Neonicotinoid insecticides have wide application in the pest control due to their high activity, low toxicity, low residue and high selectivity [1, 2]. However, with the increasing of improper application, they caused many negative effects, such as resistance [3], toxicity to bees [4] and environmental pollution [5]. Therefore, it is necessary to find novel neonicotinoid candidate with novel scaffolds, high-efficiency and low toxicity. The core structure of neonicotinoids consists of four segments, that is, a heterocycle, a linkage, a guanidine/amidine and an electro-withdrawing group. Nitromethylene analogue of imidacloprid (NMI) is a good precursor for structural modifications (Scheme 1).

|
Download:
|
| Scheme 1. Molecular design of 1, 8-naphthyridine as neonicotinoid insecticides. | |
1, 8-Naphthyridine (NAP) derivatives (Scheme 1) [5] have wide biological activity, such as anticancer [6-9], antibacterial [10-13], anti-inflammatory [14-16], antioxidant activities [17, 18], inhibitors of enzymes [19-21], as well as antagonists or agonists of receptors [22-25]. Previously, our research group developed a new method to synthesis NAP derivatives via three components of heterocyclic ketone aminals (HKAs), malononitrile dimmer, and aldehyde through one-pot method [5]. NMI, a HKA and an important neonicotinoid lead compound with excellent insecticidal activities, has higher insecticidal activity than imidacloprid. But its photo instability impeded its commercialization. Therefore, a plenty of chemical modifications have been conducted to improve its physiochemical properties generating many excellent insecticide candidates. Previous study proved that many derivatizations on NMI maintain its high activity. In combination of structure features of NMI and our newly-developed synthetic methods for NAP construction, we herein described a series of NAP derivatives as novel neonicotinoid insecticides.
2. Results and discussionNine 1, 8-naphthyridines were easily synthesized via one-pot method according to our previously-reported procedure. Products 4 was prepared through NMI, various aldehydes and malononitrile dimmer. Malononitrile dimmer 3 and aldehydes 2 underwent Knoevenagel condensation at the beginning of the reaction, then the aza-ene reaction took place between NMI 1 and Knoevenagel adducts of malononitrile dimmer 3 and aldehydes 2. For 4a-4c, part of the products was precipitated from the reaction mixture and were collected by filtration. The remaining 4a-4c in filtrate were subject to column chromatography followed by recrystallization to wipe off trace impurities in the end. The compounds 4d-4i can be easily obtained by filtration and recrystallization. The structures of these compounds were well confirmed by NMR and high-resolution mass spectrometry (HRMS). As previously reported [5], the substituent on the aryl ring had great influences on t"showhe yields of products 4, phenyl aldehyde with electrondonating gave lower yields and the steric hindrance of the substituent on the aryl ring also limited the yields of products 4. In this reaction, product 4a bearing a hydroxyl at the benzene ring exhibited lower yield than other substrates. The electron-donating feature of hydroxyl group might limit the Knoevenagel condensation between aldehyde 2 and malononitrile dimmer 3.
The insecticidal activities of all the prepared compounds (4a-4i) against cowpea aphid were screened. Compounds 4a, 4b, 4e, 4g and 4i had good aphicidal activity at concentration of 100 mg/L. Based on these results, the insecticidal activities of compounds 4a, 4b, 4e, 4g and 4i were further tested to obtain the median lethal concentration (LC50). The LC50 values of compounds 4a, 4b, 4e, 4g and 4i are 0.064, 0.067, 0.011, 0.21 and 0.031 mmol/L, respectively (shown in Table 1).
|
|
Table 1 Insecticidal activities of compounds 4a-4i against cowpea aphids. |
Compounds 4e and 4i have excellent insecticidal activity against cowpea aphids, while compounds 4a, 4b and 4g have moderate aphicidal activity. It is interesting to found that the compounds with mono-substitution on benzene ring have higher activities than compounds with bis-substituted benzene. In mono-substituted compounds, the substituent on the 4-position of benzene ring was favorable to the insecticidal activity. Compound with fluorine on the 4-position of benzene ring has better aphicidal activities than others. These results showed that introduction of fluorine on the 4-position of benzene has beneficial effects on the aphicidal activities. However, compound with fluorine at 2-position of benzene showed lower insecticidal activity against cowpea aphids.
3. ConclusionIn conclusion, several 1, 8-naphthyridine derivatives were designed and synthesized by one-pot method. Some of the target compounds have excellent insecticidal activities against cowpea aphids, while some compounds exhibited moderate antiaphids activities. The introduction of fluorine on the molecule could improve the insecticidal activity. We anticipate that this example can be potentially helpful in the development of future generation of novel insecticides.
4. Experimental 4.1. Chemicals and instrumentationsMelting points (mp) were recorded on a Büchi B540 apparatus (Büchi Labortechnik AG, Flawil, Switzerland) and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker AM-400 (400 MHz) spectrometer with CDCl3 or DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts are reported in δ (parts per million) values. High-resolution mass spectrometry (HRMS) data were recorded on a MicroMass GCT CA 055 instrument under electro impact (70 eV) condition. Analytical thin-layer chromatography (TLC) was carried out on precoated plates (silica gel 60 F254), the purity of synthesized compounds were checked by thin-layer chromatography (TLC) and spots were visualized with ultraviolet (UV) light.
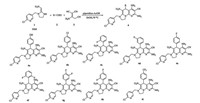
4.2. Synthetic routes and experimental dataThe general synthetic procedures for compounds 4a-4i are depicted in Scheme 2. Unless other special noted, reactant and solvents were used as received from commercial suppliers.
|
Download:
|
| Scheme 2. Synthetic procedures and compounds list of 1, 8-naphthyridine derivatives. | |
Synthetic methods of compounds 4a-4i. As previously reported [5], 2-chloro-5-[(2-nitromethylene) imidazolidin-1-yl]methyl] pyridine 1 (1 mmol), benzaldehyde 2 (3 mmol), malononitrile dimmer 3 (3 mmol) and ethanol (10 mL) were placed in a 25 mL round-bottom flask and the reaction mixture were stirred for 36 h at 70 ℃. Completion was monitored by TLC. Then, the mixture was filtrated and the crude product was further purified by column chromatography (CH2Cl2:MeOH = 20:1). Specially, in the procedure of purification, products 4d-4i were obtained by filtration and recrystallization in methanol.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-5-(4-hydroxyphenyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4a). Yield, 15%; mp, 306.2-307.4 ℃; 1H NMR (400 MHz, DMSO-d6): δ 9.17 (s, 1H), 8.30 (s, 1H), 7.56 (d, 1H, J = 7.9 Hz), 7.25 (d, 1H, J = 8.2 Hz, ), 6.87 (d, 2H, J = 8.1 Hz), 6.56 (d, 2H, J = 8.1 Hz), 6.38 (d, 4H, J = 19.9 Hz), 5.49 (s, 1H), 4.75 (s, 2H), 4.29-3.63 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 159.57, 155.91, 154.27, 154.13, 149.33, 149.18, 148.38, 138.86, 133.30, 131.15, 127.66, 128.82, 116.68, 114.75, 106.79, 90.10, 70.22, 50.54, 50.05, 43.02, 36.56; HRMS (ES+) calcd. for C23H1935ClN8O3Na (M+Na)+, 513.1166; found, 513.1165. Calcd for C23H1937ClN8O3Na (M+Na)+, 515.1137; found, 515.1143.
2, 4-Diamino-5-(4-chlorophenyl)-7-((6-chloropyridin-3-yl) methyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4b). Yield, 23%; mp, 294.2-295.3 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.22 (s, 1H), 7.55 (d, 1H, J = 8.3 Hz), 7.19 (dd, 3H, J = 8.4 Hz), 7.02 (d, 2H, J = 8.3 Hz), 6.46 (d, 4H, J = 6.2 Hz), 5.60 (s, 1H), 4.75 (q, 2H, J = 15.7 Hz), 4.23-3.81 (m, 4H); 13C NMR (100 MHz, DMSO-d6) d 159.78, 154.37, 153.64, 149.39, 149.01, 148.55, 141.91, 138.77, 131.08, 130.90, 128.49, 127.87, 123.74, 116.60, 106.18, 92.80, 70.14, 50.81, 50.40, 42.97, 36.72 ppm; HRMS (ES+) calcd for C23H1835Cl2N8O2Na (M+Na)+, 513.0827; found, 513.0822. Calcd. for C23H1837Cl2N8O2Na (M+Na)+, 533.0798; found, 533.0789.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-5-(2-fluorophenyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4c). Yield, 45%; mp, 267.9-279.1 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.27 (s, 1H), 7.65 (d, 1H, J = 8.1 Hz), 7.52 (t, 1H, J = 7.6 Hz), 7.27 (t, 1H, J = 12.3 Hz), 7.20 (dd, 1H, J = 12.8, 6.7 Hz), 6.99 (dt, 2H, J = 19.3, 8.1 Hz), 6.44 (s, 2H), 6.10 (s, 2H), 5.51 (s, 1H), 4.85-4.64 (m, 2H), 4.19 (dd, 1H, J = 9.0 Hz), 4.10-3.88 (m, 3H); 13C NMR (100 MHz, DMSO-d6): δ 159.58, 154.09, 153.25, 149.34, 149.12, 148.59, 147.97, 140.22, 138.94, 131.05, 129.11, 128.41, 126.66, 123.82, 116.43, 115.60, 115.39, 104.78, 92.34, 70.28, 50.61, 43.06, 35.27; HRMS (ES+) calcd. for C23H1835ClFN8O2Na (M+Na)+, 515.1123; found, 515.1127. Calcd. for C23H1837ClFN8O2Na (M+Na)+, 517.1093; found, 517.1105.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-5-(3-fluorophenyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4d). Yield, 24%; mp, 269.0-271.1 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.23 (s, 1H), 7.58 (d, 1H, J = 7.8 Hz), 7.18 (dd, 2H, J = 14.9, 7.8 Hz), 6.95 (dd, 2H, J = 23.6, 9.3 Hz), 6.82 (d, J = 7.6 Hz, 1H), 6.48 (s, 4H), 5.61 (s, 1H), 4.75 (q, J = 15.8 Hz, 2H), 4.29-3.81 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 160.94, 159.79, 154.38, 153.69, 149.32, 148.99, 148.54, 145.91, 138.77, 131.01, 129. 94, 123.73, 122.45, 116.59, 113.90, 113.59, 113.20, 106.01, 92.87, 70.13, 50.59, 42.99, 37.15; HRMS (ES+) calcd. for C23H1835ClFN8O2Na (M +Na)+, 515.1123; found, 515.1124. Calcd. for C23H1837ClFN8O2Na (M +Na)+, 517.1093; found, 517.1096.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-5-(4-fluorophenyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4e). Yield, 47%; mp, 287.3-288.6 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.22 (s, 1H), 7.57 (d, 1H, J = 8.0 Hz), 7.22 (d, 1H, J = 8.2 Hz), 7.14-6.98 (m, 2H), 6.94 (t, 2H, J = 8.8 Hz), 6.45 (d, 4H, J = 10.2 Hz), 5.58 (s, 1H), 4.75 (q, 2H, J = 15.7 Hz), 4.27-3.83 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 159.74, 154.32, 153.66, 149.33, 149.01, 148.48, 139.12, 138.82, 131.10, 128.48, 123.78, 116.62, 114.67, 114.46, 106.50, 93.30, 70.14, 50.76, 50.40, 42.97, 36.60; HRMS (ES+) calcd. for C23H1835ClFN8O2Na (M+Na)+, 515.1123; found, 515.1125. Calcd. for C23H1837ClFN8O2Na (M+Na)+, 517.1093; found, 517.1086.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-5-(2, 6-difluorophenyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4f). Yield, 53%; mp, 293.4-294.2 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.27 (s, 1H), 7.69 (d, 1H, J = 8.2 Hz), 7.46-7.17 (m, 2H), 6.94 (t, 2H, J = 8.3 Hz), 6.53 (s, 2H), 5.66 (d, 3H, J = 21.6 Hz), 4.80 (q, 2H, J = 15.6 Hz), 4.29-3.90 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 161.53, 159.59, 159.12, 154.04, 152.95, 149.33, 147.90, 139.10, 130.88, 128.73, 123.84, 117.55, 116.14, 111.82, 111.57, 103.70, 90.90, 70.42, 50.73, 42.98, 29.96. HRMS (ES+) calcd. for C23H1735ClF2N8O2Na (M+Na)+, 533.1029; found, 533.1028. Calcd. for C23H1737ClF2N8O2Na (M+Na)+, 535.0999; found, 535.0994.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-5-(3, 4-difluorophenyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4g). Yield, 26%; mp, 284.1-285.9 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.16 (s, 1H), 7.59 (d, 1H, J = 8.0 Hz), 7.30-7.00 (m, 3H), 6.79 (s, 1H), 6.47 (d, 4H, J = 16.2 Hz), 5.55 (s, 1H), 4.75 (dd, 2H, J = 43.6, 15.7 Hz), 4.25-3.90 (m, 4H); 13C NMR (101 MHz, DMSO-d6): δ 159.84, 154.33, 153.29, 149.34, 148.90, 148.46, 140.33, 138.78, 131.06, 123.61, 123.02, 116.87, 116.69, 116.55, 115.88, 110.92, 106.06, 92.63, 70.09, 51.16, 50.37, 42.96, 36.71; HRMS (ES+) calcd. for C23H1735ClF2N8O2Na (M+Na)+, 533.1029; found, 533.1031. Calcd. for C23H1737ClF2N8O2Na (M+Na)+, 535.0999; found, 535.1004.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-5-(2, 4-difluorophenyl)-6-nitro-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8]naphthyridine-3-carbonitrile (4h). Yield, 25%; mp, 279.9-281.4 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.22 (s, 1H), 7.74-7.45 (m, 2H), 7.30 (d, 1H, J = 8.1 Hz), 7.03-6.73 (m, 2H), 6.45 (s, 2H), 6.15 (s, 2H), 5.50 (s, 1H), 4.78 (dd, 2H, J = 15.5 Hz), 4.13 (dd, 4H, J = 18.3, 11.8 Hz); 13C NMR (100 MHz, DMSO-d6): δ 159.62, 154.11, 152.89, 149.42, 149.08, 148.00, 138.92, 132.15, 131.03, 125.34, 123.78, 116.43, 110.43, 104.67, 104.18, 103.92, 103.64, 91.98, 70.29, 50.92, 50.56, 43.02, 34.99; HRMS (ES+) calcd. for C23H1735ClF2N8O2Na (M+Na)+, 533.1029; found, 533.1033. Calcd. for C23H1737ClF2N8O2Na (M+Na)+, 535.0999; found, 535.1003.
2, 4-Diamino-7-((6-chloropyridin-3-yl)methyl)-6-nitro-5-(4-(trifluoromethyl)phenyl)-5, 7, 8, 9-tetrahydroimidazo[1, 2-a][1, 8] naphthyridine-3-carbonitrile (4i). Yield, 45%; mp, 313.3-314.9 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.21 (s, 1H), 7.52 (dd, 3H, J = 16.8, 8.2 Hz), 7.20 (t, 2H, J = 15.6 Hz), 7.13 (d, 1H, J = 8.2 Hz), 6.49 (s, 4H), 5.71 (s, 1H), 4.76 (dd, 2H, J = 42.2, 15.8 Hz), 4.26-3.85 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 159.87, 154.45, 153.56, 149.28, 149.03, 148.69, 147.60, 138.75, 130.99, 127.41, 125.74, 124.86, 123.66, 116.57, 105.84, 99.50, 92.51, 70.17, 50.98, 50.37, 42.97, 37.17; HRMS (ES+) calcd. for C24H1835ClF3N8O2Na (M+Na)+, 565.1091; found, 565.1089. Calcd. for C24H1837ClF3N8O2Na (M+Na)+, 567.1062; found, 517.1071.
4.3. Biological activityThe insecticidal activities of the title compounds against cowpea aphid were tested by the method of leaf dip bioassay [26]. The aqueous solution of each compound were prepared which plus 0.1% Triton X-100 (0.1 mg/L) as a surfactant and DMSO as solvent in the solution, then diluted it with 0.1% Triton X-100 (0.1 mg/L) to obtain the solution with different concentration (500 mg/L, 100 mg/L, 20 mg/L, 4 mg/L, 0.8 mg/L and others). Several adults of aphid were selected, avoid light, and given a starvation treatment about an hour in the dark. Then let them eat broad bean seedling until their mouthparts pierce the bean sprout (2-3 hours). Then, the leaves of the horsebean plant with 40-60 aphids was dipped for 3 s in each concentration 3 times, repeat this three times in each group. After treatment, the burgeoning shoots were placed in a conditioned room (25 ± 1) ℃, 50% RH) for 48 hours. Water containing Triton X-100 (0.1 mg/L) was used as a control. The state of aphid was observed and aphid mortality was recorded after 48 h. The toxicity experiment of each compound was repeated three times, and the results were calculated by SPSS [27]. Median lethal concentration (LC50) were determined by Probit regression analysis program and expressed in parts per million (ppm) [28].
AcknowledgmentsThis work was financial supported by National Natural Science Foundation of China (Nos. 21372079, 21472046), Shanghai Pujiang Program (No. 14PJD012).
| [1] | K. Wang, X. Qian, J. Cui. ChemInform abstract:design, synthesis, and bioactivity of cyanonitrovinyl neonicotinoids as potential insecticides. Cheminform 42 (2011) 1117–1122. |
| [2] | L. Furlan, D. Kreutzweiser. Alternatives to neonicotinoid insecticides for pest control:case studies in agriculture and forestry. Environ. Sci. Pollut. Res. 22 (2015) 135–147. DOI:10.1007/s11356-014-3628-7 |
| [3] | R. Nauen, I. Denholm. Resistance of insect pests to neonicotinoid insecticides:Current status and future prospects. Arch. Insect Biochem. Physiol. 58 (2005) 200–215. DOI:10.1002/(ISSN)1520-6327 |
| [4] | X. Liu, X. Wu, Z. Long, et al., Photodegradation of imidacloprid in aqueous solution by metal-free catalyst graphitic carbon nitride using an energy-saving lamp. J. Agri. Food. Chem. 19 (2015) 4754–4760. |
| [5] | Z. Li, X. Shao, F. Sun, F. Zhu. One-Pot, Three-component synthesis of 1, 8-naphthyridine derivatives from heterocyclic ketene aminals, malononitrile dimer, and aryl aldehydes. Synlett 26 (2015) 2306–2312. DOI:10.1055/s-00000083 |
| [6] | L. Fu, X. Feng, J.J. Wang, et al., Efficient synthesis and evaluation of antitumor activities of novel functionalized 18-naphthyridine derivatives. Acs. Comb. Sci. 17 (2015) 24–31. DOI:10.1021/co500120b |
| [7] | E. Jeanneau, - Nicolle, M. Benoit, - Guyod, A. Namil, G. Leclerc. New thiazolo[32-a] pyrimidine derivatives, synthesis and structure-activity relationships. Eur. J. Med. Chem. 27 (1992) 115–120. DOI:10.1016/0223-5234(92)90099-M |
| [8] | Y. Tsuzuki, K. Tomita, K. Shibamori, et al., Synthesis and structure-activity relationships of novel 7-substituted 14-dihydro-4-oxo-1-(2-thiazolyl)-1. 8-naphthyridine-3-carboxylic acids as antitumor agents. Part 2. J. Med. Chem. 45 (2002) 5564–5575. DOI:10.1021/jm010057b |
| [9] | L. Yang, S. Wang, D. Sun, et al., Development of a biomimetic chondroitin sulfate-modified hydrogel to enhance the metastasis of tumor cells. Sci. Rep. 6 (2016) 1–13. DOI:10.1038/s41598-016-0001-8 |
| [10] | A.A. Santilli, A.C. Scotese, J.A. Yurchenco. ChemInform Abstract:synthesis and antibacteial evaluation of 1, 2, 3, -tetrahydro-4-oxo-1, 8-naphthridine-3-carboxylic acid esters, carbonitriles, and carboxamides. J. Med. Chem. 7 (1976) 1038–1041. |
| [11] | S. Nishigaki, N. Mizushima, F. Yoneda. Synthetic antibacterials. 3. Nitrofurylvinyl-18-naphthyridine derivatives. J. Med. Chem. 14 (1971) 638–640. |
| [12] | S.B. Singh, D.E. Kaelin, P.T. Meinke, et al., Structure activity relationship of C-2 ether substituted 15-naphthyridine analogs of oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part-5). Bioorg. Med. Chem. Lett. 17 (2015) 3630–3635. |
| [13] | L.Z. Gao, Y.S. Xie, T. Li, W.L. Huang, G.Q. Hu. Synthesis and antibacterial activity of novel[12, 4]triazolo[3, 4-h] [1, 8]naphthyridine-7-carboxylic acid derivatives. Chin. Chem. Lett. 26 (2014) 149–151. |
| [14] | B. Li, J.R. Harjani, N.S. Cormier, et al., Besting vitamin E:Sidechain substitution is key to the reactivity of naphthyridinol antioxidants in lipid bilayers. J. Am. Chem. Soc. 135 (2013) 1394–1405. DOI:10.1021/ja309153x |
| [15] | T. Kuroda, F. Suzuki, T. Tamura, K. Ohmori, H. Hosoe. A novel synthesis and potent antiinflammatory activity of 4-hydroxy-2(1H)-oxo-1-phenyl-18-naphthyridine-3-carboxamides. J. Med. Chem. 35 (1992) 1130–1136. DOI:10.1021/jm00084a019 |
| [16] | S. Bekkering, B.A. Blok, L.A. Joosten, et al., In vitro experimental model of trained innate immunity in human primary monocytes, Clin. Vaccine Immunol. 12(23(12)) (2016) 349-16. |
| [17] | C. Manera, A.M. Malfitano, T. Parkkari, V. Lucchesi, S. Carpi, et al., New quinolone-and 1, 8-naphthyridine-3-carboxamides as selective CB2 receptor agonists with anticancer and immuno modulatory activity. Eur. J. Med. Chem. 97 (2015) 10–18. DOI:10.1016/j.ejmech.2015.04.034 |
| [18] | T.G. Nam, C.L. Rector, H.Y. Kim, et al., Tetrahydro-18-naphthyridinol analogues of alpha-tocopherol as antioxidants in lipid membranes and low-density lipoproteins. J. Am. Chem. Soc. 129 (2007) 10211–10219. DOI:10.1021/ja072371m |
| [19] | E.J. Barreiro, C.A. Camara, H. Verli, et al., Design, synthesis, and pharmacological profile of novel fused pyrazolo[4, 3-d]pyridine and pyrazolo[3, 4-b] [1, 8]naphthyridine isosteres:a new class of potent and selective acetylcholinesterase inhibitors. J. Med. Chem. 46 (2003) 1144–1152. DOI:10.1021/jm020391n |
| [20] | L.R.C. De, J. Egea, J. Marco-Contelles, et al., Synthesis, inhibitory activity of cholinesterases, and neuroprotective profile of novel 1, 8-naphthyridine derivatives. J. Med. Chem. 53 (2010) 5129–5143. DOI:10.1021/jm901902w |
| [21] | Q. You, Z. Li, C. Huang, et al., Discovery of a novel series of quinolone and naphthyridine derivatives as potential topoisomerase i inhibitors by scaffold modification. J. Med. Chem. 52 (2009) 5649–5661. DOI:10.1021/jm900469e |
| [22] | A.K. Dhar, R. Mahesh, A. Jindal, T. Devadoss, S. Bhatt. Design, synthesis, and pharmacological evaluation of novel 2-(4-substituted piperazin-1-yl)1, 8 naphthyridine 3-carboxylic acids as 5-ht 3 receptor antagonists for the management of depression. Chem. Biol. Drug Des. 84 (2014) 721–731. DOI:10.1111/cbdd.12370 |
| [23] | P.L.F. Laura Betti, C. Tiziana, G. Gino, et al., study on affinity profile toward native human and bovine adenosine receptors of a series of 18-naphthyridine derivatives. J. Med. Chem. 47 (2004) 3019–3031. DOI:10.1021/jm030977p |
| [24] | F.W. Hartner, Y. Hsiao, K.K. Eng, et al., Methods for the synthesis of 5, 6, 7, 8-tetrahydro-1, 8-naphthyridine fragments for avb3 integrin antagonists. J. Org. Chem. 69 (2004) 8723–8730. DOI:10.1021/jo0486950 |
| [25] | P.L. Ferrarini, C. Mori, C. Manera, et al., A novel class of highly potent and selective A1 adenosine antagonists:structure-affinity profile of a series of 18-naphthyridine derivatives. J. Med. Chem. 43 (2000) 2814–2823. DOI:10.1021/jm990321p |
| [26] | M. Mohan, G.T. Gujar. Local variation in susceptibility of the diamondback moth, Plutella xylostella (Linnaeus) to insecticides and role of detoxification enzymes. Crop Prot. 22 (2003) 495–504. DOI:10.1016/S0261-2194(02)00201-6 |
| [27] | H. Tang, P. Ji. Using the statistical program r instead of spss to analyze data. ACS Symp. Ser. 1166 (2015) 135–151. |
| [28] | S.H. Chae, S.I. Kim, S.H. Yeon, S.W. Lee, Y.J. Ahn. Adulticidal activity of phthalides identified in cnidium officinale rhizome to b-and q-biotypes of bemisia tabaci. J. Agric. Food. Chem. 59 (2011) 8193–8198. DOI:10.1021/jf201927t |
 2017, Vol. 28
2017, Vol. 28