between the adult and pediatric eye render some, though not all, therapies designed for treatment of adult ocular diseases potentially appropriate for children, if assigned the proper changes in dosage.
|
Download:
|
| Fig. 1. Illustration that represents the different structures of the eye, divided in the anterior and posterior segments. The different barriers that drugs need to overcome after topical installation are indicated with a red star, and a detailed representation is also provided. The ocular targets to treat a specific disease are indicated with a green star, if they are in the anterior segment, or with a yellow star, if they are located in the posterior segment. Reproduced with permission [4]. Copyright 2015, Elsevier | |
3. Pediatric ocular diseases 3.1. Primary congenital glaucoma
Primary congenital glaucoma is an eye disease that results in elevated intraocular pressure, damage to the optic nerve, and risks of vision loss resulting from "cupping" (enlargement of optic cup) [6-8]. Typically diagnosed within the first year of life, the occurrence of primary congenital glaucoma is predicted in approximately 1 out of every 10, 000 births within the United States alone. This particular form of glaucoma is representative of 50%-70% of all known congenital cases. In developing nations, where properdiagnosis and treatment continue to face limitations, this disease is of even higher prevalence. Children can present with glaucoma both symptomatically and asymptomatically. In the former case, photophobia, corneal opacification, epiphora, diminished visual acuity, and secondary systemic symptomology (e.g., fatigue, irritability, loss of appetite) are common. When children do not present with symptoms, corneal and eye enlargement can often still be observed. The intraocular pressure associated with glaucoma arises from fluid buildup as a result of improper fluid production, circulation, and drainage among the ciliary body and ocular drainage canals. Aside from a number of surgical interventions aimed at reducing eye pressure, clinical trials have been conducted to explore methods, primarily topically applied formulations, for day-to-day management of intraocular pressure (IOP) and ocular hypertension.
3.2. ConjunctivitisAmong children, conjunctivitis, dubbed "red eye", is one of the most common infections of the eye [9]. Approximately 70%-80% of all conjunctivitis cases in children arise from bacterial infection [10]. Symptoms include burning, itching, foreign body sensations, discharge, overproduction of tears, and variable presence of papillae (bacterial and allergic conjunctivitis) or follicles (viral or chemical conjunctivitis) [11]. Topically administered antibiotics are most commonly recommended for rapid resolution of the infection and prevention of transmission [12]. Though mild cases have the potential of resolving on their own, the availability of antibiotics specific to children suffering from more severe cases of bacterial conjunctivitis is limited. Despite ongoing global efforts to invigorate clinical trials in the pediatric population, the safety and efficacy of most topical antibiotics have been proven and subsequently approved based on studies in adults [13]. Topical administration of the treatment was favorable in the way of safety, comfort, and tolerance [10]. Historically, bacterial conjunctivitis has also been treated successfully via topical sulfacetamide, trimethoprim-polymyxin B, gentamicin, tobramycin, and erythromycin ointments [11].
3.3. Dry eye diseaseDry eye disease is a group of disorders characterized by immoderate tear evaporation or decreased tear production [14]. Dry eye often occurs secondary to other diseases such as conjunctivitis, keratitis, blepharitis, etc. Symptoms include redness, irritation, soreness, and intermittent loss of visual acuity [15]. The display of symptoms can range from asymptomatic to the general symptoms delineated above, depending on its primary etiology. In pediatric patients, dry eye disease has not been widely recognized due to its comparatively lower prevalence [16]. However, studies report that the difference in dry eye symptom prevalence among children (below age of 20) and adults is 18.7% and 30.1%, respectively [17]. The discrepancy between these values suggests that dry most commonly manifests in children asymptomatically. Thus, this disease can be easily overlooked in children, a risk which can lead to severe restriction of early visual development, inflammation, and ocular surface damage such as corneal scarring. The severity of symptoms in children with dry eye or conditions similar to it is reportedly less. It is postulated that this is due to the fact that lacrimal units in children have less damage than those in adults. Thus, children's ability to produce tears in greater volume and with better composition may serve to less their sensitivity to dry eyerelated symptoms [16]. Dry eye disease is commonly treated via topical therapies, such as artificial tears. The specificities of the disease in children sometimes require anti-inflammatory or immunosuppressive drugs in light of its manifestation as an inflammation of the ocular surface.Many of the drugs developed for this purpose to date do not have pediatric indications.
3.4. Ocular neovascularizationProliferation of blood vessels in the eye can result in deprivation of visual acuity, if not blindness among children. Though ocular neovascular diseases broadly include diabetic retinopathy and agerelated macular degeneration, these diseases typically substantiate as comorbidities of other diseases during mid to late adulthood. Better representative of pediatric manifestations of this disease category is retinopathy of prematurity (ROP) (Fig. 2) [18]. ROP occurs in preterm infants (typically < 28 week gestational age at birth) who experience disruption of neovascularization due to hypoxic suppression of growth factors, subdued maternal-fetal interaction, and other complications of birth [18]. The derangement of vasoproliferation can lead to leaky, underdeveloped vessels that promote retinal detachment. Depending on the severity of the detachment, ROP can result in blindness or irreversible visual impairment. Approximately 10% of births worldwide occur preterm. ROP is predicted to affect over 2/3 of premature infants, though only around 6% of cases require intervention. The most common intervention is surgical lasermediated ablation of the unvascularized peripheral retina. This approach is the current gold standard for treatment of ROP as it boasts promising outcomes for long-term visual acuity [19]. Other techniques include oxygen cryablation. Historically, oxygen supplementation via neonatal incubators has been another approach that has ultimately been determined to contribute to the pathogenesis of ROP. A major push for alternative treatment strategies can be observed from clinical trial data. Thus far, antivascular endothelial growth factor (VEGF) treatments, e.g., bevacizumab, have proven to be a dual-edged sword, given the adverse effects of VEGF knockdown on early-stage retinal development [20]. Thus, the need for novel alternatives to VEGF therapies persists for ROP treatment.

|
Download:
|
| Fig. 2. Progression of retinopathy of prematurity. (A) Oxygen tension is low in utero and vascular growth is normal. (B) Phase 1: After birth until roughly 30 weeks postmenstrual age, retinal vascularization is inhibited because of hyperoxia and loss of the nutrients and growth factors provided at the maternal–fetal interface. Blood-vessel growth stops and as the retina matures and metabolic demand increases, hypoxia results. (C) Phase 2: The hypoxic retina stimulates expression of the oxygen-regulated factors such as erythropoietin (EPO) and VEGF, which stimulate retinal neovascularization. Insulin-like growth factor 1 (IGF-1) concentrations increase slowly from low concentrations after preterm birth to concentrations high enough to allow activation of VEGF pathways. (D) Resolution of retinopathy might be achieved through prevention of phase 1 by increasing IGF-1 to in-utero concentrations and by limiting oxygen to prevent suppression of VEGF; Alternatively, VEGF can be suppressed in phase 2 after neovascularization with laser therapy or an antibody. EPO = erythropoietin. ω-3 PUFA = ω-3 polyunsaturated fatty acids. Reproduced with permission [18]. Copyright 2013, Elsevier | |
3.5. Optic glioma
Optic glioma represents a subset of neoplastic diseases that can result in atrophy within the optic chiasm and retrochiasmatic pathways of the visual system, causing severe visual impairment or vision loss. A study conducted by Falsini et al. suggested that topical administration of murine nerve growth factor (NGF) can significantly improve the prognosis for patients suffering from visual complications associated with low-grade optic gliomas or other neurodegenerative diseases [21].
4. Routes of administrationThough many conditions of the eye with early onset necessitate surgical intervention, the four major routes for therapeutic delivery include transcleral/periocular, intravitreal, systemic, and topical (Fig. 3) [22]. Critical factors in evaluating the suitability of a given drug delivery platform for pediatric patients include patient compliance, comfort, and invasiveness. Transcleral/periocular delivery capitalizes on the large surface area of the sclera (approx. 17 cm2) for treatment of poster segment diseases [5]. Even more direct is treatment via the intravitreal route, which is typically achieved through injection of therapeutically effective doses directly into the vitreous humor, the most invasive and thus least desirable approach. Alternatively, the systemic route, though less invasive, poses a number of side effects due to the requirement for high dosages in order to achieve therapeutic effects.

|
Download:
|
| Fig. 3. Routes of drug administration to eye. Reproduced with permission [22]. Copyright 2010, Springer | |
The topical route of therapeutic administration holds a high degree of appeal in the realm of pediatric ocular drug delivery. Topical formulations remain dominate in clinical trials (Table 1). Topical administration of drugs can be realized via contact lenses and eye drops [23]. Contact lenses prove little feasibility in the way of compliance among infants and children. Thus, within the pediatric population, eye drops are the most common method for drug delivery and serve as the topic of discussion in the context of several globally prevalent pediatric ocular diseases.
|
|
Table 1 Recently completed and ongoing clinical trials for treatment of pediatric ocular diseases according to ClinicalTrials.gov |
Since eye drops do not mandate self-administration, the burden of frequent administration often falls on the shoulders of parents or guardians. Thus, obstacles to the topical delivery route include reduction of quality of life for children and/or parents, and resultantly poor patient compliance or premature dosage discontinuation. Child compliance is largely a function of parental health literacy and medication adherence. A study by Freedman et al. demonstrated that parental health literacy holds very heavy implications for topical ocular hypotensive medication adherence among children suffering from glaucoma, with lower health literacy rates correlated to lower adherence (e.g., dosing errors, missed dosages, etc.) [24]. Case studies have also illustrated the risks of children ingesting ophthalmic preparations, thereby resulting in severe systemic poisoning or, in extreme cases, fatalities [25]. The combined knowledge suggests that the use of eye drops is characterized with a competing duality of noninvasiveness and patient autonomy. Topical formulations are most often used for treatment of anterior segment eye diseases. However, they generally have low levels of bioavailability ( < 10%) due to the barriers such as tear film and the cornea. The use of nanoparticles in ocular drug delivery has greatly enhanced the bioavailability of topically administered drugs.
5. Barriers and challenges to drug delivery systems and nanomedicinesA variety of nanoparticles and nanostructured carriers have been successfully applied to delivery ocular drugs [26-32]. Nanomedicines are able to deliver high drug payloads to the target ocular tissue and exert sustained efficay via controlled release. The delivery specficity can be enchanced by targeted delivery. However, ocular nanomedicines have been developed primarily for adults.
The eyes are equipped with several defense barriers that restrict the entry of exogenous substances. The efficacy of ocular drugs largely depends on whether they can overcome the anatomical and physiological barriers that protect the eye. The sensation of foreign substances, such as an eye drop, triggers a reflexive closure of the eyelid. Low bioavailability can result when the formulation is flushed out due to blinking or exuded via tears. Since the lacrimal system of children is more active than that of adults [16], this obstacle is particularly challenging for topical application of pediatric ocular therapy. Once the drug reaches the anterior portion of the eye, the next barrier to overcome is the blood vessels of the conjunctiva, which absorb the drug systemically, further reducing the bioavailability of the drug to its target site.
Intracellular and extracellular barriers begin with the corneal barrier: epithelium (5 layers), stroma, and endothelium. The epithelium consists of cells connected by tight junctions. It reduces the bioavailability of topically administered drugs, even when in the presence of nanoparticles or permeation enhancers [33]. The retina houses the blood-retinal barrier (BRB), which is a densely vascularized gateway containing interconnected tight junctions. The primary purpose of the BRB is to protects the posterior eye from penetration by foreign contaminants, proteins, and gases other than oxygen [33]. The barrier of nanoparticles to retinal pigment epithelium (RPE) is illustrated in Fig. 4 [20]. Despite these drawbacks, topical administration continues to provide the most promising outlook for management and treatment of pediatric ocular diseases, given that children generally require smaller dosages than adults in order to achieve similar levels of therapeutic efficacy.

|
Download:
|
| Fig. 4. Schematic overview of nanoparticle transport to the RPE. After leaving the fenestrated choriocapillaris the nanoparticle has to cross the five-layered Bruch's membrane, consisting of a central elastic layer, two collagenous layers and the RPE and choriocapillaris basal laminae. Reproduced with permission [20]. Copyright 2015, Elsevier | |
6. Conclusions
Current trends in pediatric ocular drug delivery move toward a general lack of appropriate formulations for children with ocular diseases. Despite similar anatomy, the pathophysiology of pediatric eye in certain diseases is substantially different from the adult eye. There is a great need to develop pediatric ocular nanomedicines. Aside from the common barriers to topical drug delivery, pediatric ocular therapy presents the additional barriers of patient literacy, compliance, and autonomy for effective treatment [34]. There is a continued need for cultivation of more specific therapeutics that cater to ocular disease treatment without hindering early ocular development or long-term visual acuity. It is also crucial that effective communication continues among pediatric patients, guardians and care providers on the importance of compliance throughout the treatment process.
AcknowledgmentThis work was supported by the National Institutes of Health (No. R01EY024072).
| [1] |
A. Patel, K. Cholkar, V. Agrahari, et al., World J. Pharmacol. 2(2013) 47-64. DOI:10.5497/wjp.v2.i2.47 |
| [2] |
H. Basmak, N. Yildirim, S. Topbas, et al., Pediatric ophthalmology/eye and disorders, in:Ö. Özdemir (Ed.), Complementary Pediatrics[J]. InTech, Rijeka(2012), pp. 3-30. |
| [3] |
H.K. Batchelor, J.F. Marriott, Br. J. Clin. Pharmacol. 79(2015) 405-418. DOI:10.1111/bcp.12268 |
| [4] |
S. Reimondez-Troitino, N. Csaba, M.J. Alonso, et al., Eur. J. Pharm. Biopharm. 95(2015) 279-293. DOI:10.1016/j.ejpb.2015.02.019 |
| [5] |
J.J. Kang-Mieler, C.R. Osswald, W.F. Mieler, Expert Opin. Drug Deliv. 11(2014) 1647-1660. DOI:10.1517/17425247.2014.935338 |
| [6] |
E.P. Aponte, N. Diehl, B.G. Mohney, Arch. Ophthalmol. 128(2010) 478-482. DOI:10.1001/archophthalmol.2010.41 |
| [7] |
R. Cascella, C. Strafella, C. Germani, et al., Biomed. Res. Int. 2015(2015) 321291. |
| [8] |
J.Y. Yu Chan, B.N. Choy, A.L. Ng, et al., J. Curr. Glaucoma Pract. 9(2015) 92-99. DOI:10.5005/jp-journals-10008 |
| [9] |
R.S. Gold, Pediatr. Ann. 40(2011) 95-105. DOI:10.3928/00904481-20110117-09 |
| [10] |
D. Bremond-Gignac, H. Nezzar, P.E. Bianchi, et al., Br. J. Ophthalmol. 98(2014) 739-745. DOI:10.1136/bjophthalmol-2013-303888 |
| [11] |
K. LaMattina, L. Thompson, Dis.-Mon. 60(2014) 231-238. DOI:10.1016/j.disamonth.2014.03.002 |
| [12] |
A. Sheikh, B. Hurwitz, C.P. van Schayck, et al., Cochrane Database Syst. Rev.(2012), CD001211. |
| [13] |
D. Bremond-Gignac, F. Chiambaretta, S. Milazzo, Ophthalmol. Eye Dis. 3(2011) 29-43. |
| [14] |
R.A. Sharma, R. Mather, CMAJ 186(2014) 1090. DOI:10.1503/cmaj.131590 |
| [15] |
L. Chen, L. Pi, J. Fang, et al., Acta Ophthalmol. 94(2016) e727-e730. DOI:10.1111/aos.13093 |
| [16] |
S.B. Han, H.K. Yang, J.Y. Hyon, et al., Graefe's Arch. Clin. Exp. Ophthalmol. 251(2013) 791-796. DOI:10.1007/s00417-012-2097-2 |
| [17] |
M.J. Doughty, D. Fonn, D. Richter, et al., Optom. Vis. Sci. 74(1997) 624-631. DOI:10.1097/00006324-199708000-00023 |
| [18] |
A. Hellstrom, L.E. Smith, O. Dammann, Lancet 382(2013) 1445-1457. DOI:10.1016/S0140-6736(13)60178-6 |
| [19] |
W.V. Good, R.J. Hardy, V. Dobson, et al., Pediatrics 116(2005) 15-23. DOI:10.1542/peds.2004-1413 |
| [20] |
R. Hennig, A. Goepferich, Eur. J. Pharm. Biopharm. 95(2015) 294-306. DOI:10.1016/j.ejpb.2015.02.027 |
| [21] |
B. Falsini, A. Chiaretti, G. Barone, et al., Neurorehabil. Neural Repair 25(2011) 512-520. DOI:10.1177/1545968310397201 |
| [22] |
R. Gaudana, H.K. Ananthula, A. Parenky, et al., AAPS J. 12(2010) 348-360. DOI:10.1208/s12248-010-9183-3 |
| [23] |
C. Dreno, T. Gicquel, M. Harry, et al., Ann. Pharm. Fr. 73(2015) 31-36. DOI:10.1016/j.pharma.2014.06.006 |
| [24] |
R.B. Freedman, S.K. Jones, A. Lin, et al., Arch. Ophthalmol. 130(2012) 306-311. DOI:10.1001/archopthalmol.2011.1788 |
| [25] |
C. Rangan, G. Everson, F.L. Cantrell, Pediatr. Emerg. Care 24(2008) 167-169. DOI:10.1097/PEC.0b013e3181668aee |
| [26] |
E.J. Campos, A. Campos, J. Martins, et al., Nanomedicine 13(2017) 2101-2113. DOI:10.1016/j.nano.2017.04.008 |
| [27] |
M. G. Lancina Ⅲ, H. Yang, Can. J. Chem. (2017), doi: http://dx.doi.org/10.1139/cjc-2017-0193.
|
| [28] |
Y. Fan, B. Zhuang, C. Wang, et al., Colloids Surf. B 140(2016) 1-10. DOI:10.1016/j.colsurfb.2015.11.059 |
| [29] |
R. Gaudana, J. Jwala, S.H. Boddu, et al., Pharm. Res. 26(2009) 1197-1216. DOI:10.1007/s11095-008-9694-0 |
| [30] |
C.A. Holden, P. Tyagi, A. Thakur, et al., Nanomedicine 8(2012) 776-783. DOI:10.1016/j.nano.2011.08.018 |
| [31] |
H. Yang, C.T. Leffler, J. Ocul. Pharmacol. Ther. 29(2013) 166-172. DOI:10.1089/jop.2012.0197 |
| [32] |
H. Yang, P. Tyagi, R.S. Kadam, et al., ACS Nano 6(2012) 7595-7606. DOI:10.1021/nn301873v |
| [33] |
S.P. Kambhampati, R.M. Kannan, J. Ocul. Pharmacol. Ther. 29(2013) 151-165. DOI:10.1089/jop.2012.0232 |
| [34] |
F. Fortinguerra, A. Clavenna, M. Bonati, BMC Pediatr. 12(2012) 8. |
 2017, Vol. 28
2017, Vol. 28