b Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China
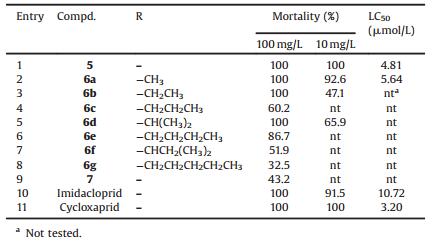
Neonicotinoid insecticides are the newest class of synthetic insecticides in the past two decades and the biggest selling insecticide class worldwide through compounds such as imidacloprid (1) and thiamethoxam (2) (Fig. 1) [1-3], replacing the organophosphates, carbamates, pyrithroids and other classes of compounds. There are several reasons for their superiority over the aforementioned traditional insecticides. Namely: lower mammalian toxicity, broad insecticidal spectrum, and good systemic properties [4, 5]. They are now registered globally in more than 120 countries and extensively used in seed treatment and soil treatment, or directly applied to plant foliage for crop protection [6].
|
Download:
|
| Fig. 1. Chemical structures of imidacloprid and thiamethoxam. | |
The target site for all neonicotinoids is the nicotinic acetylcholine receptor (nAChR), a ligand gated ion channel responsible for rapid communication at cholinergic synapses. However, that neonicotinoids act as agonists of insect nAChRs [7] has significant increases in resistance and cross-resistance observed in various insect species after frequent field applications in recent years [8-11]. There has been a renewed interest in searching for novel neonicotinoid insecticides to overcome resistance while maintaining attractive physical properties and biological profiles [12-15].
In the search for novel neonicotinoid insecticides, sulfoxaflor (3) was described by Dow AgroSciences [16-19]. It has been reported that this compound lack cross-resistance on insect pests that have developed resistance to one or more classes of insecticides, including imidacloprid and other neonicotinoids. Cycloxaprid (4), one of the most effective neonicotinoid insecticides, is currently under development for agricultural pest control and contain oxabridged configuration [20]. It is especially effective against imidacloprid-resistant pests and appears to activate a different site compared to other neonicotinoid insecticides on nAChRs [21]. This novel oxabridged skeleton structure caused our extensive interest and attention, and we presumed that bridged ring was an important factor for their biological activities.
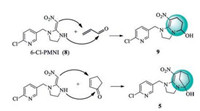
In our previous work [22], we synthesized compound 9 via Michael addition and nucleophilic attack of an NH group on a carbonyl carbon from 6-Cl-PMNI ((2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl) methyl)pyridine, 8) and acrylaldehyde (Scheme 1). If acrylaldehyde was replaced by cyclopentenone, bridged compound 5 was obtained by the same reaction. Thus, in this letter, a series of novel bridged compounds were designed and synthesized. Their insecticidal activity was tested against Aphis craccivora.
|
Download:
|
| Scheme 1. Synthetic strategy of target compound 5. | |
2. Results and discussion 2.1. Synthesis
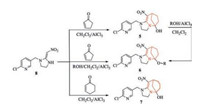
As shown in Scheme 2, compound 5 and 7 may be obtained from 6-Cl-PMNI with cyclopentenone and cyclohexenone, respectively, by Michael addition and cyclization reaction according to reported methods [22], but the yield of them was not high (50-60%) owing to uncompleted reaction. To optimize this reaction, several kinds of catalysts, such as HCl, CH3COOH, AlCl3, FeCl3 and BF3 were studied; and the solvents of this reaction such as MeCN, THF, alcohols and dichloromethane were also optimized; The result showed that AlCl3 and dichloromethane were suitable to carry out the reaction over 80% yield. Compared with compound 5, eight-membered bridged compound 7 has higher yield and reaction rate owing to weaker ring tension.
|
Download:
|
| Scheme 2. The synthetic route for the preparation of 5–7. | |
Compound 6 could be prepared by the etherification reaction of compound 5 (started from 6-Cl-PMNI) with alkyl alcohol in the presence of AlCl3. However, to increase the yield and simplify the process, we developed one-pot multicomponent reaction. The search for one-pot condensation reaction parameters started with 6-Cl-PMNI, cyclopentenone, and alkyl alcohol in molar ratios from 1:1:5 to 1:1:6 in boiling dichloromethane from 2 h to 8 h, desirable product could be obtained in 70%-80% yield. The structures of the title compounds were well characterized by 1H NMR, 13C NMR, HRMS and IR.
2.2. Insecticidal activitiesCompounds 5, 6a, 6b, 6d exhibited good insecticidal activities against Aphis craccivora (Table 1) and had 100% mortality at 100 mg/L, especially compounds 5 and 6a showed >90% mortality at 10 mg/L. The LC50 values of compounds 5 and 6a were 4.81 and 5.64 mmol/L, respectively, which were lower than that of imidacloprid (10.72 mmol/L) and slightly higher than cycloxaprid (3.20 mmol/L). We concluded that compounds 5, 6a and cycloxaprid showed higher potency compared with imidacloprid. It indicated that the oxygen atom of cycloxaprid did not play an important role, and bridged skeleton was the key factor for the high activity of these compounds. For R group, modifying the substitution with longer alkyl group would lead to decrease for biological activity. Unexpectedly, compound 7 constructed by cyclohexenone had moderate activity against Aphis craccivora. The difference of ring size and conformation between 5 and 7 could possibly account in part for the higher potency of 5 than that of 7. Further study is underway.
|
|
Table 1 Insecticidal activity results of the synthetic compounds 5-7 against Aphis craccivora. |
3. Conclusion
In summary, a series of bridged neonicotinoid analogues were designed and synthesized. All of the target compounds were obtained in optimized reaction conditions. The seven-membered bridged compounds 5 and 6a exhibited excellent insecticidal activities against Aphis craccivora. On the basis of the LC50 values, compounds 5 and 6a showed higher bioactivity than imidacloprid, which indicated that the design of novel bridged skeleton was an effective strategy to discover new candidates as pesticides.
4. Experimental 4.1. Materials and methodsAll starting materials and reagents were commercially available and used without further purification otherwise stated. Melting points were obtained with a digital melting point WRS-1 B (Shen Guang Instrument Co., Ltd. Shanghai, China) and were uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker Avaace Ⅲ (400 MHz) spectrometer with CDCl3 as the solvent and TMS as the internal standard. Chemical shifts were reported in δ. Highresolution mass spectra were recorded under electron impact (70 eV) condition using a MicroMass GCT CA 055 instrument. IR spectra were recorded on a PE spectrum one with dried KBr as the adjuvant. Analytical thin-layer chromatography (TLC) was carried out on precoated plates (silica gel GF254), and spots were visualized with ultraviolet (UV) light.
4.2. General procedure for synthesis of compounds 5 and 7To a mixture of 8 (1.0 g, 4 mmol) and the corresponding cyclopentenone or cyclohexenone (4.2 mmol) in CH2Cl2 (30 mL), a catalytic amount of AlCl3 was added. Then the above mixture was refluxed. When the reaction was complete according to TLC analysis, the mixture was concentrated in vacuo, and water (20 mL) was added to the residue, which was extracted with DCM (3 × 40 mL). The combined organic phase was dried over anhydrous Na2SO4, concentrated under reduced pressure. The residue was subjected to flash chromatography on silica gel, eluting with dichloromethane/methanol to afford pure products, for example, 1-((6-chloropyridin-3-yl)methyl)-9-nitro-2, 3, 5, 6, 7, 8-hexahydro-1H-5, 8-methanoimidazo[1, 2-a]azepin-5-ol (5): Yield, 81%; mp, 141-143 ℃; 1H NMR (CDCl3, 400 MHz): δ 8.34 (d, 1H, J1 = 2.0 Hz, PyH), 7.82 (dd, 1H, J1 = 2.4 Hz, J2 = 8.0 Hz, Py-H), 7.39 (d, 1H, J = 8.4 Hz, Py-H), 5.29 (s, 1H), 5.12 (d, 1H, J = 15.2 Hz, Py-CH2), 4.52 (d, 1H, J = 15.2 Hz, Py-CH2), 3.91-3.94 (m, 1H, CH), 3.22 ~ 3.89 (m, 4H, NCH2-CH2-N), 1.8 ~ 3.1 (m, 6H); 13C NMR (CDCl3, 100 MHz): δ 159.1, 149.7, 147.1, 138.5, 130.3, 124.6, 109.5, 96.4, 68.9, 52.4, 48.6, 41.3, 38.6, 35.6, 32.1; IR (KBr, cm-1) 3453, 2987, 2874, 1567, 1388, 1256, 1211 1093, 968; HRMS (ES+) Anal. Calcd. For C15H18N4O335Cl (M + H)+, 337.1067; Found: 337.1059.
4.3. General procedure for synthesis of compounds 6a-6gTo a mixture of 8 (1.0 g, 4 mmol), cyclopentenone (4.2 mmol), and the corresponding alkyl alcohol (20 mmol) in CH2Cl2 (30 mL), a catalytic amount of AlCl3 was added. Then the above mixture was refluxed. When the reaction was complete according to TLC analysis, the mixture was concentrated in vacuo, and water (20 mL) was added to the residue, which was extracted with DCM (3 × 40 mL). The combined organic phase was dried over anhydrous Na2SO4, concentrated under reduced pressure. The residue was subjected to flash chromatography on silica gel, eluting with dichloromethane/acetone to afford pure products, for example, 1-((6-chloropyridin-3-yl)methyl)-5-methoxy-9-nitro-2, 3, 5, 6, 7, 8-hexahydro-1H-5, 8-methanoimidazo[1, 2-a]azepine (6a): Yield, 78%; mp, 112-113 ℃; 1H NMR (CDCl3, 400 MHz): δ 8.31 (d, 1H, J1 = 2.4 Hz, Py-H), 7.84 (dd, 1H, J1 = 2.3 Hz, J2 = 8.3 Hz, Py-H), 7.36 (d, 1H, J = 8.0 Hz, Py-H), 5.19 (d, 1H, J = 15.2 Hz, Py-CH2), 4.49 (d, 1H, J = 15.2 Hz, Py-CH2), 3.86-3.90 (m, 1H, CH-), 3.16-3.31 (m, 4H, NCH2-CH2-N), 3.21 (s, 3H, -OCH3), 1.76-2.15 (m, 6H); 13C NMR (CDCl3, 100 MHz): δ 159.3, 151.2, 149.1, 139.4, 131.1, 124.5, 111.9, 97.8, 53.3, 68.1, 52.5, 49.5, 42.1, 39.4, 36.8, 36.5; IR (KBr, cm-1) 2939, 1559, 1462, 1314, 1293, 1099, 915, 826; HRMS(ES+) Anal. Calcd. For C16H20N4O335Cl (M+H)+, 351.1224; Found: 351.1221.
The characterization data of product 7 and derivatives (6b-6g) were summarized in the Supporting information.
4.4. Insecticidal activity testingThe insecticidal activity of all compounds against Aphis craccivora was tested by the leaf-dipping method. All bioassays were performed on representative test organisms reared in the laboratory. All compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted with distilled water containing Triton X-100 (0.1 mg/L) to obtain series concentrations of 100, 10, 5, 1 mg/L and others for bioassays. For comparative purposes, imidacloprid and cycloxaprid were tested under the same conditions. Horsebean plant leaves with 40-60 apterous adults were dipped in diluted solutions of the chemicals containing Triton X-100 (0.1 mg/L) for 5 s, and the excess dilution was sucked out with filter paper; the burgeons were placed in the conditioned room (25 ± 1 ℃, 50% RH). Water containing Triton X-100 (0.1 mg/L) was used as control. The mortality rates were evaluated 24 h after treatment. Each treatment had three repetitions, and the data were adjusted and subjected to probit analysis.
AcknowledgmentsThis work was financial supported by Shandong Provincial Natural Science Foundation (No. ZR2015BL009), The Key Research and Development Program of Shandong Provincial (No. 2016GGX107006), National Natural Science Foundation of China (No. 21501066), and the Opening Project of Shanghai Key Laboratory of Chemical Biology.
| [1] | A. Guan, C.L. Liu, X.P. Yang, M. Dekeyser. Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev. 114 (2014) 7079–7107. DOI:10.1021/cr4005605 |
| [2] | J. Li, X.L. Ju, F.C. Jiang. Pharmacophore model for neonicotinoid insecticides. Chin. Chem. Lett. 19 (2008) 619–622. DOI:10.1016/j.cclet.2008.03.011 |
| [3] | H.F. Shen, X. Chen, P. Liao, et al., Design synthesis, and insecticidal bioactivities evaluation of pyrrole-and dihydropyrrole-fused neonicotinoid analogs containing chlorothiazole ring. Chin. Chem. Lett. 26 (2015) 509–512. DOI:10.1016/j.cclet.2015.03.017 |
| [4] | M. Tomizawa, J.E. Casida. Molecular recognition of neoniconoid insecticide:the determinants of life or death. Acc. Chem. Res. 42 (2009) 260–269. DOI:10.1021/ar800131p |
| [5] | X.S. Shao, S.S. Xia, K.A. Durkin, J.E. Casida. Insect nicotinic receptor interactions in vivo with neonicotinoid, organophosphorus and methylcarbamate insecticides and a synergist. Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 17273–17277. DOI:10.1073/pnas.1316369110 |
| [6] | P. Jeschke, R. Nauen, M. Schindler, A. Elbert. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59 (2011) 2897–2908. DOI:10.1021/jf101303g |
| [7] | P. Jeschke, R. Nauen, M.E. Beck. Nicotinic acetylcholine receptor agonists:a milestone for modern crop protection. Angew. Chem. Int. Ed. 52 (2013) 9464–9485. DOI:10.1002/anie.v52.36 |
| [8] | A. Elbert, R. Nauen. Resistance of bemisia tabaci (Homoptera:aleyrodidae) to insecticides in southern Spain with special reference to neonicotinoids. Pest. Manage. Sci. 56 (2000) 60–64. DOI:10.1002/(ISSN)1526-4998 |
| [9] | N. Rauch, R. Nauen. Identification of biochemical markers linked to neonicotinoid cross-resistance in bemisia tabaci (Hemiptera:Aleyrodidae). Arch. Insect. Biochem. Physiol. 54 (2003) 165–176. DOI:10.1002/(ISSN)1520-6327 |
| [10] | R. Nauen, I. Denholm. Resistance of insect pests to neonicotinoid insecticides:current status and future prospects. Arch. Insect Biochem. Physiol. 58 (2005) 200–215. DOI:10.1002/(ISSN)1520-6327 |
| [11] | S. Sanada, - Morimura, S. Sakumoto, R. Ohtsu, et al., Current status of insecticide resistance in the small brown planthopper Laodelphax striatellus, in Japan Taiwan, and Viatnam. Appl. Entomol. Zool. 46 (2011) 65–73. DOI:10.1007/s13355-010-0009-7 |
| [12] | Y. Zhu, M.R. Loso, G.B. Watson, et al., Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 59 (2011) 2950–2957. DOI:10.1021/jf102765x |
| [13] | Y. Zhu, R. B. Rogers, Insecticidal N-substituted sulfoximines, WO Patent 2006060029, (2006). |
| [14] | P. Jeschke, R. , Velten, T. Schenke, et al. Substituted enaminocarbonyl compounds, WO Patent 2007115643, (2007). |
| [15] | W.M. Zhang, J.D. Barry, D. Cordova, et al., Discovery synthesis, and evaluation of N-substituted amino-2(5H)-oxazolones as novel insecticides activating nicotinic acetylcholine receptors. Bioorg. Med. Chem. Lett. 24 (2014) 2188–2192. DOI:10.1016/j.bmcl.2014.03.037 |
| [16] | H.B. Yu, Z.F. Qin, H. Dai, et al., Synthesis and insecticidal activity of Nsubstituted (1, 3-thiazole) alkyl sulfoximine derivatives. J. Agric. Food Chem. 56 (2008) 11356–11360. DOI:10.1021/jf802802g |
| [17] | M. R. Loso, B. M. Nugent, J. X. Huang, et al. Preparation of insecticidal Nsubstituted (6-haloalkylpyridin-3-yl)alkyl sulfoximines, WO Patent 2007095229, (2007). |
| [18] | M. R. Loso, B. M. Nugent, J. X. Huang, Insecticidal N-substituted (Heteroaryl) cycloalkyl sulfoximines, WO Patent 2008027073, (2008). |
| [19] | Y. M. Zhu, M. R. Loso, B. M. Nugent, J. X. Huang, R. B. Rogers, Multi-substituted pyridyl sulfoximines and their use as insecticides, WO Patent 2008057129, (2008). |
| [20] | X.S. Shao, H. Fu, X.Y. Xu, et al., Divalent and oxabridged neonicotinoids constructed by dialdehydes and nitromethylene analogues of imidacloprid:Design, synthesis, crystal structure, and insecticidal activities. J. Agric. Food. Chem. 58 (2010) 2696–2702. DOI:10.1021/jf902531y |
| [21] | X.S. Shao, Z.J. Ye, H.B. Bao, et al., Advanced research on cis-neonicotinoids. CHIMIA 65 (2011) 957–960. DOI:10.2533/chimia.2011.957 |
| [22] | Z.Z. Tian, X.S. Shao, Z. Li, X.H. Qian, Q.C. Huang. Synthesis:insecticidal activity and QSAR of novel nitromethylene neonicotinoids with tetrahydro-pyridine fixed cis configuration and exo-ring ether modification. J. Agric. Food Chem. 55 (2007) 2288–2292. DOI:10.1021/jf063418a |
 2017, Vol. 28
2017, Vol. 28