b Department of Oncology and Cancer Center, West China Hospital, Sichuan University, Chengdu 610041, China;
c Zhejiang Provincial Key Laboratory of Orthopaedics, Wenzhou 325027, China
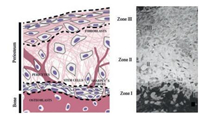
Periosteum is a dense connective tissue membrane covering the outer surface of most bone except the joint [1]. The tissue structure of the periosteum can be divided into two different layers (Fig. 1) [2]: the outer fibrous layer mainly contains fibroblasts, blood vessel networks and well-organized and directional collagen fibers aligned in the direction of bone growth [3], while the inner cambial layer, attaches directly to the bone surfaces and consists of multipotent mesenchymal stem cells (MSCs) and osteogenic progenitor cells, which are also known as periosteum-derived stem cells (PDSCs) [2, 4]. The periosteum has been shown to be important to the integrity, modelling and remodelling of bone, particularly during fracture repair [5]. Due to the superior ability of the periosteum to provide osteoprogenitor cells and shield scar tissues, periosteum transplantation has been performed in clinical medicine. However, as for other grafts, the availability of the periosteum, donor site morbidity and immunological rejection are major problems [6, 7]. By introducing the concept of tissue engineering in periosteum-related therapy, it is hoped to employ the periosteum more widely in bone defect therapy [8]. The tissueengineered periosteum can be used to assist the native graft or bionic bone scaffold, and it can be directly used as a substitute for the natural periosteum [9, 10].
|
Download:
|
| Fig. 1. Schematic representation (left) and light micrograph (right) depicting the three zones of the periosteum as well as the distribution of cell populations and extracellular matrix fibers that contribute to the biological and mechanical properties of the periosteum. Reproduced with permission [2]. Copyright 2012, John Wiley and Sons | |
Kinds of artificial periosteum, or tissue-engineered periosteum, have been developed, using small intestinal submucosa, acellular dermis, induced membrane, cell sheets, and polymeric scaffolds, and so on. Here, we review the development of artificial periosteum and classify it into three approaches based on the material source, that is, native tissues, scaffold-free cell sheets and natural or synthetic polymeric scaffold-cell composites.
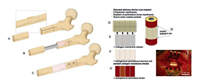
2. Native tissues 2.1. Periosteal autograftPeriosteal autografts have exhibited promising results when used to promote bone repair. Karaoglu et al. investigated the effect on bone healing of fresh cancellous autograft, demineralized deepfrozen allograft, and demineralized deep-frozen allograft covered with free autogenous periosteum, and found that ossification could be more easily achieved if demineralized deep-frozen allograft is covered with free autogenous periosteum when faced with the need for quicker and better quality bone integration (Fig. 2) [11, 12]. Autografts have been the gold standard for repairing bone defect, while their clinical application is still limited due to inaccessibility [13].
|
Download:
|
| Fig. 2. A typical procedure for treatment of bone defects harnessing the regenerative power of periosteum using autologous periosteum (A–C) in situ or periosteum substitute membrane, which comprises of a delivery device (D) and collagen membrane sheets (E), or collagen sheets seeded with periosteal cells (F), or autologous periosteal strips (G). (H, I): The complete implant is sutured in situ to itself and to neighboring periosteum. Reproduced with permission [12]. Copyright 2016, John Wiley and Sons | |
In addition, some methods have been used to pretreat periosteal grafts to enhance autograft healing and repair. Kearney et al. applied extracorporeal shock waves (ESW) therapy as a noninvasive, inexpensive, and rapid method for stimulating the proliferation of cambium cells, which are also known as osteoprogenitor cells in the periosteum [14]. Stimulated with ESW therapy 4 days before surgery, the periosteal cells at the defect site have a significant 2.7 fold increase in cambium cell number and a 4-fold increase in cambium cell thickness compared with non-treated control. Elevation and transplantation of the pretreated periosteum with an inorganic bovine bone scaffold to the bone defect site significantly promote bone regeneration.
2.2. Periosteal allograftPeriosteal allograft is also perfect guiding bone tissue regeneration membrane except that it may cause some host response [15]. Olivos-Meza et al. applied subperiosteal injections of transforming growth factor-beta1 (TGF-β1) percutaneously in the medial side of the proximal tibia 7 days prior to surgery, then transplanted the periosteal allografts for osteochondral defect repair. After 6 weeks, complete filling of the defects with regenerated tissue was observed in both groups, while the total histologic score in the TGF-β1-treated group was significantly higher than the control group. The most notable improvements were in structural integrity and subchondral bone regeneration [16].
To overcome the host response issue of allografts, decellularization may be a workable trial. Rapp et al. decellularized the periosteum via a proprietary formula to remove all antigenic material and then seeded with adipose-derived stromal cells (ASCs) or periosteal-derived stromal cells (PSCs) [17]. After incubated for 14 days, the acellular periosteum was implanted with xenograft bone chips or growth factors to repair 5-mm diameter calvarial defects. The acellular allo-periosteum was found to be a biomimetic scaffold that permits pluripotent cell adherence, migration and proliferation in vitro and promotes bone defects repair in vivo. Similarly, Chen et al. obtained a decellularized periosteum by employing a combination of physical methods as well as chemical and enzymatic solutions [18]. The cellular components were effectively removed and the native extracellular matrix properties (collagen, glycosaminoglycan (GAG), microarchitecture and mechanical properties) were reserved with no significant alterations. The decellularized periosteum could support periosteum-derived cells adhere, proliferate and infiltrate into it, and didn't elicit a severe immunogenic response in vivo, therefore, could provide a naturally compatible scaffold for use.
2.3. Porcine small intestinal submucosaPorcine small intestinal submucosa (SIS) is a bioderived acellular collagen membranous matrix, which has been used in humans in over sixty different surgical procedures [19]. The characteristics of elastic texture, biodegradability and immunocompatible properties in SIS make it optimal use as scaffold in tissue-engineered bone membrane to guide the process of bone regeneration. It is also reported that SIS can enhance angiogenesis through releasing vascular endothelial growth factor (VEGF) during biodegradation in vivo. Several studies have evaluated in vitro bioactivity [20], osteogenesis [19, 21], immunocompatibility, and the ability to repair bone defect of tissueengineered periosteum which was fabricated by coupling either bone marrow mesenchymal stem cells (MSCs) or MSCs-induced osteoblasts with SIS. Both MSCs and osteoblasts can adhere and survive on SIS scaffold, and have an active osteogenesis. It can conclude that SIS with MSCs or MSCs-induced osteoblasts is promising for in vivo bone defect reparation via a supposed biomimetic procedure of intramembranous ossification with tolerable immune reaction, while pure SIS is incapable of repairing bone defect in rabbit model [22].
2.4. Induced membraneTo reconstruct large bone defects in clinical practice, Masquelet et al. described a treatment that induces a membrane to form around the bone defect, so called induced membrane (IM) technique. In the first stage, a polymethylmethacrylate (PMMA) cement spacer is inserted into the boy defect, and over a period of 6-8 weeks, a foreign-body reaction cause a membrane to form that encapsulates the defect/spacer. Then, in a second operation, the membrane is opened, the foreign-body PMMA spacer is removed and the resulting cavity surrounding the defect is filled with autologous bone [23]. Kinds of growth factors (VEGF, TGF-β1) and osteoinductive factors (BMP-2) was produced, and maximum BMP-2 production was obtained 4 weeks after the implantation, and, at this time, induced membranes favored human bone marrow stromal cells differentiation to the osteoblastic lineage [24]. Furthermore, after membrane formation, 1 cm2 biopsy was taken together with matched, healthy diaphyseal periosteum for comparative analysis. Both tissue shared similar morphology although induced membrane was significantly thicker than periosteum. The frequency of lymphocytes, pericytes and cells expressing markers consistent with bone marrow MSCs were 31.3 and 15.5-fold higher in IM, respectively [25, 26].
By use a composite of induced membrane and the biphasic calcium phosphate material, which was proved only an osteoconductive scaffold, in a subcutaneous implantation model, the induced membrane was found to be not osteoinductive. However, clinical interest in this technique remains for prevention of soft tissue invagination and for preserving bone shape [27].
It was concluded that the induced membrane is a thick, vascularized structure that resembles periosteum with a cellular composition and molecular profile facilitating large defect repair, and the cellular composition and growth factor content in induced membrane depends on the location where the membrane is induced.
2.5. Acellular dermisAcellular human dermis is a material contains extracellular components including fibronectin and vitronectin but not simple collagen matrices, thus promoting rapid revascularization. A periosteum-like material was synthesized using acellular dermal matrix and MSCs or osteoblasts, to overcome the disadvantages of periosteal grafts like limited donor sites [28]. After 6 week's implantation in a preliminary porcine segmental bone defect model, new bone formation was achieved with minimal to no soft tissue invasion into the defect site, indicating that the dermal membrane material may be used as a scaffold to deliver cells or osteoinductive proteins for periosteum regeneration by allowing for cellular repopulation, revascularization and bone defect restoration [29].
2.6. OmentumHigh flexibility of the greater omentum is useful for reconstructive surgery, which make it suitable for tissue engineering. Some studies tried to evaluate bone tissue engineering with wrapped omentum with periosteum concurrent with adipose derived adult stem cells (ASCs) in dog model, and have shown that the wrapped omentum with periosteum concurrent with ASCs lead to a favorable bone tissue formation [30]. These studies indicated that omentum provides the necessary nutrient environment for the migrated host cells at the earlier stage of bone formation process.
3. Scaffold-free cell sheetCell sheet technology has been applied in tissue engineering for several years to regenerate damaged soft tissues, including corneal epithelia, periodontal ligament, and bladder epithelia and so on. Cell sheet technology consists of primarily cell adhesion to and detachment from the dish surface by control the hydrophobicity of the surface. This allows for a non-invasive harvest of high viability cells in an intact monolayer that includes deposited extracellular matrix (ECM) [31]. The stromal microenvironment of periosteum is composed of various highly organized extracellular matrix (ECM). So several studies have been carried out to fabricate a vascularized bone graft for bone regeneration by integrating biomimetic cell sheet engineered periosteum and biodegradable scaffold. The ECM composition is mostly depends on the cell type used, so kinds of cell sources have been investigated to fabricate cell sheet as engineered periosteum.
3.1. Osteoblasts from periosteaUchiyama et al. used osteoblasts from periostea to from cell sheet on temperature-responsive culture dishes and then transplanted it in a rat calvarial defect model. A limited bone regeneration was observed in several small regions within the central portions of the defects that were covered by the transplantable cell sheets, compared with that bone was regenerated only from the periphery of the defect edges [32].
3.2. Bone marrow stromal cells (BMSCs)Bone marrow stromal cells (BMSCs) are a promising cell source for bone tissue engineering, because they can easily isolated from autologous tissue and contain a population of adult stem cells with osteogenic capacity. Several studies have shown the cell sheets comprising multi-layered BMSCs assembled with different kinds of scaffolds, such as porous β-tricalcium phosphate ceramic [33], calcium phosphate [34, 35], and allografts [36], to improve bone healing. Taken together, the engineered periosteum made up of BMSCs cell sheets showed sporadic mineralized nodules, elevated ALP activity, and up-regulated gene expression of osteogenic markers in vitro. Moreover, after implantation in subcutaneous pockets or bone defect sites, the in vivo bone-forming capability was improved.
As the native periosteum is composed of highly organized ECM fibers, whether the substrate alignment of medium used to fabricate cell sheet can influence in vitro osteogenesis of human mesenchymal stem cells (hMSCs) was investigated [37]. Collagencoated polydimethylsiloxane (PDMS) substrates with (aligned) and without nanopattern (flat) were used to fabricate a MSCs cell sheet. It was found that the hMSCs cell sheet significantly increased alkaline phosphatase activity and calcium deposition, and can bind significantly higher amounts of growth factors including TGF-β1, bFGF and VEGF. However, the alignment of the substrates did not show significant influence on osteogenic activity and growth factor binding.
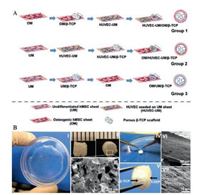
3.3. Human umbilical vascular endothelial cells (HUVECs)Nevertheless, the engineered periosteum made up of osteoblasts cell sheets or BMSCs cell sheets demonstrated limited vascularization ability. To mimic the function of high vascularization ability and similar structure of native periosteum, Kang et al. used hMSCs and HUVECs as cell sources to form cell sheets (Fig. 3) [38]. The hMSCs were chosen to form cell sheet matrix due to its stabilization ability for new formed blood vessels as a pericyte and the osteogenic differentiation potential. HUVECs were seeded to an undifferentiated hMSCs sheet to generate a prevasculairzed cell sheet (biomimetic fibrous layer), and hMSCs were cultured in osteogenic medium to form an osteogenic mineralized cell sheet (biomimetic cambium layer). The in vivo studies, which integrated the hMSCs/HUVECs sheet with beta-tricalcium phosphate (β-TCP) scaffold to engineer a graft, indicate that the hMSCs/HUVECs sheet enhanced angiogenesis and functional anastomosis between the in vitro performed human capillary networks and the mouse host vasculature, and ectopic osteogenesis.
|
Download:
|
| Fig. 3. Step-by-step procedures for preparing hMSCs-HUVEC sheets/β-TCP composite grafts. (A) Preparing three cell sheet/β-TCP grafts. (B) Macroscopic view of an hMSCs sheet on a dish (Ⅰ) and a porous β-TCP scaffold (Ⅱ). SEM image demonstrates the morphology of β-TCP pores (Ⅲ). Point forceps were used to wrap the cell sheet onto a β-TCP scaffold (Ⅳ), thus generating a HUVEC-UM/OM/β-TCP graft (Ⅴ). SEM images show a very dense extracellular matrix of cell sheet on a β-TCP scaffold (Ⅵ, Ⅶ). Reproduced with permission [38]. Copyright 2014, American Chemical Society | |
On the other hand, cell sheets have also an intrinsic shortcoming that they have poor mechanical strength compared with that demanded for large defects [39]. So that, Ishijima et al. tried to fabricate a titanium-reinforced cell sheets [40]. It is prepared by culturing rat calvarial periosteum-derived cells on temperatureresponsive culture dishes and attached to fifty-mm-thick titanium plates containing apertures. Single-sided cell sheets or doublesided cell sheets on as-made titanium contracted and deformed within 4 days of incubation, while the titanium-reinforced cell sheets on photofunctionalized titanium were structurally stable up to 14 days, developed the expected osteogenic phenotypes, and maintained structural integrity without functional degradation.
4. Scaffold-cell compositesDirect delivery of MSCs using cell sheets technology could often leads to detachment of tissue segments, poor graft localization and limited cell survival. To overcome these complications and emulate periosteum function, it was necessary to develop appropriate scaffold that could optimally support the initial adhesion and subsequent cell growth. Numerous biocompatible and biodegradable biomaterials have been investigated, including natural polymers, synthetic polymers, and their combination, in forms of membrane, sponge, hydrogel, and so on.
4.1. Natural polymersAmong the most common natural polymeric scaffolds investigated are collagen, chitosan, fibrin and cellulose, which are typically employed in membrane or sponge forms.
4.1.1. CollagenCollagen membranes are the most commonly used barrier membrane materials to guide bone regeneration in the treatment of bone defects. Collagen membranes are preferred owing to their hemostatic function, which leads to early wound stabilization, chemotactic properties toward fibroblasts, and permeability, which facilitates nutrient transport [41, 42]. Hattori et al. developed a bio-artificial periosteum composed of osteogenic cells and collagen sponge and found new bone formation in the central part as well as at the bone edge in the defect sites covered with the bioartificial periosteum [43]. Using a collagen membrane with a porous hydroxyapatite scaffold to serve as an osteoconductive scaffold, Guda et al. also found a threefold increase in the density of the regenerated bone than that in the scaffold group after 8 weeks implantation [44]. In addition, the use of periosteum-mimetic collagen wrap showed significant benefits of increased interfacial bone in-growth (149% greater) and periosteal remodelling (49%).
Based on these studies, there were also some improvements to develop collagen-based engineered periostea. Cobalt chloride (CoCl2), a hypoxia mimicking agent which can active the hypoxia inducible factor (HIF-1), was used to pre-treat BMSCs to promote vascularization. CoCl2 pre-treated BMSCs and osteogenesis differentiated BMSCs were seeded in a collagen scaffold to construct a dual-layered periosteum [45]. Both the ectopic osteogenesis in subcutaneous implantation after 2 weeks and orthotopic osteogenesis and vascularization in skull bone defects implantation after 4 weeks were significantly enhanced. Kajiwara et al. developed artificial periosteum-like membranes made from tilapia fish collagen and hydroxyapatite composites as a drug carrying support [46]. The tensile strength and adsorption ability of proteins of the membrane depended on the compositions ratio of collagen and hydroxyapatite. Shi et al. fabricated freestanding collagen micro patterned membrane from PDMS templates to simulate the inner layer of the periosteum that contain highly longitudinally oriented structure, and then seeded with mesenchymal stem cells and endothelial cells, thus making a pseudo-periosteum. The pseudo-periosteum-covered collagen/bioactive glass scaffolds showed remarkable osteogenesis when compared with the pseudo-periosteum-free scaffolds [47].
4.1.2. ChitosanChitosan, a deacetylated derivative of the natural abundant polysaccharide chitin, is widely used in bone tissue engineering and surface coating, for its biodegradability, antibacterial activity, and promote wound healing [48]. Chitosan can be used alone or combine with other components, to fabricate various forms of scaffolds such as electrospun fibrous membrane, polyelectrolyte multilayers, hydrogel and sponge.
An ultra-thin coating based on polyelectrolyte multilayers of chitosan and polyanionic glycosminoglycans (e.g., heparin) could be used to bind, stabilize, and deliver heparin-binding growth factors [49]. Furthermore, Almodóvar et al. have reported theses chitosan-heparin ultra-thin coatings could be applied directly to cortical bone allograft surfaces, helping promote MSCs attachment and had significant antibacterial activity against S. aureus and E. coli [50]. Similarly, Romero et al. compared the cytocompatibility among these chitosan-heparin coating, chitosan foam coating and electrospun chitosan nanofibers. Then they investigated whether these three coating membrane could serve as periosteum mimics and combined with porous chitosan scaffolds to deliver osteoprogenitor cells and improve bone graft healing [51]. Based on chitosan membrane, a chitosan-tricalcium phosphate-gelatin scaffold could also be constructed as a mechanical properties reinforced tissue-engineered periostea [52]. Using the chitosanheparin membrane-based tissue engineered periosteum to locally deliver FGF-2, TGF-β1 and adipose-derived mesenchymal stem cells to mouse femur defect was also evaluated [53].
4.1.3. FibrinThe platelet-rich fibrin (PRF) can also serve as a resorbable membrane in implantology to cover bone augmentation sites [54, 55]. Gassling et al. firstly measured the biocompatibility and ability to support and promote the proliferation of human periosteal cells on PRF membranes [56]. The biocompatibility of the PRF-based membranes was slightly inferior, while the metabolic activity and proliferation level of periosteal cells was higher, when compared with the commonly used collagen membrane Bio-Gide as scaffolds for periosteal tissue engineering.
A platelet-rich plasma (PRP) membrane incorporating BMSCs which could release vascular endothelial growth factor (VEGF) and platelet derived growth factor-BB (PDGF-BB) was also used as periosteal substitute for regeneration of compromised bone defects [57]. It was found that the PRP/BMSCs gel membrane could significantly induce the migration of endothelial cells, and increased the expression of bone morphogenetic protein 2 as well as proangiogenic factors such as VEGF and interleukin-8 in vitro, and enhanced ectopic and orthotopic bone regeneration.
In addition to these natural component mentioned above, cellulose and so on, have also been evaluated the potential ability to fabricate periosteum biomimetic, while natural-derived polymers may exhibit poor mechanical properties and pathogenic impurities, limiting their clinical application.
4.2. Synthetic polymerSynthetic scaffolds can be easily designed and fabricated with a variety of programmable features (e.g., porosity, pore size, mechanical properties and degradation rate) which can be tailed to intended application sites.
Among these synthetic materials, polylactic-co-glycolic acid (PLGA) is one of the most popularly materials used in biomedical products and devices such as suture materials for surgical procedures and is approved by the Food and Drug Administration. PLGA-based fiber is suited for fabrication of a multi-layered membrane simulating the highly organized ECM in periosteum for bone graft repair and reconstruction. The effects of PLGA-based electrospun fibers diameter and orientation (random and aligned) on differentiation and ECM organization of BMSCs, in attempt to provide rationale for fabrication of a periosteum mimetic for bone defect repair [58]. Besides, a method of combination of micropatterning and spin coating was also introduced to develop flexible PLGA nanosheets (Fig. 4) [59]. The microgrooved PLGA nanosheets could be adhered onto various scaffolds and implanted with a high stability, and could effectively regulate the cell alignment of stem cells cultured on them. These features enable microgrooved PLGA nanosheets to serve as stable and biomimic templates for the generation of artificial periostea for bone repair. Ardjomandi et al. have evaluated the biological functionality of 2D coating in plates and 3D coating in β-TCP scaffolds consisting of PLGA and graphene oxide (GO) by varying parameters [60]. The addition of GO significantly reinforce the PLGA coating membrane which served as a periostea, and reduce the brittleness of the β-TCP scaffolds without affecting its cytotoxicity.

|
Download:
|
| Fig. 4. Generation of microgrooved PLGA nanosheets. (A) A schematic for the fabrication of microgrooved PLGA nanosheets. (B) Thickness of flat part of PLGA nanosheets fabricated with different PLGA concentrations. (C) The height of the microgrooved PLGA nanosheets with different groove widths using a 10 mg/mL PLGA solution; and the groove spacing is 100 μm (in red color), 50 μm (in blue color), 30 μm (in green color), and 20 μm (in purple color). (D) Images of the PLGA and PVA bilayer film and the microgrooved PLGA nanosheet. (E) SEM images of PLGA nanosheets with different groove spacings (scale bars: 100μm). Reproduced with permission [59]. Copyright 2014, John Wiley and Sons | |
Besides, various kinds of polymer, such as poly (L-lactic acid) (PLLA) [61], dopamine-coated parafilm [62, 63], polycaprolactone [64-66], polyglycolic acid [55, 67], poly[(R)-3-hydroxybutyric acid] (PHB) [68], and expanded-polytetrafluoroethylene (e-PTFE) [69], have been made into fibrous membranes and seeded with kinds of cells to fabricate engineered periosteum, to investigate their effects on cell biological functions and bone regeneration.
Not only can be functioned as a barrier membrane and carrier for stem cells to guide bone regeneration, polymer membrane can also be used as drug delivery system [70]. Chou et al. produced a PLGA membrane as artificial periosteum to release lidocaine, vancomycin, and ceftazidime in the treatment of segmental femoral open fractures [71]. Su et al. developed an artificial periosteum that forms dexamethasone (DEX)-containing polyvinyl alcohol (PVA) nanofiber obtained from silk fibrous scaffold, and evaluated its effect on bone healing of osteogenic differentiation in stem cells originating from human exfoliated deciduous teeth (SHEDs) in vitro [72]. After 21 days of induced culture, the expression of alkaline phosphatase activity and calcium mineralization notably increased, as well as the gene expression of Runx2, OPN and OCN and osteoblastic protein expression of BMP-2. The artificial periosteum made from PVA nanofibers that contains DEX on a silk scaffold could effectively induce SHEDs differentiated into osteoblasts by DEX stable released.
4.3. Natural-synthetic compositeIn some studies, natural and synthetic polymer were combine used to play the advantages of each materials, achieving a promising scaffold with proper porosity, biodegradation rate and mechanical properties [73]. Kawase et al. have demonstrated a collagen-coated poly (L-Lactide-co-caprolactone) (LCL) film, and tested its function as a scaffold of periosteal cell sheets to improve the periodontal regeneration [74]. Surface collagen coating modified the hydrophobic nature of LCL and substantially improve the initial adhesion. Kawase et al. have tested the effects of scaffold made of salmon collagen-coated e-PTFE mesh on improving the osteogenic activity of periosteal cell sheets [75]. After implanted the scaffold-periosteal sheets complex subcutaneously into nude mice, periosteal sheets efficiently form osteoid around the mineral deposits, suggesting the collagen-coated e-PTFE mesh augmented the osteogenic activity of human periosteal sheets both in vivo and in vitro compared with e-PTFE mesh.
4.4. HydrogelIn contrast to traditional membrane or sponge-formed scaffolds, hydrogels have mechanical properties and hydration of the native ECM environment, making them ideal for many tissue engineering applications.
Poly (ethylene glycol) (PEG) hydrogel are easily modified to allow for degradation and inclusion of biomolecules and other celladhesion ligands to promote specific cell function, so it can be designed to have consistent hydration, elastic properties, and provide similar cellular persistence as the periosteum. Hoffman Michael et al. utilized hydrolytically degradable PEG hydrogels to transplant and localize MSCs to allograft surfaces, creating a periosteum mimetic [76, 77]. It demonstrated this approach resulted in about 2.4 fold in increased vascularization, 2.8 fold in endochondral bone formation, and 1.8 fold in biomechanical strength, as compared to untreated allografts, after 16 weeks implantation. Furthermore, they used PEG hydrogels as a tissue engineered periosteum to localize a mixture population of 50:50 MSCs and osteoprogenitor cells to better mimic native periosteum cell population and paracrine factor production, and showed expedite allograft healing [78]. Baldwin et al. transplanted human BMSCs with a star-PEG heparin hydrogel carrier at the surgical site in a mice femoral cortical windows defect model. It was found that human BMSCs retained their undifferentiated phenotype in vivo, and human endothelial cells developed into mature functional vessels and connected to host vasculature [79].
Chun et al. developed a 3D injectable hydrogel-bioceramic composite consisting of gelatin-3-(4-hydroxyphenyl) propionic acid (Gtn-HPA) and carboxymethyl cellulose-tyramine (CMC-Tyr), incorporated with fish scale-derived calcium phosphate (CaP) for bone regeneration [80]. The Gtn-HPA/CMC-Tyr hydrogel system served as a periosteum led to significant improvement in various functional properties favorable for bone tissue regeneration. A series of systematic investigations showed that the hydrogelbioceramic composite with 10% CaP (w/v) was optimal for an enhanced mechanical property, efficient drug delivery, and a harmonized swelling ration with its degradation rate resulted in improved cell proliferation.
5. ConclusionPeriosteum, as a store for progenitor cells and local growth factors and a barrier to shield scar tissues, plays a significant role in modelling and remodelling of bone, particularly during bone formation and bone defect regeneration. It is regarded as an indispensable part of bone grafts or tissue-engineered bones. Due to the source limitation of autografts and allografts, kinds of artificial periosteum, or so called tissue-engineered periosteum, have been developed. So far, progress has been made on periosteum tissue engineering, with a focus on using different biomaterial source to construct bionic periosteum comparable to the native periosteum, either in mimetic structure or in similar function.
An ideal artificial periosteum should have a periosteal-mimetic structure, would either directly deliver or recruit locally endogenous factors that improve bone fracture healing, including osteoinductive signals factors, osteogenic cells, and antimicrobial factors. Among the previous reported artificial periosteum, native tissues like SIS, acellular dermis and omentum are adopted mainly because they contain an acellular collagen membranous extracellular matrix (ECM), which is similar to periosteum matrix and could support osteogenic cells adhesion and proliferation. While, induced membrane, periostea cells or bone marrow stromal cells (BMSCs) derived cell sheet mainly directly delivery both ECM containing osteoinductive signals factors and osteogenic cells. Go a step further, scaffold-based tissue engineering technology enable us to fabricate a periosteum-mimetic structure on demand, as well as deliver various cells and osteoinductive or angiogenetic growth factors to improve bone regeneration. Experiments have been carried out both in vitro and in vivo to examine the performance of these tissue-engineered periosteum.
Nevertheless, bionic research on the microstructure of biological functions of the periosteum remains need to be further investigated. One distinct disadvantage of present artificial periosteum exists in the fact that it is difficult to effectively anchor to or integrate with bone scaffolds. The use of 2D ultrathin polymeric nanosheets may be a promising candidate for generating artificial periosteum due to its unique feature of flexibility [59, 62, 81, 82]. Furthermore, another promising approach may be using 3D-bioprinting technology to print a bone-periosteum integrate structure, avoiding the anchor problem [83]. Taking the advantage of 3D printing technology which is to arrange the position of cells and scaffolds precisely, we can fabricate the two layers of periosteum with strong adhesive strength to anchor to the bone surface, or even directly fabricate a bone-periosteum integrate structure, which is a complex work and require additional effort.
Besides, we need to further explore the biologic function of periosteum in bone formation. A recent study has shown the role of periosteum in magnesium-induced bone formation [84]. The intramedullary implantation of magnesium induces local production of CGRP, which is a neuropeptide released by sensory nerves in periosteum, help improving bone fracture healing. Once periosteum is removed, new bone would no longer form at defect regions. This finding indicates that there still are some mechanisms need us to investigate about the function of periosteum in bone regeneration.
AcknowledgmentsThis work was financially supported by National Natural Science Foundation of China (Nos. 31525009 and 31271021), National 863 Project (No. 2015AA020316), Sichuan Innovative Research Team Program for Young Scientists (No. 2016TD0004), Zhejiang Provincial Science and Technology Grant (No. 2017C33100), and Zhejiang Provincial Natural Science Foundation of China (No. LY17H060010).
| [1] |
M.R. Allen, J.M. Hock, D.B. Burr, Bone 35(2004) 1003-1012. DOI:10.1016/j.bone.2004.07.014 |
| [2] |
H. Chang, M.L.K. Tate, Stem Cells Transl. Med. 1(2012) 480-491. DOI:10.5966/sctm.2011-0056 |
| [3] |
J. Foolen, C. van Donkelaar, N. Nowlan, et al., J. Orthop. Res. 26(2008) 1263-1268. DOI:10.1002/jor.v26:9 |
| [4] |
K.N. Malizos, L.K. Papatheodorou, Injury 36(2005) S13-19. |
| [5] |
E. Seeman, Osteoporos. Int. 18(2007) 123-128. DOI:10.1007/s00198-006-0296-6 |
| [6] |
J. Xie, Y. Hou, Y. Yao, et al., J. Biomed. Nanotechnol. 11(2015) 1826-1835. DOI:10.1166/jbn.2015.2119 |
| [7] |
S. Dhivya, J. Ajita, N. Selvamurugan, J. Biomed. Nanotechnol. 11(2015) 1675-1700. DOI:10.1166/jbn.2015.2115 |
| [8] |
J. Liao, K. Shi, Q. Ding, et al., J. Biomed. Nanotechnol. 10(2014) 3085-3104. DOI:10.1166/jbn.2014.1934 |
| [9] |
N. Li, J. Song, G. Zhu, et al., Biomater. Sci. 4(2016) 1554-1561. DOI:10.1039/C6BM00481D |
| [10] |
X. Zhang, H.A. Awad, R.J. O'Keefe, R.E. Guldberg, E.M. Schwarz, Clin. Orthop. Relat. Res. 466(2008) 1777-1787. DOI:10.1007/s11999-008-0312-6 |
| [11] |
S. Karaoglu, A. Baktir, S. Kabak, H. Arasi, Injury 33(2002) 679-683. DOI:10.1016/S0020-1383(02)00086-4 |
| [12] |
S.R. Moore, C. Heu, N.Y. Yu, et al., Stem Cells Transl. Med. 5(2016) 1739-1749. DOI:10.5966/sctm.2016-0004 |
| [13] |
M. Kanou, T. Ueno, T. Kagawa, et al., Ann. Plast. Surg. 54(2005) 71-78. DOI:10.1097/01.sap.0000139562.42726.dd |
| [14] |
C.J. Kearney, H.P. Hsu, M. Spector, Tissue Eng. Part A 18(2012) 1500-1508. DOI:10.1089/ten.tea.2011.0573 |
| [15] |
X. Liu, C. Zhou, T. Lou, Chin. J. Stomatol. 35(2000) 385-387. |
| [16] |
A. Olivos-Meza, J.S. Fitzsimmons, M.E. Casper, et al., Osteoarthr. Cartil. 18(2010) 1183-1191. DOI:10.1016/j.joca.2010.06.003 |
| [17] |
S.J. Rapp, D.C. Jones, P. Gerety, J.A. Taylor, Surgery 152(2012) 595-605. DOI:10.1016/j.surg.2012.07.019 |
| [18] |
K. Chen, X. Lin, Q. Zhang, et al., Acta Biomater. 19(2015) 46-55. DOI:10.1016/j.actbio.2015.02.020 |
| [19] |
L. Zhao, J.L. Zhao, L. Wan, S.K. Wang, Strateg. Trauma Limb Reconstr. 3(2008) 57-64. DOI:10.1007/s11751-008-0034-z |
| [20] |
L. Zhao, J. Zhao, J. Yu, et al., Mater. Sci. Eng. C Mater. Biol. Appl. 58(2016) 1170-1176. DOI:10.1016/j.msec.2015.09.086 |
| [21] |
C. Zhang, S. Wang, G. Ren, et al., Chin. J. Reparative Reconstr. Surg. 28(2014) 384-388. |
| [22] |
L. Zhao, J. Zhao, S. Wang, J. Wang, J. Liu, J. Biomed. Mater. Res. Part B 97(2011) 1-9. |
| [23] |
P. Pelissier, D. Martin, J. Baudet, S. Lepreux, A.C. Masquelet, Br. J. Plast. Surg. 55(2002) 596-598. DOI:10.1054/bjps.2002.3936 |
| [24] |
P.H. Pelissier, A.C. Masquelet, R. Bareille, S.M. Pelissier, J. Amedee, J. Orthop. Res. 22(2004) 73-79. DOI:10.1016/S0736-0266(03)00165-7 |
| [25] |
R.J. Cuthbert, S.M. Churchman, H.B. Tan, et al., Bone 57(2013) 484-492. DOI:10.1016/j.bone.2013.08.009 |
| [26] |
D. Henrich, C. Seebach, C. Nau, et al., J. Tissue Eng. Regen. Med. 10(2016) E382-E396. DOI:10.1002/term.v10.10 |
| [27] |
S. Catros, N. Zwetyenga, R. Bareille, et al., J. Orthop. Res. 27(2009) 155-161. DOI:10.1002/jor.v27:2 |
| [28] |
B. Schönmeyr, N. Clavin, T. Avraham, V. Longo, B.J. Mehrara, Tissue Eng. Part A 15(2009) 1833-1841. DOI:10.1089/ten.tea.2008.0446 |
| [29] |
D. Beniker, D. McQuillan, S. Livesey, et al., Orthopedics 26(2003) S591-S596. |
| [30] |
A.B. Sadegh, E. Basiri, A. Oryan, P. Mirshokraei, Cell Tissue Bank 15(2014) 127-137. DOI:10.1007/s10561-013-9383-z |
| [31] |
K. Nishida, M. Yamato, Y. Hayashida, et al., Transplantation 77(2004) 379-385. DOI:10.1097/01.TP.0000110320.45678.30 |
| [32] |
H. Uchiyama, M. Yamato, R. Sasaki, et al., J. Tissue Eng. Regen. Med. 5(2011) 483-490. DOI:10.1002/term.340 |
| [33] |
D. Ma, H. Yao, W. Tian, et al., Clin. Oral Implants Res. 22(2011) 1193-1199. DOI:10.1111/clr.2011.22.issue-10 |
| [34] |
F.N. Syed-Picard, G.A. Shah, B.J. Costello, C. Sfeir, J. Oral Maxillofac. Surg. 72(2014) 1078-1083. DOI:10.1016/j.joms.2014.02.005 |
| [35] |
J. Venkatesan, B. Lowe, S. Anil, et al., J. Nanosci. Nanotechnol. 16(2016) 8881-8894. DOI:10.1166/jnn.2016.12730 |
| [36] |
T. Long, Z. Zhu, H.A. Awad, et al., Biomaterials 35(2014) 2752-2759. DOI:10.1016/j.biomaterials.2013.12.039 |
| [37] |
Q. Xing, Z. Qian, B. Kannan, M. Tahtinen, F. Zhao, ACS Appl. Mater. Interfaces 7(2015) 23239-23247. DOI:10.1021/acsami.5b07386 |
| [38] |
Y. Kang, L. Ren, Y. Yang, ACS Appl. Mater. Interfaces 6(2014) 9622-9633. DOI:10.1021/am502056q |
| [39] |
F. Chen, Y. Zhou, S.T. Barnabas, M.A. Woodruff, D.W. Hutmacher, J. Biomech. 40(2007) S73-S79. |
| [40] |
M. Ishijima, M. Hirota, W. Park, et al., J. Biomater. Appl. 29(2015) 1372-1384. DOI:10.1177/0885328214567693 |
| [41] |
Y. Sun, S. Liu, Y. Fu, et al., J. Biomed. Nanotechnol. 12(2016) 2029-2040. DOI:10.1166/jbn.2016.2296 |
| [42] |
S.K. Padmanabhan, L. Salvatore, F. Gervaso, et al., J. Nanosci. Nanotechnol. 15(2015) 504-509. DOI:10.1166/jnn.2015.9489 |
| [43] |
K. Hattori, T. Yoshikawa, Y. Takakura, et al., Biomed. Mater. Eng. 15(2005) 127-136. |
| [44] |
T. Guda, J.A. Walker, B.M. Singleton, et al., Tissue Eng. Part A 19(2013) 1879-1888. DOI:10.1089/ten.tea.2012.0057 |
| [45] |
W. Fan, R. Crawford, Y. Xiao, Biomaterials 31(2010) 3580-3589. DOI:10.1016/j.biomaterials.2010.01.083 |
| [46] |
D. Kajiwara, T. Ikoma, MRS Adv.(2017), 1-6. |
| [47] |
X. Shi, S. Chen, Y. Zhao, C. Lai, H. Wu, Adv. Healthc. Mater. 2(2013) 1229-1235. DOI:10.1002/adhm.v2.9 |
| [48] |
G. Molinaro, J.C. Leroux, J. Damas, A. Adam, Biomaterials 23(2002) 2717-2722. DOI:10.1016/S0142-9612(02)00004-2 |
| [49] |
J. Almodóvar, M.J. Kipper, Macromol. Biosci. 11(2011) 72-76. DOI:10.1002/mabi.v11.1 |
| [50] |
J. Almodovar, J. Mower, A. Banerjee, et al., Biotechnol. Bioeng. 110(2013) 609-618. DOI:10.1002/bit.24710 |
| [51] |
R. Romero, L. Chubb, J.K. Travers, et al., Carbohydr. Polym. 122(2015) 144-151. DOI:10.1016/j.carbpol.2015.01.015 |
| [52] |
H. Guo, X. Li, X. Yuan, X. Ma, J. Trauma Acute Care Surg. 72(2012) E94-100. DOI:10.1097/TA.0b013e3182196a54 |
| [53] |
R. Romero, J.K. Travers, E. Asbury, et al., J. Biomed. Mater. Res. A 105(2017) 900-911. DOI:10.1002/jbm.a.35965 |
| [54] |
J. Choukroun, A. Diss, A. Simonpieri, et al., Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 101(2006) 299-303. DOI:10.1016/j.tripleo.2005.07.012 |
| [55] |
S. Koshinuma, S. Murakami, M. Noi, et al., Exp. Anim. 65(2016) 473-483. DOI:10.1538/expanim.16-0031 |
| [56] |
V. Gassling, T. Douglas, P.H. Warnke, et al., Clin. Oral Implants Res. 21(2010) 543-549. DOI:10.1111/clr.2010.21.issue-5 |
| [57] |
R.M. El Backly, S.H. Zaky, A. Muraglia, et al., Tissue Eng. Part A 19(2013) 152-165. DOI:10.1089/ten.tea.2012.0357 |
| [58] |
S. Lyu, C. Huang, H. Yang, X. Zhang, J. Orthop. Res. 31(2013) 1382-1389. DOI:10.1002/jor.v31.9 |
| [59] |
X. Shi, T. Fujie, A. Saito, et al., Adv. Mater. 26(2014) 3290-3296. DOI:10.1002/adma.v26.20 |
| [60] |
N. Ardjomandi, A. Henrich, J. Huth, et al., Biomed. Mater. 10(2015) 045018. DOI:10.1088/1748-6041/10/4/045018 |
| [61] |
T. Kawase, T. Tanaka, T. Nishimoto, et al., J. Biomed. Mater. Res. A 98(2011) 100-113. |
| [62] |
X. Shi, L. Li, S. Ostrovidov, et al., ACS Appl. Mater. Interfaces 6(2014) 11915-11923. DOI:10.1021/am5029236 |
| [63] |
Y. Deng, Y. Sun, Y. Bai, et al., J. Biomed. Nanotechnol. 12(2016) 602-618. DOI:10.1166/jbn.2016.2096 |
| [64] |
X. Liu, S. Liu, S. Liu, W. Cui, J. Biomed. Mater. Res. Part B 102(2014) 1407-1414. DOI:10.1002/jbm.b.v102.7 |
| [65] |
Y.W. Tarng, B.F. Huang, F.C. Su, Int. Orthop. 36(2012) 863-868. DOI:10.1007/s00264-011-1291-x |
| [66] |
H. Park, D.-J. Lim, S.-H. Lee, et al., J. Biomed. Nanotechnol. 12(2016) 2076-2082. DOI:10.1166/jbn.2016.2306 |
| [67] |
Y. Tsumanuma, T. Iwata, K. Washio, et al., Biomaterials 32(2011) 5819-5825. DOI:10.1016/j.biomaterials.2011.04.071 |
| [68] |
Z. Karahaliloglu, B. Ercan, E.N. Taylor, et al., J. Biomed. Nanotechnol. 11(2015) 2253-2263. DOI:10.1166/jbn.2015.2106 |
| [69] |
R.M. El Backly, D. Chiapale, A. Muraglia, et al., Front. Bioeng. Biotechnol. 2(2014) Article 80. |
| [70] |
J. Wang, C. He, N. Cheng, et al., J. Nanosci. Nanotechnol. 15(2015) 4844-4850. DOI:10.1166/jnn.2015.9844 |
| [71] |
Y.C. Chou, Y.S. Cheng, Y.H. Hsu, Y.H. Yu, S.J. Liu, Int. J. Nanomed. 11(2016) 941-953. DOI:10.2217/nnm-2015-0012 |
| [72] |
W.T. Su, W.L. Chiou, H.H. Yu, T.Y. Huang, Mater. Sci. Eng. C Mater. Biol. Appl. 58(2016) 1036-1045. DOI:10.1016/j.msec.2015.09.077 |
| [73] |
M.K. Pilehrood, A. Atashi, H. Sadeghi-Aliabadi, et al., J. Nanosci. Nanotechnol. 16(2016) 9000-9007. DOI:10.1166/jnn.2016.12740 |
| [74] |
T. Kawase, K. Yamanaka, Y. Suda, et al., J. Periodontol. 81(2010) 1653-1662. DOI:10.1902/jop.2010.100194 |
| [75] |
T. Kawase, K. Okuda, H. Kogami, et al., J. Mater. Sci. Mater. Med. 21(2010) 731-739. DOI:10.1007/s10856-009-3896-9 |
| [76] |
M.D. Hoffman, C. Xie, X. Zhang, D.S. Benoit, Biomaterials 34(2013) 8887-8898. DOI:10.1016/j.biomaterials.2013.08.005 |
| [77] |
M.D. Hoffman, D.S. Benoit, Clin. Orthop. Relat. Res. 471(2013) 721-726. DOI:10.1007/s11999-012-2695-7 |
| [78] |
M.D. Hoffman, D.S. Benoit, Biomaterials 52(2015) 426-440. DOI:10.1016/j.biomaterials.2015.02.064 |
| [79] |
J.G. Baldwin, F. Wagner, L.C. Martine, et al., Biomaterials 121(2017) 193-204. DOI:10.1016/j.biomaterials.2016.11.016 |
| [80] |
Y.Y. Chun, J.K. Wang, N.S. Tan, et al., Macromol. Biosci. 16(2016) 276-287. DOI:10.1002/mabi.v16.2 |
| [81] |
W. Zhang, J. Yu, H. Chang, J. Mater. Chem. B 3(2015) 4959-4964. DOI:10.1039/C5TB00087D |
| [82] |
V.N. Vernekar, R. James, K.J. Smith, et al., J. Nanosci. Nanotechnol. 16(2016) 8953-8965. DOI:10.1166/jnn.2016.12738 |
| [83] |
H. Cui, M. Nowicki, J.P. Fisher, L.G. Zhang, Adv. Healthc. Mater. 6(2017) 1601118. DOI:10.1002/adhm.v6.1 |
| [84] |
Y. Zhang, J. Xu, Y.C. Ruan, et al., Nat. Med. 22(2016) 1160-1169. DOI:10.1038/nm.4162 |
 2017, Vol. 28
2017, Vol. 28 


