b Dafeng Marine Industrial Institute of Nanjing Tech University, Yancheng 224145, China
The 5-substituted uracils and uracil nucleosides are important scaffolds in pharmaceutical and life sciences [1]. In addition to being the core structure of some anticancer and antiviral drugs [2, 3], these nucleosides can be used as building blocks in chemically modified small interfering RNA (si-RNA) [4-6]. Due to the unique properties of the fluorine atom, fluorine has emerged as a "magic element" in medicinal chemistry [7-9]. The introduction of a fluorine atom or a trifluoromethyl (CF3) group into uracils or uracil nucleosides has been extensively studied. For instance, 5-fluorouracil and its derivatives are popular anti-metabolic drugs [10-12]. Trifluridine (TFT) is an antiviral drug (Fig. 1) [13, 14]. However, the incorporation of other fluoroalkylated functional groups into uracils or uracil nucleosides is still very rare [15-18]. Generally, the preparation of perfluoroalkylated uracils is the cross-coupling of 5-bromo or iodo uracils with perfluoroalkyl reagents such as perfluoroalkyl copper/zinc (Scheme 1) [18]. Though those methods are reliable, there are still some intrinstic drawbacks. Firstly, both the cross-coupling partners required additional steps to prepare. Furthermore, the free amino or hydroxyl group on uracils and uracil nucleosides should be protected before used.

|
Download:
|
| Fig. 1. Examples of bioactive fluorinated uracils and uracil nucleosides | |
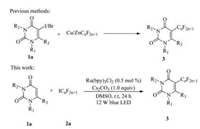
Over the past few years, visible light induced photoredox catalysis has emerged as an efficient and eco-friendly tool in chemistry [19-22]. The addition of fluoroalkyl radicals via a single electron transfer (SET) process by using transition metal catalysis [23-27] or visible-light photoredox catalysis [28-33] have become an efficient strategy for accessing fluoroalkylated compounds. Very recently, we have developed an efficient and general method for the synthesis of difluoroalkylated uracils and uracil nucleosides. This process is promoted by visible light irradiation of a mixture of uracils, BrCF2R and base in the presence of fac-Ir(ppy)3 [34]. To continue our research, we tried to introduce perfluoroalkyl group via this method by using perfluoroalkyl bromide as substrate, but only trace yield was obtained. To resolve this problem, herein we developed an efficient method for the preparation of 5-perfluoroalkylation uracils and uracil nucleosides through visible light induced reaction by using Ru(bpy)3Cl2 as catalyst and perfluoroalkyl iodine as perfluoroalkyl reagent (Scheme 1). The significant advantages of this method are high efficiency, environmentally benign conditions, thus providing a facile route for application of this strategy in medicinal chemistry and life sciences.
|
Download:
|
| Scheme 1. Perfluoroalkylation of uracils and uracil nucleosides | |
2. Results and discussion
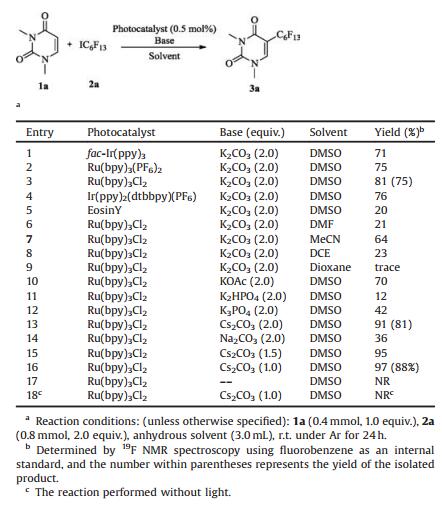
We began this study by choosing 1, 3-dimethyluracil (1a) and perfluorohexyl iodine (2a) as model substrates (Table 1). To our delight, the perfluoroalkylated product was obtained with 71% yield when the reaction was carried out with 1a (1.0 equiv.), 2a (2.0 equiv.), K2CO3 (2.0 equiv.), and fac-Ir(ppy)3 (0.5 mol%) in DMSO at room temperature under blue LED irradiation for 24 h. Various photocatalysts, including Ru(bpy)3(PF6), Ir(ppy)2(dtbbpy)(PF6), Ru (bpy)3Cl2 all work well in this reaction (Table 1, entries 1-4). Considering the effectiveness and price of those catalysts, Ru (bpy)3Cl2 was chosen as the optimum catalyst. Then, a series of reaction media were screened (Table 1, entries 6-9). DMF and MeCN were less efficient compared to DMSO. DCE and Dioxane gives low yield because of low solubility of the catalyst in these solvents. Next, different bases were tested (Table 1, entries 10-14), stronger bases, such Cs2CO3, were more effective and give the product in 91% yield. 97% yield of product was get when 1.0 equiv. of Cs2CO3 was used (Table 1, entry 16). No product was generated in the absence of visible-light or base, which indicates that a photoredox process was occured in the reaction. (Table 1, entries 17 and 18). Finally, the optimal reaction conditions were identified by using Ru(bpy)3Cl2 (0.5 mol%) as catalyst, Cs2CO3 (1.0 equiv) as base and DMSO as solvent, with 88% isolated yield of 3a obtained (Table 1, entry 16).
|
|
Table 1 Representative results for optimization of visible light-mediated reaction of 1a and C6F13I |
With the optimum reaction conditions in hand (Table 1, entries 16), a variety of uracils, uracil nucleosides and perfluoroalkyl iodines were investigated, and the representative results are illustreted in Table 2. Uracil gives the product in 82% yield which indicate the amide have little influence on the reaction efficiency (3b). Other functional groups such as methyl, ester are all compatible with the reaction condition (3c, d), and give the product in moderate yield. Perfluorobutyl iodine was also a sutable reaction partner and good yield canbe obtained (3e). The unprotected hydroxy groups have negative effect on the reaction efficiency, and only moderate yields were get when uridine and deoxyuridine were treated (3f-h) while 2', 3', 5'-tri-O-acetyluridine gives the product in excellent yield (3i).
|
|
Table 2 Direct perfluoroalkylation of uracils and uracil nucleosides a, b |
To demonstrate the utility of this method, the reaction between 2, 4-dimethyl-6-hydroxypyrimidine (4) and C6F13I were also done under the optimum reaction conditions (Scheme 2), and provided the coupling product 5 in 57% yield.

|
Download:
|
| Scheme 2. Direct perfluoroalkylation of 2, 4-dimethyl-6-hydroxypyrimidine (4) with C6F13I | |
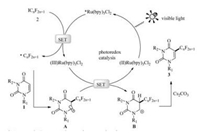
On the basis of previous literatures [24-29], a plausible reaction mechanism was depicted in Scheme 3. Initially, the excitation of Ru (bpy)3Cl2 with visible light produced a strong reductive species (*Ru(bpy)3Cl2) that performs a single electron transfer (SET) process to generate ·CnF2n+1 from ICnF2n+1 (2). Subsequent regioselective addition of ·CnF2n+1 to uracils (1) lead to the radical intermediate (A), which are further oxidized to cation species (B) via a SET process with strong oxidant Ru(Ⅲ)(bpy)3Cl2. Finally, deprotonation of B with Cs2CO3 could afford the desired perfluoroalkylated product (3).
|
Download:
|
| Scheme 3. Proposed reaction mechanism | |
3. Conclusion
In conclusion, we have developed an efficient and general method for the preparation of perfluoroalkylated uracils and uracil nucleosides through a visible light mediated reaction of perfluoroalkyl iodine with uracils and uracil nucleosides. The notable features of this protocol are its low catalyst loading, simple reaction system and excellent functional group compatibility. Further studies to apply those compounds to life sciences and medicinal chemistry are currently underway in our laboratory.
4. Experimental1H NMR, 19F NMR and 13C NMR spectra were recorded on a Agilent AM400 spectrometer. Chemical shifts (δ) are reported in ppm, and coupling constants (J) are in Hertz (Hz). NMR yield was determined by 19F NMR using fluorobenzene as an internal standard before working up the reaction. All reagents were used as received from commercial sources, and handled in air, and then refilled with an inert atmosphere of Ar at room temperature. DMF and DMSO were distilled under reduced pressure from CaH2. 1, 4-Dioxane was distilled from sodium and benzophenone immediately before use.
General procedure for the direct perfluoroalkylation of uracils and uracil nucleosides: To a 25 mL of Schlenk tube equipped with a Teflon septum were added Ru(bpy)3Cl2 (1.5 mg, 0.5 mol%), Cs2CO3 (130 mg, 1.0 equiv) and 1 (0.4 mmol, 1.0 equiv) followed by DMSO (3.0 mL) with stirring. Perfluoroalkyl iodine (2.0 equiv.) was then added subsequently under Ar. The Schlenk tube was screw capped and the reaction was then under irradiated with a 12 W blue LEDs (460 nm-470 nm). After stirring for 24 h, the reaction mixture was diluted with ethyl acetate, washed with brine, the organic phases were collected and dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified with silica gelchromatography to provide pure product. All the spectral data and original spectra are deposited in Supporting information.
AcknowledgmentsThis work was financially supported by the Young Talents Cultivation Program of the China Association for Science and Technology (No. 2015-41), The Training Programme Foundation for the Talents of the Zun Yi Science and Technology Bureau (No. 2015-40), Key Programs of Guizhou Province (125 Program, No. 2015-039), and The Natural Science Foundation of Jiangsu Province (No. BK20141265).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.039.
| [1] | L.A. Agrofoglio, I. Gillaizeau, Y. Saito. Palladium-assisted routes to nucleosides. Chem. Rev. 103 (2003) 1875–1916. DOI:10.1021/cr010374q |
| [2] | E. De Clercq, J. Descamps, P. De Somer, et al., (E)-5-(2-Bromovinyl)-2'-deoxyuridine:a potent and selective anti-herpes agent. Proc. Natl. Acad. Sci. U. S. A. 76 (1979) 2947–2951. DOI:10.1073/pnas.76.6.2947 |
| [3] | C. McGuigan, H. Barucki, S. Blewett, et al., Highly potent and selective inhibition of varicella-zoster virus by bicyclic furopyrimidine nucleosides bearing an aryl side chain. J. Med. Chem. 43 (2000) 4993–4997. DOI:10.1021/jm000210m |
| [4] | J.O. Heidenreich, W. Pieken, F. Eckstein. Chemically modified RNA:approaches and applications. FASEBJournal 7 (1993) 90–96. |
| [5] | J.P.S. Pallan, E.M. Greene, P.A. Jicman, et al., Unexpected origins of the enhanced pairing affinity of 2'-fluoro-modified RNA. Nucleic Acids Res. 39 (2011) 3482–3495. DOI:10.1093/nar/gkq1270 |
| [6] | M. Košutić, L. Jud, C. Da Veiga, et al., Surprising base pairing and structural properties of 2'-trifluoromethylthio-modified ribonucleic acids. J. Am. Chem. Soc. 136 (2014) 6656–6663. DOI:10.1021/ja5005637 |
| [7] | R.D. Chambers. Fluorine in Organic Chemistry, 2th ed., London: Blackwell, 2004 . |
| [8] | P. Kirsh. Modern Fluoroorganic Chemistry, Weinheim: Wiley-VCH, 2004 . |
| [9] | J.T. Welch, S. Eswarakrishnan. Fluorine inbioorganic Chemistry, New York: Wiley, 1991 . |
| [10] | L. Metterle, C. Nelson, N. Patel. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC):A review. J. Am. Acad.of Dermatol. 74 (2015) 552–557. |
| [11] | E. Carrillo, S.A. Navarro, A. Ramirez, et al., 5-Fluorouracil derivatives:a patent review (2012-2014). Expert. Opin. Ther. Pat. 25 (2015) 1131–1144. DOI:10.1517/13543776.2015.1056736 |
| [12] | A. Palasz, D. Ciez. The driving force:digital servo drive doubles accuracy of high-speed cut-to-length machines. Eur. J. Med. Chem. 46 (2015) 582–611. |
| [13] | H.J. Lenz, S. Stintzing, F. Loupakis. TAS-102, a novel antitumor agent:A review of the mechanism of action. Cancer Treat. Rev. 41 (2015) 777–783. DOI:10.1016/j.ctrv.2015.06.001 |
| [14] | E. De Clercq. Selective anti-herpesvirus agents. Antivir. Chem. Chemoth. 23 (2013) 93–101. DOI:10.3851/IMP2533 |
| [15] | G. Caillot, J. Dufour, M.C. Belhomme, et al., Copper-catalyzed olefinic C-H difluoroacetylation of enamides. Chem. Commun. 50 (2014) 5887–5890. DOI:10.1039/C4CC01994F |
| [16] | F. Sladojevich, E. McNeill, J. Boergel, S.L. Zheng, T. Ritter. Condensed-phase, halogen-bonded CF3I and C2F5I adducts for perfluoroalkylation reactions. Angew. Chem. Int. Ed. 54 (2015) 3712–3716. DOI:10.1002/anie.201410954 |
| [17] | M. Ivanova, A. Bayle, T. Besset, T. Poisson, X. Pannecoucke. Copper saltcontrolled divergent reactivity of. Angew. Chem. Int. Ed. 55 (2016) 14141–14145. DOI:10.1002/anie.v55.45 |
| [18] | K. Aikawa, Y. Nakamura, Y. Yokota, W. Toya, K. Mikami. Stable but reactive perfluoroalkylzinc reagents:application in ligand-free copper-catalyzed perfluoroalkylation of aryl iodides. Chem. Eur. J. 21 (2015) 96–100. DOI:10.1002/chem.201405677 |
| [19] | Y. Zhao, B. Zhao, J. Liu, et al., Oxide-modified nickel photocatalysts for the production of hydrocarbons in visible light. Angew. Chem. Int. Ed 55 (2016) 4215–4219. DOI:10.1002/anie.201511334 |
| [20] | H. Yu, R. Shi, Y. Zhao, et al., Smart utilization of carbon dots in semiconductor photocatalysis. Adv. Mater. 28 (2016) 9454–9477. DOI:10.1002/adma.201602581 |
| [21] | Y. Zhao, X. Jia, G.I.N. Waterhouse, et al., Layered double hydroxide nanostructured photocatalysts for renewable energy production. Adv. Energy. Mater. 6 (2016) . DOI:10.1002/aenm.201501974 |
| [22] | Y. Zhao, G. Chen, T. Bian, et al., Defect-rich ultrathinznal-layered double hydroxide nanosheets for efficient photoreduction of CO2 to CO with water. Adv. Mater. 27 (2015) 7824–7831. DOI:10.1002/adma.201503730 |
| [23] | Q.Q. Min, Z. Yin, Z. Feng, W.H. Guo, X. Zhang. Highly selective gemdifluoroallylation of organoborons with bromodifluoromethylated alkenes catalyzed by palladium. J. Am. Chem. Soc. 136 (2014) 1230–1233. DOI:10.1021/ja4114825 |
| [24] | Z. Feng, Q.Q. Min, H.Y. Zhao, J.W. Gu, X. Zhang. A general synthesis of fluoroalkylated alkenes by palladium-catalyzed heck-type reaction of fluoroalkyl bromides. Angew. Chem. Int. Ed. 54 (2015) 1270–1274. DOI:10.1002/anie.201409617 |
| [25] | S. Ge, W. Chaladaj, J.F. Hartwig. Pd-Catalyzed(-arylation of (, (-difluoroketones with aryl bromides and chlorides A route to difluoromethylarenes. J. Am. Chem. Soc. 136 (2014) 4149–4152. DOI:10.1021/ja501117v |
| [26] | C. Guo, R.W. Wang, F.L. Qing. Palladium catalyzed direct(-arylation of (, (-difluoroketones with aryl bromides. J. Fluorine Chem. 44 (2013) 135–142. |
| [27] | Z. Feng, F. Chen, X. Zhang. Copper catalyzed cross-coupling of iodobenzoates with bromozincdifluorophosphonate. Org. Lett. 14 (2012) 1938–1941. DOI:10.1021/ol3006425 |
| [28] | T. Chatterjee, N. Iqbal, Y. You, E.J. Cho. Controlled fluoroalkylation reactions by visible-light photoredox catalysis. Acc. Chem. Res. 49 (2016) 2284–2294. DOI:10.1021/acs.accounts.6b00248 |
| [29] | X.J. Tang, W.R. Dolbier. Efficient Cu-catalyzed atom transfer radical addition (ATRA) reactions of fluoroalkylsulfonyl chlorides with electron-deficient alkenes induced by visible light. Angew. Chem. Int. Ed. 54 (2015) 4246–4249. DOI:10.1002/anie.201412199 |
| [30] | W. Li, X. Zhu, H. Mao, et al., Visible-light-induced direct C(sp3)-H difluromethylation of tetrahydroisoquinolines with the in situ generated difluoroenolates. Chem. Commun. 50 (2014) 7521–7523. DOI:10.1039/C4CC02768J |
| [31] | Y.M. Su, Y. Hou, F. Yin, et al., VisibleLight-Mediated C-H difluoromethylation of electron-rich heteroarenes. Org. Lett. 16 (2014) 2958–2961. DOI:10.1021/ol501094z |
| [32] | Q. Lin, L. Chu, F. Qing. Direct introduction of ethoxycarbonyldifluoromethyl-Group to heteroarenes with ethyl bromodifluoroacetate via visible-Light photocatalysis. Chin. J. Chem. 45 (2013) 885–891. |
| [33] | J.D. Nguyen, J.W. Tucker, M.D. Konieczynska, C.R.J. Stephenson. Intermolecular Atom transfer radical addition to olefins mediated by oxidative quenching of photoredox catalysts. J. Am. Chem. Soc. 133 (2011) 4160–4163. DOI:10.1021/ja108560e |
| [34] | C.Y. He, J. Kong, X. Li, et al., Visible-Light-Induced direct difluoroalkylation of uracils, pyridinones and coumarins. J. Org. Chem. 82 (2017) 910–917. DOI:10.1021/acs.joc.6b02316 |
 2017, Vol. 28
2017, Vol. 28