b School of Chemistry and Chemical Engineering, Huangshan University, Huangshan 245041, China;
c Zhejiang Base of National Southern Pesticide Research Centre, Zhejiang Research Institute of Chemical Industry, Hangzhou 310023, China
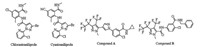
Chemical pesticides are the major methods of controlling arthropod pests which reduced the productivity of crop. To overcome the insects resistance caused by high frequent application of insecticides, there is a great demand to develop insecticidal compounds with novel modes of action [1, 2]. Ryanodine receptor (RyR), known as the calcium ion channel receptor, provides an effective pesticide target for commercial exploitation [3]. Anthranilic diamides potently activate the RyR leading to interrupt the normal contraction of the muscle via releasing stored calcium from the sarcoendoplasmic reticulum [4]. Recently, anthranilic diamides have attracted more attention especially for development of commercial insecticides (such as Chlorantraniliprole [5] and Cyantraniliprole [6], Fig. 1), and many anthranilic diamide compounds had been reported [7-13].
|
Download:
|
| Fig. 1. Structures of chlorantraniliprole, cyantraniliprole and per(poly)fluoroalkyl pyrazole derivatives with high insecticidal activity. | |
It is well-known that the pyrazolyl heterocycles derivatives have been widely used in agriculture, such as insecticidal activity [14], nematicidal activity [15-19], herbicidal activity [20], fungicidal activity [21, 22]. Moreover, some per(poly)fluoroalkyl pyrazole derivatives (such as compound A and B; Fig. 1) with high insecticidal activity have been discovered recently [23-25].
In our continuing research on biologically active heterocycles [26-30], the insecticide Chlorantraniliprole was selected as a lead compound, the Br group of Chlorantraniliprole was replaced by a per(poly)fluoroalkyl pyrazole group, a series of novel title anthranilic diamide compounds were designed and synthesized. The insecticidal activity against Mythimna separata Walker and Plutella xylostella Linnaeus of title compounds were evaluated.
2. Results and discussion 2.1. Synthesis and spectraDuring our study on cyclization of 3-chloro-2-hydrazinylpyridine with diethyl maleate, we found that appropriate concentration of sodium ethoxide used as catalyst was prepared by 2.0 equivalent sodium and 11 equivalent ethanol (corresponding to the starting compound 2), while the cyclization product 3 was isolated in poor yield with 22 equivalent ethanol. The title compounds 8a-8p were readily obtained in 52.1%-77.4% yields through the ring-opening reaction of compound 7 with different amine at room temperature.
The structures of title compounds were identified by 1H NMR and HRMS. In the 1H NMR spectra of 8a-8p, singlet peak around δ 3.85-3.90 was assigned as the methyl proton of pyrazole. Signals corresponding to the proton of the pyrazole ring were observed at δ 6.83-7.12. The proton of 5-position on the pyridine ring appeared as doublet of doublets at δ 7.37-7.40 with coupling constants 8.0 Hz and 4.7 Hz. The signal peak at δ 9.98-10.60 could be attributed to the active proton of aromatic amide. The HRMS showed an ion peak of M+H+, which was consistent with the calculated mass value of the compounds.
1-(3-Chloropyridin-2-yl)-N-(4-cyano-2-methyl-6-(methylcarbamoyl)phenyl)-3-((1-methyl-3-(perfluoroethyl)-4-(trifluoromethyl)-1H-pyrazol-5-yl)oxy)-1H-pyrazole-5-carboxamide 8a: Light yellow solid, m.p. 183-185 ℃, yield 55.1%. 1H NMR (CDCl3, 600 MHz): δ 10.60 (s, 1H, CONH), 8.42 (dd, 1H, J = 4.7, 1.5 Hz, pyridine-H), 7.86 (dd, 1H, J = 8.0, 1.5 Hz, pyridine-H), 7.59 (s, 2H, PhH), 7.38 (dd, 1H, J = 8.0, 4.7 Hz, pyridine-H), 6.74 (s, 1H, pyrazole-H), 6.27 (q, 1H, J = 4.8 Hz, NHCH3), 3.88 (s, 3H, pyrazole-CH3), 3.01 (d, 3H, J = 4.9 Hz, NHCH3), 2.25 (s, 3H, Ph-CH3); HRMS calcd. for C26H18ClF8N8O3 ([M+H]+) 677.1057, found 677.1063.
1-(3-Chloropyridin-2-yl)-N-(4-cyano-2-(isopropylcarbamoyl)-6-methylphenyl)-3-((1-methyl-3-(perfluoroethyl)-4-(trifluoromethyl)-1H-pyrazol-5-yl)oxy)-1H-pyrazole-5-carboxamide 8c: Light yellow solid, m.p. 187-189 ℃, yield 53.1%. 1H NMR (CDCl3, 600 MHz): δ 10.61 (s, 1H, CONH), 8.43 (dd, 1H, J = 4.7, 1.6 Hz, pyridine-H), 7.85 (dd, 1H, J = 8.0, 1.6 Hz, pyridine-H), 7.58 (d, 2H, J = 5.1 Hz, Ph-H), 7.38 (dd, 1H, J = 8.0, 4.7 Hz, pyridine-H), 6.73 (s, 1H, pyrazole-H), 6.02 (d, 1H, J = 7.7 Hz, NHCH), 4.27-4.19 (m, 1H, NHCH), 3.89 (s, 3H, pyrazole-CH3), 2.25 (s, 3H, Ph-CH3), 1.28 (d, 6H, J = 6.6 Hz, CH(CH3)2); HRMS calcd. for C28H22ClF8N8O3 ([M+H]+) 705.1370, found 705.1380.
2.2. Bioassay and SARThe bioactivities of compounds 8a-8p against Mythimna separata Walker are summarized in Table 1. Most of the title compounds exhibited good insecticidal activity (100%) against Mythimna separata Walker at 500 mg/L. When the concentration was reduced to 0.8 mg/L, compound 8a, 8c, 8g, 8k and 8l still exhibited 100% larvicidal activities. On the basis of the preliminary in vivo bioassays results, the compound 8a, 8c, 8e, 8g, 8k and 8l were selected for further bioassay against Plutella xylostella Linnaeus. The results indicated that when the concentration is at 0.4 mg/L, the insecticidal activity against Plutella xylostella Linnaeus is still excellent, while the concentration was at 0.08 mg/L, insecticial activity of correspondence compounds decrease, except compound 8a (100%) and 8c (100%). For LC50 testing against M. separata, the insecticide Chlorantraniliprole was selected as positive control. The LC50 of compound 8a (0.48 mg/L) and 8c (0.43 mg/L) values are the same as the positive control insecticide chlorantraniliprole (0.40 mg/L) (Table 2).
|
|
Table 1 Insecticidal activities of compounds 8a-8p and chlorantraniliprole against Mythimna separata Walker and Plutella xylostella Linnaeus. |
|
|
Table 2 The LC50 of compound 8a and 8c against M. separata. |
From Table 1, the preliminary structure and activity relationship (SAR) analysis indicated that 4-cyano-2-methyl substituted group in benzene ring exhibited higher larvicidal activities than 2-F substituted group. Also the substitutions in the aliphatic amide moiety of title compounds can influence the activity. It was found that bulky groups, such as i-butyl, i-pentyl and -(CH2)5- seriously lead to a decrease activity quickly.
3. ConclusionA new series of anthranilic diamide compounds containing polyfluoroalkyl pyrazole moiety were designed and synthesized. Their structures were confirmed and the insecticidal activity was tested. The bioassay results indicated that compound 8a and 8c possessed good insecticidal activity (100%) against Mythimna separata and Plutella xylostella at 0.8 mg/L and 0.08 mg/L respectively.
4. ExperimentalThe melting points were determined on an X-4 binocular microscope melting point apparatus (Beijing Tech Instrument Co., China) and were uncorrected. 1H NMR spectra were recorded on a Bruker AVANCE Ⅲ HD spectrometer (600 MHz) using CDCl3 or DMSO-d6 as solvent and TMS as internal standard. Chemical shift values (δ) were given in parts per million (ppm). HRMS were obtained using a UPLC/Xevo G2-XS Q-TOF Waters mass spectrometer. Unless otherwise noted, all reagents were purchased from commercial supplies and used without further purification. All solvents were dried and redistilled before use.
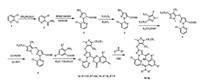
4.1. SynthesisThe synthetic route of the title compounds is outlined in Scheme 1. The intermediate 5 was synthesized by condensation reaction of the compound 4 with the 5-fluoro-1-methyl-3-(perfluoroethyl)-4-(trifluoromethyl)-1H-pyrazole in dry dimethylformamide using potassium carbonate as base according to the reported method [31]. Then the pyrazole carboxylic acid 6 was given by hydrolysis. The benzoxazinone 7 was obtained by cyclization reaction of compound 6 with the substituted 2-aminobenzoic acid in triethylamine solution as described in Ref [32].
|
Download:
|
| Scheme 1. Synthesis of the anthranilic diamides derivatives. | |
According to the literature [33], to a solution of intermediate 7 (0.5 g, 0.76 mmol) in tetrahydrofuran (15 mL), 0.99 mmol amine was added dropwise. The resulting solution was stirred at room temperature for 30 min. After completion of the reaction, the mixture was added water (40 mL), and then extracted with ethyl acetate (20 mL × 3). The combined extracts were dried by MgSO4, filtered and evaporated. The residue was separated by flash chromatography (silica gel, 30:70 ethyl acetate/hexane) to afford the corresponding pure title compounds 8a-8p. The other physical-chemical data can be obtained in Supporting information.
4.2. Insecticidal assayAll bioassays were performed on representative test organisms reared in the laboratory. The test compounds were dissolved in 5 mL DMF and 0.05 mL of Tween-80 as an emulsifier, then diluted with water to different concentrations. Evaluations were based on a percentage scale of 0-100 (0 = no activity and 100 = total kill).
Insecticidal activity against Mythimna separata Walker: The insecticidal activities of the synthesized compounds and contrast compound chlorantraniliprole against Mythimna separata Walker were tested according to the leaf-dip method using the reported procedure [9]. Leaf discs (2 cm) were cut from fresh corn leaves and then were dipped into the test solution for 10 s. After air drying, the treated leaf discs were transferred individually into a petri dish (9 cm in diameter). Each dried treated leaf disk was infested with 10 larvae of third-instar Mythimna separata Walker larvae. Each treatment was performed four times. Percentage mortalities were determined 3 days after treatment and listed in Table 1.
Insecticidal activity against Plutella xylostella Linnaeus: The insecticidal activities of compounds 8a-8p against Plutella xylostella Linnaeus were evaluated by the leaf-dip method according to the reported procedures [8, 10]. Fresh cabbage leaf discs (2 cm) were immersed for 15 s in a test solution, dried in air at room temperature and placed individually into a petri dish lined with filter paper. Ten second-instar larvae were introduced on the leaf discs. Chlorantraniliprole was tested under the same conditions as the control. Each treatment was performed four times. Percentage mortalities were evaluated 3 days after treatment. The insecticidal activity is summarized in Table 1.
AcknowledgmentThis work was supported by Key Projects of the National Science and Technology Pillar Program of China (No. 2011BAE06B01-20).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.05.015.
| [1] | E.C. Oerke. Crop losses to pests. J. Agric. Sci. 144 (2006) 31–43. DOI:10.1017/S0021859605005708 |
| [2] | B.L. Wang, H.W. Zhu, Y. Ma, et al., Synthesis, insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 61 (2013) 5483–5493. DOI:10.1021/jf4012467 |
| [3] | J.R. Bloomquist. Ion channels as targets for insecticides. Annu. Rev. Entomol. 41 (1996) 163–190. DOI:10.1146/annurev.en.41.010196.001115 |
| [4] | D. Cordova, E.A. Benner, M.D. Sacher, et al., Anthranilic diamides:a new class of insecticides with a novel mode of action:ryanodine receptor activation. Pestic. Biochem. Physiol. 84 (2006) 196–214. DOI:10.1016/j.pestbp.2005.07.005 |
| [5] | G.P. Lahm, T.M. Stevenson, T.P. Selby, et al., RynaxypyrTM:a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 17 (2007) 6274–6279. DOI:10.1016/j.bmcl.2007.09.012 |
| [6] | K.A. Hughes, G.P. Lahm, T.P. Selby, et al., Cyano anthranilamide insecticides, WO 2004067528, 2004. |
| [7] | D.A. Clark, G.P. Lahm, B.K. Smith, et al., Synthesis of insecticidal fluorinated anthranilic diamides. Bioorg. Med. Chem. 16 (2008) 3163–3170. DOI:10.1016/j.bmc.2007.12.017 |
| [8] | J. Wu, B.A. Song, D.Y. Hu, et al., Design, synthesis and insecticidal activities of novel pyrazole amides containing hydrazone substructures. Pest Manag. Sci. 68 (2012) 801–810. DOI:10.1002/ps.v68.5 |
| [9] | J.F. Zhang, J.Y. Xu, B.L. Wang, et al., Synthesis and insecticidal activities of novel anthranilic diamides containing acylthiourea and acylurea. J. Agric. Food Chem. 60 (2012) 7565–7572. DOI:10.1021/jf302446c |
| [10] | K. Chen, Q. Liu, J.P. Ni, et al., Synthesis:insecticidal activities and structureactivity relationship studies of novel anthranilic diamides containing pyridylpyrazole-4-carboxamide. Pest Manag. Sci. 71 (2015) 1503–1512. DOI:10.1002/ps.3954 |
| [11] | X.W. Hua, W.T. Mao, Z.J. Fan, et al., Novel anthranilic diamide insecticides:design, synthesis, and insecticidal evaluation. Aust. J. Chem. 67 (2014) 1491–1503. DOI:10.1071/CH13701 |
| [12] | S. Zhou, Z.H. Jia, L.X. Xiong, et al., Chiral dicarboxamide scaffolds containing a sulfiliminyl moiety as potential ryanodine receptor activators. J. Agric. Food Chem. 62 (2014) 6269–6277. DOI:10.1021/jf501727k |
| [13] | S. Zhou, Y.C. Gu, M. Liu, et al., Insecticidal activities of chiral N-trifluoroacetyl sulfilimines as potential ryanodine receptor modulators. J. Agric. Food Chem. 62 (2014) 11054–11061. DOI:10.1021/jf503513n |
| [14] | S.V. Joseph, I. Grettenberger, L. Godfrey. Insecticides applied to soil of transplant plugs for Bagrada hilaris (Burmeister) (Hemiptera:Pentatomidae) management in broccoli. Crop Prot. 87 (2016) 68–77. DOI:10.1016/j.cropro.2016.04.023 |
| [15] | W. Zhao, Z.H. Shen, J.H. Xing, et al., Synthesis and nematocidal activity of novel 1-(3-chloropyridin-2-yl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Chem. Pap. 71 (2017) 921–928. DOI:10.1007/s11696-016-0012-8 |
| [16] | W. Zhao, Z.H. Shen, T.M. Xu, et al., Synthesis, nematocidal activity and docking study of novel chiral 1-(3-chloropyridin-2-yl)-3-(difluoromethyl)-1Hpyrazole-4-carboxamide derivatives. J. Heterocycl. Chem. 54 (2017) 1751–1756. DOI:10.1002/jhet.v54.3 |
| [17] | W. Zhao, Z.H. Shen, J.H. Xing, et al., Synthesis, characterization, nematocidal activity and docking study of novel pyrazole-4-carboxamide derivatives. Chin. J. Struct. Chem. 36 (2017) 423–428. |
| [18] | W. Zhao, Z.H. Shen, J.H. Xing, et al., Synthesis and nematocidal activity of novel pyrazole carboxamide derivatives against Meloidogyne incognita. Lett. Drug Des. Discov. 14 (2017) 323–329. DOI:10.2174/1570180813666160930164327 |
| [19] | X.H. Liu, W. Zhao, Z.H. Shen, et al., Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 125 (2017) 881–889. DOI:10.1016/j.ejmech.2016.10.017 |
| [20] | Z.W. Zhai, Q. Wang, Z.H. Shen, et al., Synthesis and biological activity of 1, 2, 4-triazole thioether derivatives containing pyrazole moiety. Chin. J. Org. Chem. 37 (2017) 232–236. DOI:10.6023/cjoc201607031 |
| [21] | X.H. Liu, C.X. Tan, J.Q. Weng. Synthesis, dimeric crystal structure, and fungicidal activity of 1-(4-methylphenyl)-2-(5-((3, 5-dimethyl-1H-pyrazol-1-yl)methyl)-4-phenyl-4H-1, 2, 4-triazol-3-ylthio)ethanone. Phosphorus Sulfur Silicon Rel. Elem. 186 (2011) 558–564. DOI:10.1080/10426507.2010.508060 |
| [22] | J.X. Mu, Y.X. Shi, M.Y. Yang, et al., Design, synthesis, DFT study and antifungal activity of pyrazolecarboxamide derivatives. Molecules 21 (2016) 68. DOI:10.3390/molecules21010068 |
| [23] | W. Thielert, M. Maue, L. Pitta, et al, Synergistic insecticidal combinations containing pyrazole-5-carboxamide and diamide insecticides, WO 2015132168, 2015. |
| [24] | M. Maue, T. Harschneck, R. Fischer, et al. , Novel halogen-substituted compounds, WO 2016020441, 2016. |
| [25] | M. Maue, A. Decor, J. J. Hahn, et al. , Halogen-substituted compounds, WO 2015193218, 2015. |
| [26] | Z.W. Zhai, Y.X. Shi, M.Y. Yang, et al., Microwave assisted synthesis and antifungal activity of some novel thioethers containing 1, 2, 4-triazolo[4, 3-a] pyridine moiety. Lett. Drug Des. Discov. 13 (2016) 521–525. DOI:10.2174/157018081306160618181757 |
| [27] | S.L. Yan, M.Y. Yang, Z.H. Sun, et al., Synthesis and antifungal activity of 1, 2, 3-thiadiazole derivatives containing 1, 3, 4-thiadiazole moiety. Lett. Drug Des. Discov. 11 (2014) 940–943. DOI:10.2174/1570180811666140423222141 |
| [28] | X.H. Liu, Q. Wang, Z.H. Sun, et al., Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manag. Sci. 73 (2017) 953–959. DOI:10.1002/ps.2017.73.issue-5 |
| [29] | L.J. Zhang, M.Y. Yang, Z.H. Sun, et al., Synthesis and antifungal activity of 1, 3, 4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 11 (2014) 1107–1111. DOI:10.2174/1570180811666140610212731 |
| [30] | X. H. Liu, Y. M. Fang, F. Xie, et al. , Synthesis and in vivo fungicidal activity of some new quinoline derivatives against rice blast, Pest Manag. Sci. (2017), doi: http://dx.doi.org/10.1002/ps.4556. |
| [31] | X.H. Liu, W. Zhao, Z.H. Shen, et al., Synthesis:nematocidal activity and docking study of novel chiral 1-(3-chloropyridin-2-yl)-3-(trifluoromethyl)-1Hpyrazole-4-carboxamide derivatives. Bioorg. Med. Chem. Lett. 26 (2016) 3626–3628. DOI:10.1016/j.bmcl.2016.06.004 |
| [32] | J. Wu, D.D. Xie, W.L. Shan, et al., Synthesis and insecticidal activity of anthranilic diamides with hydrazone substructure. Chem. Pap. 69 (2015) 993–1003. |
| [33] | R. A. Berger, J. L. Flexner, Anthranilamide arthropodicide treatment, WO 2003024222, 2003. |
 2017, Vol. 28
2017, Vol. 28