b College of Chemistry, Sichuan University, Chengdu 610064, China
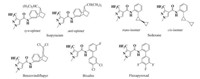
Plant diseases caused by fungal pathogens represent a worldwide issue and often lead to detrimental agricultural crop losses [1]. As one of the most important categories of agrochemicals, CF2H substituted pyrazole carboxamide fungicides have been intensively employed throughout the world to fight against highly destructive plant pathogens, such as Botrytis cinerea, Sclerotinia spp., Leveillula taurica and Spherotheca macularis [2]. From the first CF2H substituted pyrazole carboxamide fungicide isopyrazam (Syngenta) for crop protection to those newly discovered, these molecules cover sedaxane (Syngenta), benzovindiflupyr (Syngenta), bixafen (Bayer) and fluxapyroxad (BASF) (Fig. 1) [3]. The initial narrow biological spectrum was broadened with progress of the chemical structure modification [4]. The CF2H substituted pyrazole carboxamide groups of these fungicides have a common target receptor, succinate dehydrogenase (SDH, EC 1.3.5.1) [5]. They act as SDH inhibitors (SDHIs) and disrupt the mitochondrial tricarboxylic acid cycle and respiration chain [6].
|
Download:
|
| Fig. 1. Structures of some CF2H substituted pyrazole carboxamide fungicides. | |
Diarylamine represents an important structural motif for many bioactive compounds used in the agrochemical field over the years [7]. Their derivatives feature significant biological activities, including fungicidal, insecticidal, acaricidal, rodenticidal and herbicidal activities [8-12]. Therefore, diarylamine may represent a promising bioactive motif to integrate with other pharmacophores. Recently our research group discovered when the phenyl group in fenfuram (a commercial fungicide) was replaced with the diarylamine, the novel fenfuram-diarylamine hybrid exhibited better antifungal activities than fenfuram [13]. Yang et al. also discovered if the biphenyl group in bixafen was replaced with the diphenyl ether group, the novel CF2H substituted pyrazole carboxamide fungicides showed good antifungal activities [14].
Inspired by these reports, herein to extend the research on the development of novel amide derivatives as fungicides [13, 15-20], bixafen was applied as a lead molecule and the diarylamines were introduced in order to replace the biphenyl group in bixafen based on the principle of "splicing-up" bioactive substructures [19, 20]. A series of novel pyrazole carboxamides with diarylamine-modified scaffold were designed and synthesized (Fig. 2). Subsequently, in vitro and in vivo bioassays were performed to evaluate the fungicidal activities against the selected five phytopathogenic fungi Rhizoctonia solani (R. solani), Rhizoctonia cerealis (R. cerealis), Sclerotinia sclerotiorum (S. sclerotiorum), Rhizoctonia solani Kuha (R. solani Kuha) and Fusarium oxysporum vasinfectum (F. oxysporum vasinfectum). Furthermore, the principles of homology modeling and molecular docking methods were used in order to verify the potential mechanism of the target compounds.

|
Download:
|
| Fig. 2. The strategy for design of pyrazole carboxamide with diarylamine-modified scaffold as new antifungal agents. | |
2. Results and discussion 2.1. Chemical synthesis
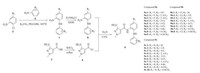
Schemes 1 and 2 detail the synthesis and chemical structures of the title compounds. Initially, the diphenylamine derivatives 7 were obtained and were transformed into the other corresponding amino-substituted diphenylamine derivatives (i.e. compounds 8) through reduction reactions [21]. Meanwhile, the important intermediate compound 4 was prepared via chlorination of the key pyrazole acid 3 (Scheme 1) [22]. Finally, the acyl chloride derivative 4 was reacted with the primary amines 8 through classical approach to afford a series of novel pyrazole carboxamide fungicides [23-25], namely compounds 9a, 9b and 9c.

|
Download:
|
| Scheme 1. Synthesis of intermediate compound 3. | |
|
Download:
|
| Scheme 2. Synthesis of the target compounds 9a, 9b and 9c. | |
The structures of the synthetic compounds were confirmed by 1H NMR, 13C NMR, IR and ESI-HRMS spectroscopic data. All of these compounds provided satisfactory spectroscopic data, which were in full accordance with their corresponding structural parameters. It should be noted in this context that, tothe bestof ourknowledge, all of the title products are first reported. Yields, physical and spectroscopic data of title compounds are given in the Supplementary data.
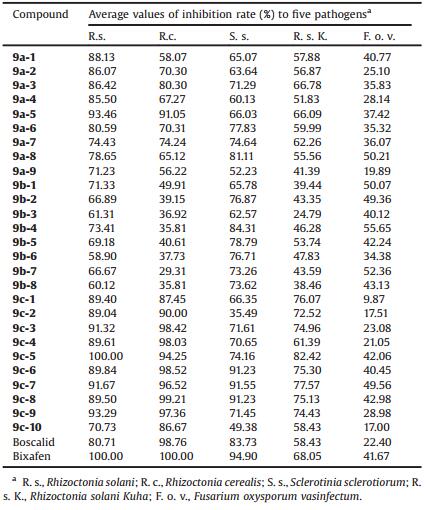
2.2. Fungicidal activities in vitroPreliminary in vitro screening results of the title compounds for their antifungal activities against five phytopathogenic fungi are listed in Table 1. The results obtained indicated that the fungicidal activities of the tested compounds against R. solani, R. cerealis and S. sclerotiorum proved to be superior to R. solani Kuha and F. oxysporum vasinfectum at concentrations of 20 mg/mL. As a consequence, bioactivities of the tested compounds were focus on the antifungal activities against the three fungi R. solani, R. cerealis and S. sclerotiorum.
|
|
Table 1 Preliminary antifungal activities of compounds at 20 mg/mL in vitro. |
In terms of the bioactivities against R. solani, the class of compounds 9c exhibited the highest activities with antifungal rates ranging between 89.04 and 100.00%, except for 9c-10 (70.73%). Meanwhile, the fungicidal activities of the series of compounds 9b were inferior to the series of compounds 9a at concentration of 20 mg/mL. In general, the antifungal activity results against R. solani followed the order: 9c> 9a> 9b. Furthermore, the fungicidal tendency of the three classes of title compounds against R. cerealis were similar to R. solani. Particularly, the class of compounds 9c showed remarkably high activities against R. cerealis at concentration of 20 mg/mL. The results revealed that the substituted group R1 was hydrogen atom in phenyl ring could be critical for enhancing the fungicidal potency of the target compounds. Furthermore, when the substituted group R1 was the fluorine atom in phenyl ring, the target compounds seemed to be more beneficial to fungicidal activity than the target compounds whose substitute group R1 was chlorine atom in phenyl ring. It could be supposed that the bioactivity of tested compound was related to the atomic radius of the substituted group R1 in phenyl ring.
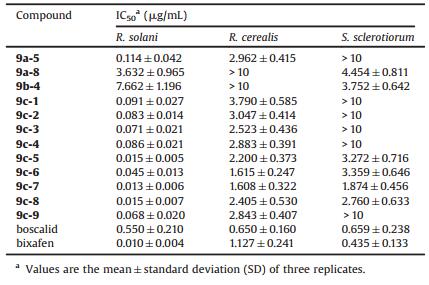
Encouraged by these preliminary findings, further studies on the antifungal activities of the selected compounds were carried out. Avarietyof IC50 (median inhibitory concentration)values were obtained by using the mycelial growth inhibitory rate method [26, 27]. As shown in Table 2, each of compounds presented different fungicidal activities against R. solani, R. cerealis and S. sclerotiorum. All of the tested compounds presented higher fungicidal activities against R. solani than the widely used SDHI boscalid (IC50=0.550 mg/mL), except for compounds 9a-8 and 9b-4, whereas they were less active than the other positive control bixafen (IC50=0.010 mg/mL). Notably, compounds 9c-5, 9c-6, 9c-7 and 9c-8 displayed the closest activities against R. solani to the positive control bixafen, with IC50 values of 0.015, 0.045, 0.013 and 0.015 mg/mL, respectively. This finding suggested when substituted group R2 was chlorine atom or bromine atom in side phenyl ring was beneficial to fungicidal activity. For example, with different substituents at the 4-position of the phenyl ring, compounds 9c-3 (IC50 = 0.071 mg/mL), 9c-6 (IC50 = 0.045 mg/mL) and 9c-8 (IC50 = 0.015 mg/mL) exhibited increased activities against R. solani. Furthermore, compounds 9c-7 and 9c-6 presented the highest antifungal activities against R. cerealis with IC50 values of 1.608 and 1.615 mg/mL, respectively, although they were a little less active than boscalid (IC50 = 0.650 mg/mL) and bixafen (IC50 = 1.127 mg/ mL). In the case of the bioactivities against S. sclerotiorum, compound 9c-7 still displayed the highest activity with an IC50 value of 1.874 mg/mL. Among all of the title compounds, 9c-7 was found to be the most promising leading structure for further optimization with the highest activities against all of the three fungi, R. solani, R. cerealis and S. sclerotiorum.
|
|
Table 2 IC50 values against R. solani, R. cerealis and S. sclerotiorum in vitro. |
2.3. Fungicidal activities in vitro
Considering the high in vitro antifungal activities against R. solani of compounds 9c-6, 9c-7 and 9c-8, they were further tested in vivo. As shown in Table 3, although the three tested compounds were less active than reference drug bixafen (IC50 = 9.24mg/mL), they still exhibited high in vivo antifungal activities with IC50 values of 38.07, 22.21 and 23.13 mg/mL, respectively. The fungicidal tendency of them is consistent both in vitro and in vivo: 9c-7 > 9c-8 > 9c-6. The results suggested that these molecules could potentially control the disease caused by R. solani and field tests are being planned.
|
|
Table 3 IC50 (mg/mL) values against R. solani in vivo. |
2.4. Binding mode analysis
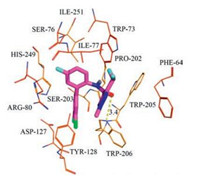
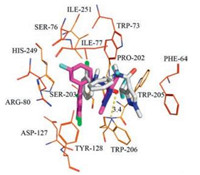
In an effort to explain the possible fungicidal mechanism of these pyrazole carboxamide containing diphenylamine moieties, molecular docking of compound 9c-7 into the homology model was performed and bixafen was docked as contrast at the same time. The theoretical binding modes of compound 9c-7 and the positive control bixafen to SDH are shown in Figs. 3-5 and all results were obtained using PyMoL 1.7.6. Compound 9c-7 was found to fit in the cavity composed of subunits B, C and D (Fig. 3). The 2, 4-dichlorophenyl group of 9c-7 bounded at the hydrophobic pocket, surrounded by residues B/Pro-202, B/Ile-251, C/Ile-77 and C/Trp-73, while the substituted pyrazole group of 9c-7 located at another hydrophobic pocket, surrounded by residues B/Trp-205, B/Trp-206, C/Phe-64 and C/Trp-73. Detailed analysis showed that two π-π interactions could be observed between 9c-7 and SDH model. The 2, 4-dichlorophenyl group of 9c-7 interacted with the side chain of residue C/Trp-73 through π-π interaction, and the interaction also existed between the substituted pyrazole group of 9c-7 and the side chain of residue C/Phe-64 at the same time. Furthermore, bixafen also fitted in the cavity composed of subunits B, C and D (Fig. 4). Further analysis results suggested that the oxygen atom in the amido bond of bixafen formed a hydrogen bond interaction with B/Trp-206 and a Cl-π interaction was formed between dichlorophenyl ring of bixafen and D/Tyr-128. The estimated binding energy between ligand 9c-7 and target protein SDH was determined as -8.0 kcal/mol, which proved to be very close to the binding energy (-8.1 kcal/mol) of bixafen and SDH. This finding could provide a suitable explanation for the high antifungal activity of compound 9c-7.

|
Download:
|
| Fig. 3. The theoretical binding mode between compound 9c-7 and SDH. | |
|
Download:
|
| Fig. 4. The theoretical binding mode between bixafen and SDH. | |
|
Download:
|
| Fig. 5. The theoretical binding mode of 9c-7 and bixafen to SDH. | |
It is believed that differences in the antifungal activity among SDHI fungicides may result from differences in their binding affinity or interactions with amino acid residues in the succinate dehydrogenase subunits involved in the inhibitor binding sites [26]. The above molecular simulations provided virtual binding energies and interactions between two inhibitors 9c-7, bixafen and receptor SDH. Efforts for the formation of a co-crystal of compound 9c-7 with SDH are currently carried out in our laboratory in order to obtain more reliable information of binding mode between 9c-7 and SDH.
3. ConclusionIn summary, a series of pyrazole carboxamides with diarylamine-modified scaffold were designed, prepared and evaluated for their fungicidal activities against five phytopathogenic fungi (R. solani, R. cerealis, S. sclerotiorum, R. solani Kuha and F. oxysporum vasinfectum). Compounds 9c-5, 9c-6, 9c-7 and 9c-8 displayed substantially strong antifungal activities against R. solani in vitro with IC50 values of 0.015, 0.045, 0.013 and 0.015 mg/mL, respectively. Specifically, compound 9c-7 presented the highest activities against all of the three fungi species (i.e. R. solani, R. cerealis and S. sclerotiorum) in vitro with IC50 values of 0.013, 1.608 and 1.874 mg/mL, respectively. And 9c-7 also exhibited the most promising antifungal activity against R. solani in vivo with an IC50 value of 22.21 mg/mL. This suggests that compound 9C-7 could act as a potential fungicide to be used for further optimization.
4. Experimental 4.1. Chemicals and instrumentsAll chemicals and reagents were purchased from commercial sources and were used as received. All reactions were monitored by thin layer chromatography (TLC) using pre-coated silica gel GF254 plates. Column chromatography purification was performed on silica gel (200-300 mesh, Qingdao Marine Chemical Ltd., Qingdao, China). Melting points (uncorrected) were determined on a SPSIC WRS-1B digital melting-point apparatus. IR spectra were recorded on a Perkin Elmer Spectrum 100 FT-IR spectrometer (KBr presser method). ESI-HRMS spectra were recorded on a Bruker Daltonics ESI-BioTOF-Q High Definition Mass Spectrometer. 1H NMR and 13C NMR spectra were collected on a Bruker AVII-400 NMR spectrometer (Bruker Company, Germany) using DMSO-d6 as solvent at room temperature. Chemical shifts (δ) are reported in parts per million (ppm) with reference to internal TMS, and coupling constants (J) are provided in Hertz (Hz).
4.2. General procedure 4.2.1. General procedure for the synthesis of compound 3Compound 3 was synthesized via two main steps (Scheme 1). First, compound 1 (60 mmol) and triethyl orthoformate (120 mmol) were placed in a round-bottom flask containing acetic anhydride (180 mmol) as solvent. The mixture was refluxed at 110-130 ℃ for 6 h and the reaction progress was monitored by TLC. A brown residue (i.e. compound 2) was obtained by vacuum distillation, used to remove impurities and solvents with low boiling points from the reaction mixture. Compound 2 was used as a reactant without further purification. Second, to a 50 mL roundbottom flask containing 30% methyl hydrazine hydrate (60 mmol) and a 30% NaOH (60 mmol) aqueous solution, a mixture of compound 2 (whole raw products of the first step) in 15-20 mL of toluene was added dropwise for 30 min at 15 ℃ under stirring. The resulting mixture was stirred for another 10 min at 25 ℃. An aqueous solution consisting of 30% NaOH (80 mmol) was added and the resulting mixture was stirred at 65 ℃ for 45 min. After reaction completion, the mixture was poured into a separatory funnel to separate the water layer. This aqueous phase was collected and added dropwise to an aqueous solution consisting of 30% HCl (90 mmol) at 95 ℃. After cooling to room temperature, the precipitate was filtered, washed with water, and was dried in vacuo to afford 3.22 g of compound 3 as a white powder.
4.2.2. General procedure for the synthesis of target compounds 9a, 9b and 9cThe main procedure is shown in Scheme 2. The raw products of compound 7 were synthesized via one-pot method in a flask containing compounds 5 (30 mmol), 6 (30 mmol), K2CO3 (15 mmol) and PEG1000 (0.45 mmol). The reactions were carried out under stirring at 185 ℃ and were monitored by TLC. After reactions completion, the desired diphenylamine derivatives 7 were obtained via column chromatographic purification. Next, compounds 7 (20 mmol), reductive iron powder (60 mmol), solid NH4Cl (60 mmol) and ethanol aqueous solution (75%, 50 mL) were added. The reactions proceeded with refluxing for 3 h at 90 ℃ and compounds 8 were obtained.
Then, compound 3 (30 mmol) and SOCl2 (thionylchloride, 30 mL) were added. The mixture was heated to reflux at 90 ℃ for 3 h. Residual SOCl2 was removed by vacuum distillation in order to obtain compound 4. Finally, the primary amines 8 (10 mmol) dissolved in dichloromethane (CH2Cl2, 5 mL) and triethylamine (Et3N, 10 mL) were added. The mixture was cooled to 0 ℃ and compound 4 (20 mmol) dissolved in dichloromethane (CH2Cl2, 10 mL) was added dropwise under stirring at a temperature not exceeding 5 ℃. The final mixture was stirred at room temperature for 10 h and the target compounds 9a, 9b and 9c were purified via column chromatography and recrystallization.
4.3. Biological tests 4.3.1. Antifungal bioassay in vitroThe five phytopathogenic fungi species (R. solani, R. cerealis, S. sclerotiorum, R. solani Kuha and F. oxysporum vasinfectum) were provided by the Institute of Pesticide and Crop Protection, Sichuan University. After retrieval from the storage tube, the strains were incubated in PDA at 25 ℃ in order to obtain new mycelia for the antifungal assay. The synthesized compounds were initially screened in vitro for their antifungal activities against the five phytopathogenic fungi by using the mycelium growth rate method [26, 27]. Each of the target compounds was dissolved in DMSO to prepare a stock solution of 20 mg/mL before mixing with molten agar below 60 ℃. The media containing the compounds at a final concentration of 20 mg/mL for the initial screening was poured into sterilized Petri dishes. After storage for appropriate days at 25 ℃, the colony diameter of each strain was measured with the original mycelial disk diameter (6 mm) subtracted from the measurement. Each measurement consisted of three replicates. The percentage inhibition was calculated as (1 -A/B) × 100%, where A is the mean colony diameter in Petri dishes with the compounds and B is the mean colony diameter in Petri dishes without the presence of the test compounds. DMSO served as the negative control, whereas the commercially available agricultural fungicides boscalid and bixafen were used as positive controls. The inhibition ratio of those compounds at the concentration of 20 mg/mL was summarized in Table 1. Afterwards, the IC50 (median inhibitory concentration) values of some compounds possessing high antifungal activities were determined against the three fungi (R. solani, R. cerealis and S. sclerotiorum), and the results were listed in Table 2. The concentration-dependent curve relates to the values of the inhibition rates (Y axis) against the test sample concentrations (mg/mL, X axis) [28].
4.3.2. Antifungal bioassay in vivoCompounds 9c-6, 9c-7 and 9c-8 were also tested in vivo for antifungal activities against R. solani. Rice plants were grown in vinyl pots (4.5 cm diameter) in a greenhouse at 25 (± 5) ℃ for 1-4 weeks. The potted plant seedlings were sprayed with the tested compound at different concentration (6.25, 12.5, 25, 50 and 100 mg/mL) and subsequently cultivated for 24 h. The blank control groups and the treated rice seedlings at the third-leaf stage were inoculated with strain R. solani (0.5 × 2.0 cm) [29]. After incubation for 5 days at 26 ℃ and 80%-90% RH, the disease severity was determined and the control efficiency was calculated [29]. Pots were arranged as a randomized complete block with three replicates per treatment.
4.4. Molecular docking 4.4.1. Homology modelingThe NCBI protein database (http://www.ncbi.nlm.nih.gov/protein/) was used to search the SDH amino acid sequence of R. solani. The employed protein sequence was CUA72490.1, CUA71217.1, CUA73421.1 and CUA73959.1 reported by Wibberg. The BLAST server (http://blast.ncbi.nlm.nih.gov) was used to search a template for the chain. SDH from avian (PDB ID: 1YQ3) was applied as the template and the homology of the amino acid sequence was aligned. Homology modeling of SDH from R. solani was carried out using MODELER 9.15 (http://salilab.org/modeller/).
4.4.2. Molecular dockingMolecular docking studies were performed to investigate the binding mode of compound 9c-7 and the positive control bixafen to SDH using Autodock vina. The 3D structures of 9c-7 and bixafen were drawn using ChemBioDraw Ultra 12.0 and ChemBio3D Ultra 12.0. The AutoDock Tools 1.5.6 package (http://mgltools.scripps.edu) was employed to generate the docking input files. The search grid of SDH was identified as center_x: 86.459, center_y: 65.6 and center_z: 85.537 with dimensions as follows: size_x = 15, size_y = 15 and size_z = 15. The value of exhaustiveness was set to 20. For Vina docking, the default parameters were used if not specified otherwise. The best-scoring pose as judged by the Vina docking score was chosen and visually analyzed using PyMOL1.7.6 software (http://www.pymol.org/).
AcknowledgmentsThis study was supported by National Key Research and Development Program of China (No. 2016YFC0502004-2) and Applied Basic Research Program of Sichuan Province (No. 2014JY0063).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.021.
| [1] | M. Schirra, S.D. Aquino, P. Cabras, et al., Control of post harvest diseases of fruit by heat and fungicides:efficacy, residue levels, and residue persistence. J. Agric. Food Chem. 59 (2011) 8531–8542. DOI:10.1021/jf201899t |
| [2] | J.C. Yang, J.B. Zhang, B.S. Chai, et al., Progress of the development on the novel amides fungicides. Agrochemicals 1 (2008) 6–9. |
| [3] | H. Walter, Pyrazole carboxamide fungicides inhibiting succinate dehydrogenase, in: C. Lamberth, J. Dinges (Eds. ), Bioactive heterocyclic compound classes: agrochemicals, E-Publishing Inc. , Germany, 2012, pp. 175-193. |
| [4] | H. Walter, H. Tobler, D. Gribkov, et al., Sedaxane, isopyrazam and solatenol®:novel broad-spectrum fungicides inhibiting succinate dehydrogenase (SDH)-synthesis challenges and biological aspects. Chimia 69 (2015) 425–434. DOI:10.2533/chimia.2015.425 |
| [5] | G. Scalliet, J. Bowler, T. Luksch, et al., Mutagenesis and functional studies with succinate dehydrogenase inhibitors in the wheat pathogen Mycosphaerella graminicola. PLoS One 7 (2012) 341–344. |
| [6] | R. Joachim, R. Heiko, C. Pierre-Yves, Modern crop protection compounds: succinate dehydrogenase inhibitors, 2nd ed. , Wiley, Germany, 2012. |
| [7] | B. C. Mac, The pesticide manual, Alton, Hants, UK, British Crop Protection Council, 2012, pp. 389. |
| [8] | B. A. Dreikorn, 3-Chloro-2, 6-dinitro-N-(substituted phenyl)-4-(trifluoromethyl)benzenamines, US Patent 4152460, 1979. |
| [9] | B. A. Dreikorn, K. E. Kramer, Diphenylamine compounds, US Patent 4407820, 1983. |
| [10] | B. A. Dreikorn, K. E. Kramer, D. F. Berard, et al. , Diphenylamines. I. Synthesis and structure-activity relationship development of novel N-(substituted-phenyl)-N-alkyl-2-(trifluoromethyl)-4, 6-dinitrobenzenamines leading to a potent miticide (El-462), in: D. R. Baker, J. G. Fenyes, J. J. Steffens (Eds. ), Synthesis and chemistry of agrochemicals Ⅲ, E-Publishing Inc. , Washington, 1992, pp. 336-341. |
| [11] | D. B. Allen, N-Alkyldiphenylamines, DE Patent 2642148, 1977. |
| [12] | D. J. Collins, J. W. Slater, J. D. Hunt, et al. , Herbicidal process using 2, 4-dinitro-6-trifluoroanilines, GB Patent 1544078, 1979. |
| [13] | H.Y. Wang, X.H. Gao, X.X. Zhang, et al., Design, synthesis and antifungal activity of novel fenfuram-diarylamine hybrids, Bioorg. Med. Chem. Lett. 27 (2017) 90–93. DOI:10.1016/j.bmcl.2016.11.026 |
| [14] | G. F. Yang, L. Xiong, Q. Chen, Pyrazole amide compound containing diphenyl ether, and application thereof, and pesticide composition, CN Patent 104557709, 2013. |
| [15] | F. Wen, H. Zhang, Z.Y. Yu, et al., Design, synthesis and antifungal/insecticidal evaluation of novel nicotinamide derivatives. Pestic. Biochem. Physiol. 982 (2010) 248–253. |
| [16] | T. Pu, H.Y. Wang, Y. Liu, et al., Design, synthesis and antifungal activity of novel 2-methyl-3-furancarboxamide derivatives. J. Sichuan Univ. (Nat. Sci. Ed.) 54 (2017) 178–184. |
| [17] | G.P. Zhou, W. Liu, H. Jin, et al., Sythesis and screening for potential against phytopathogenic fungi activity of novel amides. J. Sichuan Univ. (Nat. Sci. Ed.) 49 (2012) 871–878. |
| [18] | M.J. Chen, H. Jin, K. Tao, et al., Synthesis and bioactivity evaluation of novel benzamide derivatives containing a diphenyl ether moiety. J. Pestic. Sci. 39 (2014) 187–192. |
| [19] | Z.Y. Yu, G.Y. Shi, Q. Sun, et al., Design, synthesis and in vitro antibacterial/antifungal evaluation of novel 1-ethyl-6-fluoro-1, 4-dihydro-4-oxo-7(1-piperazinyl) quinoline-3-carboxylic acid derivatives. Eur. J. Med. Chem. 44 (2009) 4726–4733. DOI:10.1016/j.ejmech.2009.05.028 |
| [20] | F. Wen, H. Jin, K. Tao, et al., Design, synthesis and antifungal activity of novel furancarboxamide derivatives. Eur. J. Med. Chem. 120 (2016) 244–251. DOI:10.1016/j.ejmech.2016.04.060 |
| [21] | X.L. Chen, T.Q. Chen, Y.Q. Xiang, et al., Metal-free regioselective hydrobromination of alkynes through C-H/C-Bractivation. Tetrahedron Lett. 55 (2014) 4572–4575. DOI:10.1016/j.tetlet.2014.06.070 |
| [22] | W.Q. Liu, V. Megale, L. Borriello, et al., Synthesis and structure-activity relationship of non-peptidicantagonists of neuropilin-1 receptor. Bioorg. Med. Chem. Lett. 24 (2014) 4254–4259. DOI:10.1016/j.bmcl.2014.07.028 |
| [23] | Y.P. Zhu, S. Sergeyev, P. Franck, et al., Amine activation:synthesis of N-(Hetero) arylamides from isothioureas and carboxylic acids. Org. Lett. 18 (2016) 4602–4605. DOI:10.1021/acs.orglett.6b02247 |
| [24] | X.L. Deng, J. Xie, Y.Q. Li, et al., Design, synthesis and biological activity of novel substituted pyrazole amide derivatives targeting EcR/USP receptor. Chin. Chem. Lett. 27 (2016) 566–570. DOI:10.1016/j.cclet.2016.02.009 |
| [25] | X.L. Deng, L. Zhang, X.P. Hu, et al., Target-based design, synthesis and biological activity of new pyrazole amide derivatives. Chin. Chem. Lett. 27 (2016) 251–255. DOI:10.1016/j.cclet.2015.10.006 |
| [26] | Y.B. Bai, A.L. Zhang, J.J. Tang, et al., Synthesis and antifungal activity of 2-chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J. Agric. Food Chem. 61 (2013) 2789–2795. DOI:10.1021/jf3053934 |
| [27] | Y. Xiao, H.X. Li, C. Li, et al., Antifungal screening of endophytic fungi from Ginkgo biloba for discovery of potent anti-phytopathogenic fungicides. FEMS Microbiol. Lett. 339 (2013) 130–136. DOI:10.1111/fml.2013.339.issue-2 |
| [28] | Y.H. Ye, L. Ma, Z.C. Dai, et al., Synthesis and antifungal activity of nicotinamide derivatives as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 62 (2014) 4063–4071. DOI:10.1021/jf405437k |
| [29] | L.L. Wang, C. Li, Y. Zhang, et al., Synthesis and biological evaluation of benzofuroxan derivatives as fungicides against phytopathogenic fungi. J. Agric. Food Chem. 61 (2013) 8632–8640. DOI:10.1021/jf402388x |
 2017, Vol. 28
2017, Vol. 28