Ceria, a key component for heterogeneous catalysts, is an active support for catalytic metals or multicomponent alloys and efficiently contributes to achieve desired chemical reactions [1, 2]. Ceria-supported noble metal catalysts like Rh, Pd, and Pt have attracted intensive research interests owing to their excellent activities in promoting various reactions including low-temperature CO oxidation, water-gas-shift reactions (WGS) and hydrogen production from ethanol [3-5]. These applications benefit from the unique oxygen storage capacity of ceria as well as the strong metal-support interaction (SMSI) between metals and the ceria supports [1, 6-8]. However, the high price and limited reserves of these noble metals remain as major concerns, catalysts that are cheaper and harder to denature are attractive industrial alternatives. Co-based catalyst emerges as a less expensive but promising alternative for certain reactions, for example, as a mixed oxide, in CO oxidation [9] and for steam reforming of ethanol (SRE) [10, 11]. Supported cobalt catalyst represents high ethanol conversion and selectivity to H2 and CO2 at relatively low temperatures [12, 13]. It has been also extensively studied for Fischer-Tropsch synthesis (FTS) because of its high activity and selectivity for linear hydrocarbons [14, 15]. Cobalt is also a doping agent for ceria, which is demonstrated its positive role in facilitating bulk oxygen diffusion in ceria [16-20].
Ceria-supported Au is an active catalyst in many applications including WGS, NO reduction, as well as CO oxidation [21-24]. Interestingly, ceria-supported gold modified with cobalt used as catalysts exhibited unique properties compared to individual Co and Au counterparts due to the synergistic effects between the two metals as well as the interaction between the metals and oxide supports [25]. The presence of Co could improve the catalytic activity of Au-based catalyst. For example, gold supported on Co3O4-modified ceria has been reported enhanced catalytic activities compared to conventional Au/ceria catalysts in the CO oxidation reaction [26-28]. Gold incorporation at Co3O4/CeO2 catalysts have been identified as promising catalysts for WGS, which have been observed to increase the capacity to release lattice oxygen from the catalysts to oxidize CO at low temperatures in oxygen-enhanced WGS reaction (OWGS) [29]. In addition, gold catalysts supported on ceria doped with cobalt oxide could serve as an excellent catalyst in complete benzene oxidation and obtain very high and stable catalytic activity [30, 31]. Despite the importance of Co modification to Au the catalysts on ceria, however, the atomic level understanding of the influence of Co on Au catalyst is still unknown. The Co-Au bimetallic clusters on CeOx(111) (1.5 < x ≤ 2) present a model system for understanding the nature of metal-metal interactions and metal-support interactions.
We report here the results of a systematic investigation of the interactions between Co and ceria thin film surfaces and the thermal stability of pure and bimetallic Co-Au particles on ceria films using techniques including scanning tunneling microscopy (STM), synchrotron radiation photoemission spectroscopy (SRPES) and X-ray photoelectron spectroscopy (XPS). In particular, the effect of Co on the sintering behavior of Au nanoparticles is examined. The bimetallic particles are prepared by sequential deposition of Au on Co pre-deposited ceria surfaces. Both fully oxidized CeO2(111) and partially reduced CeO1.82(111) thin films grown on Cu(111) are used as model of ceria supports in order to examine the effect of the degree of ceria reduction on the sintering behavior of Co-Au.
2. Results and discussionTo study the interaction between Co and ceria, we first used XPS and SRPES to investigate the electronic structure evolution upon deposition of Co on ceria at 300 K at different coverages, as shown in Fig. 1a. Changes in the ceria oxidation state as a function of Co coverage on CeO2(111) surface are monitored by the Ce 3d XPS spectra (Fig. 1b). The satellite peaks (u''', u'', u, v''', v'', v) are associated with the tetravalent Ce cations [32]. The presence of Ce3+ cations in the films results in four additional peaks indicated as u', u0, v', and v0 [32]. The labels u and v refer to the 3d3/2 and 3d5/2 spin-orbit components, respectively. The deposition of Co at 300 K induces a well detectable growth of the reduced component, which is best visible as an intensity enhancement in the valleys at ~885.0 and ~903.5 eV (Fig. 1b). By peak fitting the spectra shown in Fig. 1b, the contribution of the Ce3+ states can be derived at different Co coverages. As can be seen in Fig. 1c, the intensity of Ce3+ increases sharply below 0.3 monolayer (ML), followed by a slower increase up to θCo ~ 0.68 ML. This clearly indicates that an electron transfer from Co to ceria occurs upon Co deposition, especially at low coverages. Fig. 1d shows the valence band spectra for Co deposited on CeO2(111) at different Co coverages. The main feature of the spectrum from the clean substrate (bottom curve) is the broad peak ranging from 3 to 8 eV which can be ascribed to O 2p structure [33, 34]. With increasing Co coverage, this broad peak shifts toward higher binding energy side due to the coupling of Co states. In addition, a peak located at ~1.5 eV below the Fermi level, which is attributed to Ce 4f, gradually increases in intensity [34]. This peak is only related to the reduced state Ce3+. The appearance of this state as well as its gradual increase in intensity indicates that the CeO2 surface is reduced upon Co deposition, in agreement with the results from Ce 3d spectra. Moreover, the density of state [35] near EF, which is attributed to the DOS near Fermi edge EF of metallic Co [36], gradually increases with increasing Co coverage.

|
Download:
|
| Fig. 1. (a) Ball model of Co deposited on CeO2(111) surface, red, ivory and blue balls represent oxygen, cerium, and cobalt, respectively; (b) Ce 3d XPS spectra (hv = 1486.6 eV) of 2 nm thick CeO2(111) layer before and after the deposition of different amounts of Co at 300 K; (c) the intensity ratio of Ce3+ 3d peak to that of total Ce 3d peak calculated from the fitting of spectra presented in (b) as a function of Co coverage; (d) valence band photoemission spectra of Co on CeO2(111) at different Co coverages taken at a photon energy of 170 eV; (e) Co 2p XPS spectra (hv = 1486.6 eV) for different amounts of Co deposited on CeO2(111) surface; (f) Change of work function versus Co coverage on CeO2. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) | |
XPS spectra of the Co 2p aftereach deposition stepareplotted in Fig. 1e. According to the literature [37-40], for metallic cobalt (Co0), an asymmetric Co 2p3/2 peak is observed at 778.0-778.5eV, while for Co2+ the Co 2p3/2 peak locates at 780-781eV with a strong satellite at 786-787eV. The Co 2p3/2 peak for Co3+ also locates at 780-781eV but with no satellite [37-40]. As can be clearly seen in the Fig. 1e, in the initial stage (θCo=0.15 ML) of Co deposition on CeO2(111), the appearance of a Co 2p3/2 peak at 781.0eV with a strong satellite at 786.1eV can be assigned to Co2+. The shoulder at 778.6eV is attributed to metallic Co. Moreover, at 0.15 ML, the doublet separation between Co 2p3/2 peak at 781.0eV and Co 2p1/2 peak at 796.9eV is 15.9eV, which fits well with previous results on Co2+ [7]. For metallic Co, the doublet separation between Co 2p3/2 peak at 778.6eV and Co 2p1/2 peak at 793.7eV is 15.1eV, similar to the value reported previously for metallic Co (15eV) [7]. As the Co coverage increases, both the Co2+ and the metallic Co peaks gain intensity, but the latter becomes dominant in the Co 2p region at higher Co coverages (Fig. 1e). The oxidation of Co upon deposition is in good agreement with the aforementioned Ce 3d result. Fig. 1f shows the relative work function changes of CeO2(111) upon Co deposition at 300K. After deposited 0.15 ML Co, the work function decreases by 0.35eV due to the electron transfer from Co to ceria. Further deposition of Co induces the increase of work function. This can be attributed to the appearance of metallic Co on the surface. Finally at high Co coverages (>2.5 ML), the absolute work function reaches 5.75eV, in close agreement with the value reported for metallic Co (5.76eV) [41]. Overall, the work function measurements further confirm that at low Co coverages the Co is partially oxidized, in consistent with the result from the Co 2p observations.
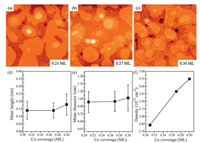
Fig. 2a-c shows the STM results of three different coverages of Co (0.21, 0.27, and 0.30 ML) deposited on CeO2 at 300K. The corresponding particle diameters, heights, and densities are plotted in Fig. 2e-f. As can be seen, Co grows randomly on the CeO2 surface for the whole coverage range investigated. A representative STM image for the low coverage (θCo=0.21 ML) is shown in Fig. 2a: the Co clusters appear as bright spots on top of the long-range modulated ceria film and are uniformly distributed on the surfaces. The apparent height and average diameter of the Co clusters amount to be about 0.14 ±0.06nm and 0.99 ±0.21nm, as inferred from Fig. 2d and e, respectively. When increasing the Co coverage to 0.27 ML, the island density increases but the size of Co clusters keep almost the same, which is about 1.00 ± 0.20nm in diameter and 0.14 ±0.05nm in height (Fig. 2b). Further increasing the Co coverage to 0.30 ML (Fig. 2c) leads to the average size of Co clusters increasing to 1.07 ±0.26nm in diameter and 0.18 ±0.07nm in height. On the other hand, Co clusters exhibit a monotonic increase in cluster density during the whole deposition (Fig. 2f). On the basis of these observations, we conclude that Co follows a 2D-like growth mode on CeO2(111) surface at the submonolayer coverage.
|
Download:
|
| Fig. 2. STM images of Co deposited on CeO2 with three different coverages at 300K: (a) 0.21 ML, (b) 0.27 ML, (c) 0.30 ML. All images are 100nm ×100nm. (d) Mean diameter, (e) mean height, and (f) density of Co particles on CeO2 surface at different Co coverages | |
Detailed STM studies allow to directly extract the information of thermally induced morphological changes of the Co nanoparticles on CeO2(111). Shown in Fig. 3 is the STM results for 0.3 ML Co deposited on CeO2(111) at 300K followed by annealing to 800K in three steps. As seen in the Fig. 3a, at 300K relatively small Co particles with an average width of about 1.07 ± 0.26nm and a height of 0.18 ±0.07nm are formed on the ceria surface. Upon heating the surface to 500K, larger clusters with an average diameter of ~1.52 ±0.42nm and a height of ~0.26 ± 0.07nm emerge accompanied by the decrease of cluster density (Fig. 3b). Further annealing to 600K, the number of clusters on the surface continues to decrease while the cluster size keeps almost the same, which is about 1.44 ± 0.39 nm in diameter and 0.23 ± 0.07 nm in height (Fig. 3c). Eventually, as shown in Fig. 3d, upon heating to 800 K, only a few relatively small Co particles can be found on the surface. Heating to high temperatures also results in a strong intensity loss of Co 2p XPS spectra (Fig. S2 in Supporting information). For Co/CeO2(111) surfaces, the diffusion of Co2+ into the subsurface of CeO2(111) coexisting with the agglomeration of Co0 upon heating to higher temperatures has been reported in the previous study of Vari et al. [7]. Co could even diffuse through the ceria layer deep into the Cu crystal [7]. However, the possibility of formation of CoCu alloy can be ruled out [42].

|
Download:
|
| Fig. 3. STM images of 0.3 ML Co deposited on CeO2(111) at 300 K (a) and after annealing to 500 (b), 600 (c) and 800 K (d), respectively. All images are 100 nm × 100 nm. Plots of mean particle height and diameter (e) and particle density (f) of 0.3 ML Co deposited on CeO2(111) as a function of annealing temperatures | |
In order to explore the influence of pre-deposited Co on the stability of Au nanoparticles on ceria, we prepared Co-Au bimetallic clusters on ceria by depositing more mobile Au atoms (ΘAu = 0.5 ML) on less mobile Co (ΘCo = 0.8 ML) pre-covered CeO2(111). Compositional changes in the bimetallic clusters are investigated by XPS after subsequently step-by-step annealed to high temperatures in UHV (annealing time = 5 min/step). At each annealing temperature, all XPS signal intensities are normalized to that at room temperature so that changes in intensity can be observed on the same scale. For comparison, studies of the thermal stability of pure Au nanoparticles on ceria were performed first. In addition, we also studied the influence of ceria stoichiometry on the Au thermal stability by depositing 0.5 ML Au on 2 nm thick CeO2(111) and CeO1.82(111) surfaces at 300 K followed by annealing to different temperatures in UHV for 5 min. After the surface was cooled down to room temperature, the Au 4f XPS spectra were collected. Shown in Fig. 4a are the Au 4f peak intensities normalized to the value at 300 K as a function of temperature. As can be seen, with increasing annealing temperature, the Au 4f intensity for Au on both CeO2(111) and CeO1.82(111) decreases at a slower rate below 600 K but after that drops rapidly. However, in general the Au 4f intensity for the Au/CeO2 system decreases more rapidly than what is observed for the Au/CeO1.82 system at the same temperature. This is attributed to the oxygen vacancies on the reduced CeO2-x surface which can stabilize the Au particles through a stronger interaction [43]. When comparing the normalized Au 4f intensity of the pure Au clusters on ceria with those of bimetallic Co-Au clusters on the same ceria surface, one can see that below 700 K the decrease of Au 4f intensity from the Co-Au/CeO2 surface follows almost identically with that of pure Au on CeO2 at each annealing temperature (Fig. 4b). However, above 700 K, the Au 4f intensity of the pure Au cluster drops more rapidly than that of the bimetallic cluster. This implies that the predeposited Co on CeO2 suppresses the sintering of post-deposited Au particles at high temperatures in comparison with that of pure Au particles. Interestingly, at the same annealing temperature on CeO1.82(111), the Au 4f intensity of the bimetallic Co-Au clusters drops more quickly than that of the pure Au clusters in the whole temperature range (Fig. 4c). A possible explanation is that the inward diffusion of Co is more hindered on the partially reduced ceria thin film (Fig. S2), making Au more easily encapsulated in the presence of surface Co or diffusing into the bulk of bimetallic clusters upon annealing, similar to the case of Pt-Co bimetallic clusters on the titania support [44].

|
Download:
|
| Fig. 4. Au 4f intensities as a function of annealing temperature for (a) pure Au on CeO2 and CeO1.82; (b) pure Au, and Co-Au clusters on CeO2; (c) pure Au, and Co-Au clusters on CeO1.82. At each temperature, the samples were annealed for 5 min and then cooled down to 300 K for collecting the spectra. All the intensities were normalized to those at 300 K | |
The XPS results presented above suggest that the addition of Co to the Au/CeO2 surface significantly affects the thermal stability of Au at elevated temperatures in comparison with that of pure Au particles. In order to further prove the information gained from XPS, an STM study during step-wise annealing of the sample should provide more straightforward structural/morphological information. The deposition of 0.25 ML Co on CeO2 results in clusters with average diameter and heights of 1.17 ± 0.23 and 0.17 ± 0.07 nm and a cluster density of 2.7 ×1013/cm2 (Fig. 5a). Subsequent deposition of 0.18 ML Au on this Co pre-covered CeO2 surface leads to only a small (~4%) increase in cluster density up to 2.8 × 1013/cm2 (Fig. 5b), indicating that the majority of Au atoms nucleate at existing Co sites. This is consistent with the observed increase in average cluster diameter (1.23 ± 0.26 nm) and height (0.22 ± 0.08 nm) as the incoming Au atoms are incorporated into Co clusters to form bimetallic clusters. In the absence of Co clusters, the growth of 0.18 ML Au on CeO2 (Fig. S3) results in much larger clusters (diameter: 1.13 ± 0.36 nm, height: 0.35 ± 0.11 nm) with a lower cluster density (1.0 ×1013/cm2), providing further evidence that Au nucleates at the existing Co clusters in Fig. 5b.Upon heating, the particles aggregate to form larger ones at the expense of the particle density. The particle density decreases by 66% when the surface was annealed to 500 K (Fig. 5c). The mean particle sizes are 1.26 ± 0.30 nm wide and 0.36 ± 0.11 nm high. Shown in Fig. 5d is the STM image taken after the surface was further heated to 600 K. Obviously, the increase in the particle size but decrease in the particle density was observed. Further annealing to 800 K, the particle density decreases by 89% (Fig. 5e). The particles have an average diameter and height of 1.65 ± 0.36 and 0.47 ± 0.16 nm, respectively. For comparison, same amount of pure Au deposited on CeO2(111) at 300 K followed by heating to 800 K is shown in Fig. 5f. Clearly, the pure Au particles undergo more severe sintering on CeO2(111) than the Co-Au particles, indicating that the Co pre-deposited on ceria surface can improve the thermal stability of gold particles on the ceria surface, in good agreement with those XPS results. Based on the STM results and the aforementioned XPS results, a schematic representation of sintering behavior of Au on ceria and Co pre-deposited ceria surfaces is shown in Fig. S5.

|
Download:
|
| Fig. 5. STM images of (a) 0.25 ML Co deposited on CeO2(111) at room temperature; (b) 0.18 ML Au on CeO2(111) pre-deposited with 0.25 ML Co; and subsequently annealed to (c) 500 K; (d) 600 K; (e) 800 K; (f) 0.18 ML Au deposited on CeO2(111) at 300 K following by annealing to 800 K. All images are 100 nm × 100 nm and recorded at room temperature | |
3. Conclusion
The interaction of Co with ultrathin ceria thin films and its influence on the thermal stability of Au particles on ceria were investigated at the atomic level. After small amount of cobalt is deposited on CeO2 at 300 K, Co is oxidized to Co2+ and simultaneously the ceria substrate is partially reduced. With increasing the Co coverage, XPS results suggest an increasing fraction of metallic Co0. At high temperatures, diffusion of Co2+ ions into the CeO2 thin film is found, accompanied with the agglomeration of metallic Co0 into larger particles. Annealing of Au particles on CeO2(111) leads to significant sintering of Au small particles into large ones. Oxygen defects on ceria can enhance the thermal stability of Au particles on CeO1.82(111). The Co-Au bimetallic clusters were prepared on both stoichiometric CeO2(111) and reduced CeO1.82(111) by deposition of Au on Co pre-covered surfaces. Comparing with the bare ceria surface, on the stoichiometric CeO2(111) surface, the existing of Co can improve the thermal stability of Au particles at high temperature, while on the reduced CeO1.82(111) surface, the thermal stability of Au in Co-Au particles is even lower than that for the pure Au particles on CeO1.82.
4. ExperimentalAll of the experiments were performed in two separate ultrahigh vacuum (UHV) systems. STM measurements were performed in a system that comprises three chambers with base pressures all below 8×10-11Torr. The SRPES and XPS measurements were performed at the Catalysis and Surface Science Endstation at the BL11U beamline in the National Synchrotron Radiation Laboratory. The beamline is connected to an undulator and equipped with two gratings that offer soft X-rays from 20 to 600eV with a resolution (E/ΔE) better than 104 at 29eV. Detailed descriptions of these two systems could be found in our previous works [45, 46]. The valence-band spectra were measured by using synchrotronradiationlight with photon energyof 170eV. A sample bias of -10V was applied in order to observe the secondary electron cutoff.
The Cu(111) single crystal (Mateck, 8mm diameter, 2mm thickness) was cleaned using several cycles of Ar+ ion sputtering and annealing until no impurity could be detected by XPS, and LEED gave a sharp (1×1) pattern with low background. The 2nm thick well-ordered CeO2(111) thin films were grown on the Cu(111) substrate at gradually increased substrate temperature by depositing Ce from a multi-pocket electron beam evaporator in the 2×10-7mbar of oxygen environment followed by annealing at 1000K for 5min. Our previousstudy has provedthat theceria films grown by this recipe are fully oxidized and well-ordered, exhibiting large and flat terraces with few surface defects [47]. The partially reduced ceria can be obtained by decreasing the oxygen pressure during the Ce evaporation.
Au was deposited from a gold foil (Alfa Aesar, 99.999%) using a knudsen-cell evaporator, while Co was deposited from a homemade source using Co wire (Alfa Aesar, 99.994%). The Au and Co flux were calibrated with a quartz crystal microbalance (QCM) before each deposition. A coverage of one monolayer (ML) of Co or Au is defined as the packing density of the Co(0001) (1.83 ×1015 atoms/cm2) or Au(111) (1.40 ×1015atoms/cm2) surface, respectively [25]. The deposition rates were 0.2 ML/min for Co and 0.2ML/min for Au.
All the STM images were collected using an etched tungsten tip at room temperature in a constant current mode (0.01-0.05nA, 3-4V). During the STM experiments, great efforts were made to minimize the tip convolution effect. The STM images were processed using the WSXM program [48]. Particle size distribution (PSD) analysis was achieved by commercial software SPIP.
AcknowledgmentThe authors gratefully acknowledge the National Natural Science Foundation of China (Nos. 21473178 and 21403205) and National Basic Research Program of China (No. 2013CB834605) for the financial support of this work.
Appendix A. Supplementary dataSupplementary data associatedwith this article can be found, in the online version, at10.1016/j.cclet.2017.04.012.
| [1] | A. Trovarelli. Catalyticpropertiesof ceria and CeO2-containing materials. Catal. Rev. Sci. Eng. 38 (1996) 439–520. DOI:10.1080/01614949608006464 |
| [2] | C.T. Campbell, C.H.F. Peden. Chemistry. Oxygenvacancies and catalysis on ceria surfaces. Science 309 (2005) 713–714. DOI:10.1126/science.1113955 |
| [3] | O. Pozdnyakova-Tellinger, D. Teschner, J. Kroehnert, et al., Surface waterassisted preferential CO oxidation on Pt/CeO2 catalyst. J. Phys. Chem. C 111 (2007) 5426–5431. DOI:10.1021/jp0669862 |
| [4] | T.F. Hou, B. Yu, S.Y. Zhang, et al., Hydrogen production from ethanol steam reforming over Rh/CeO2 catalyst. Catal. Commun. 58 (2015) 137–140. DOI:10.1016/j.catcom.2014.09.020 |
| [5] | N.L. Wieder, M. Cargnello, K. Bakhmutsky, et al., Studyofthewater-gas-shiftreaction on Pd@CeO2/Al2O3 core-shell catalysts. J. Phys. Chem. C 115 (2011) 915–919. DOI:10.1021/jp102965e |
| [6] | J. Kaspar, P. Fornasiero, M. Graziani. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 50 (1999) 285–298. DOI:10.1016/S0920-5861(98)00510-0 |
| [7] | G. Vari, L. Ovari, C. Papp, et al., The interaction of cobalt with CeO2(111) prepared on Cu(111). J. Phys. Chem. C 119 (2015) 9324–9333. DOI:10.1021/acs.jpcc.5b00626 |
| [8] | J.C. Conesa, A. Martinez-Arias, M. Fernandez-Garcia, et al., Surface structure and redox chemistry of ceria-containing automotive catalytic systems. Res. Chem. Intermed. 26 (2000) 103–111. DOI:10.1163/156856700X00138 |
| [9] | S. Royer, D. Duprez. Catalytic oxidation of carbon monoxide over transition metal oxides. Chemcatchem 3 (2011) 24–65. DOI:10.1002/cctc.201000378 |
| [10] | E. Martono, M.P. Hyman, J.M. Vohs. Reaction pathways for ethanol on model Co/ZnO(0001) catalysts. PCCP 13 (2011) 9880–9886. DOI:10.1039/c1cp20132h |
| [11] | M.S. Batista, R.K.S. Santos, E.M. Assaf, et al., Characterization of the activityand stability of supported cobalt catalysts for the steam reforming of ethanol. J. Power Sources 124 (2003) 99–103. DOI:10.1016/S0378-7753(03)00599-8 |
| [12] | H. Song, U.S. Ozkan. The role of impregnation medium on the activity of ceriasupported cobalt catalysts for ethanol steam reforming. J. Mol. Catal. A:Chem. 318 (2010) 21–29. DOI:10.1016/j.molcata.2009.11.003 |
| [13] | B. Bayram, I.I. Soykal, D. von Deak, et al., Ethanol steam reforming over Cobased catalysts:investigation of cobalt coordination environment under reaction conditions. J. Catal. 284 (2011) 77–89. DOI:10.1016/j.jcat.2011.09.001 |
| [14] | K. Shimura, T. Miyazawa, T. Hanaoka, et al., Fischer-Tropschsynthesis over TiO2 supported cobalt catalyst:effect of TiO2 crystal phase and metal ion loading. Appl. Catal. A:Gen. 460 (2013) 8–14. |
| [15] | S.Y. Yu, W.L. Huang, Y. Ma, et al., Characterization of cobalt-based catalyst supported on CeO2 nanocubes for Fischer-Tropsch synthesis. Integr. Ferroelectr. 138 (2012) 32–37. DOI:10.1080/10584587.2012.688425 |
| [16] | J. Wang, M. Shen, J. Wang, et al., CeO2-CoOx mixed oxides:structural characteristics and dynamic storage/release capacity. Catal. Today 175 (2011) 65–71. DOI:10.1016/j.cattod.2011.03.004 |
| [17] | G. Li, Q. Wang, B. Zhao, et al., Modification of Ce0.67Zr0.33O2 mixed oxides by coprecipitated/impregnated Co:effect on the surface and catalytic behavior of Pd only three-way catalyst. J. Mol. Catal. A:Chem. 326 (2010) 69–74. DOI:10.1016/j.molcata.2010.04.008 |
| [18] | L.F. Liotta, G. Di Carlo, G. Pantaleo, et al., Catalytic performance of Co3O4/CeO2 and Co3O4/CeO2-ZrO2 compositeoxides for methane combustion:influence of catalyst pretreatment temperature and oxygen concentration in the reaction mixture. Appl. Catal. B:Environ. 70 (2007) 314–322. DOI:10.1016/j.apcatb.2005.12.023 |
| [19] | Z.Q. Zou, M. Meng, Q. Li, et al., Surfactants-assisted synthesis and characterizations of multicomponent mesoporous materials Co-Ce-Zr-O and Pd/Co-Ce-Zr-O used for low-temperature CO oxidation. Mater. Chem. Phys. 109 (2008) 373–380. DOI:10.1016/j.matchemphys.2007.12.004 |
| [20] | J.Y. Luo, M. Meng, J.S. Yao, et al., One-step synthesis of nanostructured Pddoped mixed oxides MOx-CeO2(M=Mn, Fe, Co, Ni, Cu) for efficient CO and C3H8 total oxidation. Appl. Catal. B:Environ. 87 (2009) 92–103. DOI:10.1016/j.apcatb.2008.08.017 |
| [21] | M.F. Camellone, S. Fabris. Reaction mechanisms for the CO oxidation on Au/CeO2 catalysts:activity of substitutional Au3+/Au+ cations and deactivation of supported Au+ adatoms. J. Am. Chem. Soc. 131 (2009) 10473–10483. DOI:10.1021/ja902109k |
| [22] | M. Baron, O. Bondarchuk, D. Stacchiola, et al., Interaction of gold with cerium oxide supports:CeO2(111) thin films vs CeOx nanoparticles. J. Phys. Chem. C 113 (2009) 6042–6049. DOI:10.1021/jp9001753 |
| [23] | Q. Fu, H. Saltsburg, M. Flytzani-Stephanopoulos. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301 (2003) 935–938. DOI:10.1126/science.1085721 |
| [24] | L. Ilieva, G. Pantaleo, R. Nedyalkova, et al., NO reduction by CO over gold catalysts based on ceria supports, prepared by mechanochemical activation, modified by Me3+(Me=Al orlanthanides):effectof waterin the feed gas. Appl. Catal. B:Environ. 90 (2009) 286–294. DOI:10.1016/j.apcatb.2009.03.021 |
| [25] | R.P. Galhenage, S.C. Ammal, H. Yan, et al., Nucleation, growth, and adsorbateinduced changes in composition for Co-Au bimetallic clusters on TiO2. J. Phys. Chem. C 116 (2012) 24616–24629. DOI:10.1021/jp307888p |
| [26] | T.R. Reina, A.A. Moreno, S. Ivanova, et al., Influence of vanadium or cobalt oxides on the CO oxidation behavior of Au/MOx/CeO2-Al2O3 systems. Chemcatchem 4 (2012) 512–520. DOI:10.1002/cctc.v4.4 |
| [27] | N.K. Gamboa-Rosales, J.L. Ayastuy, Z. Boukha, et al., Ceria-supported Au-CuO and Au-Co3O4 catalysts for CO oxidation:an 18O/16O isotopic exchange study. Appl. Catal. B:Environ. 168-169 (2015) 87–97. DOI:10.1016/j.apcatb.2014.12.020 |
| [28] | H. Wang, H. Zhu, Z. Qin, et al., Deactivation of a Au/CeO2-Co3O4 catalyst during CO preferential oxidation in H2-rich stream. J. Catal. 264 (2009) 154–162. DOI:10.1016/j.jcat.2009.04.003 |
| [29] | N.K. Gamboa-Rosales, J.L. Ayastuy, A. Iglesias-González, et al., Oxygenenhanced WGS over ceria-supported Au-Co3O4 bimetallic catalysts. Chem. Eng. J. 207-208 (2012) 49–56. DOI:10.1016/j.cej.2012.06.142 |
| [30] | L. Ilieva, P. Petrova, T. Tabakova, et al., Gold catalysts on ceria doped with MeOx (Me=Fe, Mn, Co and Sn) for complete benzene oxidation:effect of composition and structure of the mixed supports. React. Kinet. Mech. Catal. 105 (2012) 23–37. DOI:10.1007/s11144-011-0368-2 |
| [31] | L. Ilieva, P. Petrova, T. Tabakova, et al., Relationship between structural properties and activity in complete benzene oxidation over Au/CeO2-CoOx catalysts. Catal. Today 187 (2012) 30–38. DOI:10.1016/j.cattod.2012.03.006 |
| [32] | M. Romeo, K. Bak, J. Elfallah, et al., XPS study of the reduction of cerium dioxide. Surf. Interface Anal. 20 (1993) 508–512. DOI:10.1002/(ISSN)1096-9918 |
| [33] | D.R. Mullins, P.V. Radulovic, S.H. Overbury. Ordered cerium oxide thin films grown on Ru(0001) and Ni(111). Surf. Sci. 429 (1999) 186–198. DOI:10.1016/S0039-6028(99)00369-6 |
| [34] | D.D. Kong, G.D. Wang, Y.H. Pan, et al., Growth, structure, and stability of Ag on CeO2(111):synchrotron radiation photoemission studies. J. Phys. Chem. C 115 (2011) 6715–6725. DOI:10.1021/jp112392y |
| [35] | A.U. Nilekar, Y. Xu, J. Zhang, et al., Bimetallic and ternary alloys for improved oxygen reduction catalysis. Top. Catal. 46 (2007) 276–284. DOI:10.1007/s11244-007-9001-z |
| [36] | S. Banik, S. Barman, S.K. Rai, et al., Electronic structure of buried Co-Cu interface studied with photoemission spectroscopy. J. Appl. Phys. 112 (2012) 5. |
| [37] | L. Óvári, S. Krick Calderon, Y. Lykhach, et al., Near ambient pressure XPS investigation of the interaction of ethanol with Co/CeO2(111). J. Catal. 307 (2013) 132–139. DOI:10.1016/j.jcat.2013.07.015 |
| [38] | M.P. Hyman, J.M. Vohs. Reaction of ethanol on oxidized and metallic cobalt surfaces. Surf. Sci. 605 (2011) 383–389. DOI:10.1016/j.susc.2010.11.005 |
| [39] | E. Martono, J.M. Vohs. Supporteffects in cobalt-based ethanol steamreforming catalysts:reaction of ethanol on Co/CeO2/YSZ(100) model catalysts. J. Catal. 291 (2012) 79–86. DOI:10.1016/j.jcat.2012.04.010 |
| [40] | S.S.Y. Lin, D.H. Kim, M.H. Engelhard, et al., Water-induced formation of cobalt oxides over supported cobalt/ceria-zirconia catalysts under ethanol-steam conditions. J. Catal. 273 (2010) 229–235. DOI:10.1016/j.jcat.2010.05.016 |
| [41] | H.L. Skriver, N.M. Rosengaard. Surface-energy and work fuction of elemental metals. Phys. Rev. B 46 (1992) 7157–7168. DOI:10.1103/PhysRevB.46.7157 |
| [42] | G.L. Zhou, M.H. Yang, C.P. Flynn. Epitaxial growth of metastable Co-Cu alloys by a surface pump mechanism. Phys. Rev. Lett. 77 (1996) 4580–4583. DOI:10.1103/PhysRevLett.77.4580 |
| [43] | Y.H. Zhou, E.W. Peterson, J. Zhou. Growth and structure of Ni-Au bimetallic particles on reducible CeO2(111). Top. Catal. 58 (2015) 134–142. DOI:10.1007/s11244-014-0352-y |
| [44] | R.P. Galhenage, H. Yan, A.S. Ahsen, et al., Understanding the growth and chemical activity of Co-Pt bimetallic clusters on TiO2(110):CO adsorption and methanol reaction. J. Phys. Chem. C 118 (2014) 17773–17786. DOI:10.1021/jp505003s |
| [45] | Q. Xu, S.W. Hu, D.L. Cheng, et al., Growth and electronic structure of Sm on thin Al2O3/Ni3Al(111) films. J. Chem. Phys. 136 (2012) . |
| [46] | W.J. Wang, S.W. Hu, Y. Han, et al., Interaction of Zr with oxidized and partially reduced ceria thin films. Surf. Sci. 653 (2016) 205–210. DOI:10.1016/j.susc.2016.07.007 |
| [47] | S.W. Hu, Y. Wang, W.J. Wang, et al., Ag nanoparticles on reducible CeO2(111) thin films:effect of thickness and stoichiometry of ceria. J. Phys. Chem. C 119 (2015) 3579–3588. DOI:10.1021/jp511691p |
| [48] | I. Horcas, R. Fernandez, J.M. Gomez-Rodriguez, et al., WSXM:A software for scanning probemicroscopyand a toolfor nanotechnology. Rev. Sci. Instrum. 78 (2007) 013705. DOI:10.1063/1.2432410 |
 2017, Vol. 28
2017, Vol. 28 


