b College of Chemistry, Beijing Normal University, Beijing 100875, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
There has been a burgeoning interest in the development of artificial receptors for anions because anions play the critical roles in biological and chemical events [1-7]. A variety of anion receptors have been developed. Among them, foldamer-based receptors are especially attractive due to their helical structures mimicking that of proteins [8, 9]. For foldamer receptors, encapsulation of anion is typically achieved by helical folding of oligomer induced by the interaction between oligomer and anion [10, 11]. This binding style provides foldamer receptor an advantage over rigid ones in binding various anions with different shapes and geometries but, on the other hand, make the binding behavior more challenging due to the larger conformational entropy penalty that the foldamers pay on complexation [12-14]. In the past decades, towards a better understanding of the corresponding anion binding, much effort has been devoted to establishing the structure-activity relationships of receptors based on foldamer. In these contexts, a wide variety of H-bond donors [15-17] for anion binding have been examined; effects of important structural factors on anion-binding, including size and shape of the binding cavity [18-21], length of backbone [22, 23], and conformational preorganization [24, 25], have been also extensively studied. In contrast, the influence of terminal substituents on the anion binding capability for foldamer based receptors has not been systematically revealed yet, although such influence has been widely observed in a number of foldamer systems [26, 27].
We [28-36] and others [37-40] have previously demonstrated that aryl-1, 2, 3-triazole foldamers can act as appropriate hosts for halide anion recognition. Besides, when amide H-bond donor was further incorporated into the main chain, enhanced affinities of the resultant oligo(aryl-triazole-amide)s foldamers for halide anions were obtained. Terminal substituents on the oligomers clearly affect the anion binding, as evidenced by the observation that initially increasing the chain length of short aryl-triazole-amide backbone gave rise to a decrease in anion binding capabilities [30]. We thus envisioned that aryl-triazole and aryl-triazole-amide oligomers are good platforms to study the influence of terminal substituent on anion binding.
In this contribution, we report the anion-binding properties of aromatic triazole foldamer and its amide derivatives 1-4 (Fig. 1). The molecules were designed to possess an identical oligo(aryltriazole)s core but different terminal substituents: while receptor 1 has two methyl ester groups on the termini, receptors 2-4 equip with two N-butyl, N-benzyl, and N-pyrenylmethyl benzamide groups, respectively. A 2-(2-methoxyethoxy)ethoxy side chain was introduced at each phenyl group to assure good solubility of the receptors in polar solvents. The synthetic route was depicted in Scheme 1.

|
Download:
|
| Fig. 1. Receptors based on foldamer 1-4 studied herein. | |

|
Download:
|
| Scheme1. Synthetic route to receptors 1-4. | |
2. Results and discussion
The anion-binding properties of receptors 1-4 were first investigated in CDCl3 by 1H NMR spectroscopy. However, variable-concentration (VC) 1H NMR experiments (Figs. S2-S5 in Supporting information) revealed that all the receptors except 1 aggregated obviously in CDCl3. Such aggregations preclude the assessment of the anion-binding affinities for the receptors in CDCl3. To alleviate these aggregations, several commercial available deuterated solvents were tested, and the mixture of DMSO-d6 and CDCl3 (v/v = 15/85) was finally chosen for the titration experiments. In such solvent mixture, aggregations of the receptors are negligible, as indicated by results of the VC 1H NMR experiments (Figs. S6-S9 in Supporting information). Spherical halide anions, including chloride, bromide, and iodide anions, were selected as the guest anions for the anion binding studies.
Despite DFT (UB3LYP/3-21G) [41] calculations predicted that receptor 1 could complex one chloride anion with a folded conformation in the gas phase (Fig. S1 in Supporting information), addition of tetrabutylammonium salt of chloride anion to a 1.0 mmol/L solution of 1 in 15/85 (v/v) DMSO-d6/CDCl3 gave no noticeable changes in chemical shifts of the triazole Ha and aromatic proton Hb, indicating no complexation takes place (Fig. S10 in Supporting information). Similar phenomena were also observed in the cases of bromide (Fig. S11 in Supporting information) and iodide anions (Fig. S12 in Supporting information). The weak anion-binding capability of 1 under the studied conditions is attributed to inherently weak 1, 2, 3-triazole H-bond donors, steric hindrance of the terminal methyl benzoate groups and the influence of DMSO in the solvent mixture.
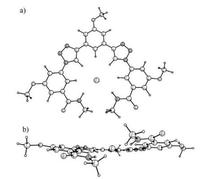
We anticipated that receptor 2, bearing two moderate triazole H-bond donors and two strong amide NH donors, would possess much higher anion affinities compared to that of 1. The simplified structure of the chloride complex of 2 in the gas phase (Fig. 2), wherein all PEG and two terminal n-butyl groups were replaced with methyl ones, was studied at UB3LYP/3-21G level of theory. The results showed that the optimized geometry of the receptor in the complex adopted a folded conformation to complex one chloride anion in the inner cavity. In the complex, the chloride anion was bound to 2 through H-bonding with two triazole groups, two amide NH, and three aryl protons. Notably, H-bonding between chloride and amide NH can help to stabilize the helical conformation of the receptor thus being able to reduce the influence of steric hindrance of the terminal benzoyl groups on anion-binding.
|
Download:
|
| Fig. 2. Energy-minimized structure of 2·Cl- (RB3LYP/3-21G, gas phase). a) top view; b) side view. To reduce the computational costs, all PEG and terminal n-butyl units were replaced with methyl ones. | |
The 1H NMR titration spectra of receptor 2 with chloride anion as shown in Fig. 3 are representative of the features observed for the studied halide anions. As expected, addition of chloride anions to the solution of receptor 2 produced moderate downfield shifts for the triazole proton Ha, central phenylene proton Hb and the amide proton Hc (Figs. 3 and 4), indicating that these protons primarily serve in binding the anions. Titrations of bromide and iodide anions gave rise to similar, but stronger, downshifts of Ha, Hb, and Hc. Fitting analysis of the binding curves in a 1:1 binding model yielded the association constants (Ka) of 90 ± 2.0, 153 ±3.84 and 142 ± 3.86 L/mol for chloride, bromide and iodide anions, respectively (Table 1). The results exhibited that the binding of 2 to bromide and iodide anions are slightly stronger than that to the chloride ones. This is reasonable because the receptor 2 may possess a comparatively large cavity, which is more suitable for hosting bromide or iodide anions. Meanwhile, chlorides are more strongly solvated than that of bromides and iodides due to the presence of the highly competitive solvent DMSO, resulting in the weakest binding with the receptor compared to that of bromide and iodide anions.

|
Download:
|
| Fig. 3. Changes in the 1H NMR spectrum (400 MHz, DMSO-d6/CDCl3 = 15:85 (v/v)) of receptor 2 ([2] = 1 mmol/L) with the addition of TBACl at 298 K. | |

|
Download:
|
| Fig. 4. Change in 1H chemical shift of partial proton signals of receptor 2 in 15:85 (v/ v) DMSO-d6/CDCl3 upon increasing the concentration of chloride anions at 298 K. [2] = 1 mmol/L. | |
|
|
Table 1 Binding constants Ka (L/mol) of receptors 1-4 with various halide at room temperature in 15:85 (v/v) DMSO-d6/CDCl3.a |
Basically, the binding affinities of receptor 2 for studied anions have been greatly enhanced compared with those of receptor 1. This impressive enhancement confirms the role of the additional two amide NH donors, highlighting the requirement of the incorporation of strong H-bond donors at the termini of main backbone to facilitate anion recognition for a foldamer receptor.
1H NMR titration experiments for receptors 3 and 4 also displayed continuous downfield shifts of all the protons those are supposed to be located at the inner cavities (Figs. S20-S37 in Supporting information), indicating that simple 1:1 complexes of these two receptors to the anions were formed in these cases. As expected, the titration curves fit quite well to a 1:1 binding model in all the studied cases. The results are summarized in Table 1.
Comparing the binding affinities of 3 and 4 for halide anions to those of 2, it is clear that there is a strong influence of terminal substituents on the anion binding of the foldamer receptors. The benzyl terminal group incorporated in receptor 3 possesses a large steric hindrance compared to the n-butyl ones that in 2, resulting in a slight decrease in the binding affinities for halide anions. Interestingly, despite pyrenylmethyl groups have an even larger steric hindrance in comparison with that of benzyl ones, receptor 4 bearing two pyrenylmethyl terminal groups has even higher affinities for halide anions than those of receptor 2. The enhanced binding ability of 4 could attribute to the strong π-π stacking interaction between two terminal pyrenylmethyl groups, which is capable of stabilizing the folded conformation of the aryl-triazoleamide oligomers thus reducing the conformational entropy penalty that the receptor should pay on complexation.
On the other hand, in each studied cases of 2-4, the binding constants of the receptor for bromide and iodide anion are almost same, but are a little larger than that for chloride one. For example, the binding constant of 4 for bromide and iodide anions is about 180 L/mol, which is larger that of chloride (93 L/mol). This phenomenon indicates that the terminal substituents do not significantly influence the binding selectivity of the foldamer receptor. This is understandable, considering that anion-binding selectivity of receptors mainly stems from the size and shape of its inner cavity as well as the binding environment, and that, in the studied cases, different terminal substituents would not give rise to much change in the size and shape of the cavity of the oligo(aryltriazoleamide)s foldamers.
3. ConclusionIn summary, we have demonstrated that incorporating amide Hbond donors at the terminal of a short oligo(aryltriazole)s foldamer can greatly enhance the anion-binding ability of the foldamer receptor. The terminal substituent groups have a considerable influence on the binding affinities of the resultant oligo(aryltriazoleamide)s foldamer receptors forhalide anions. The steric hindrance of the terminal substituents was found to reduce the binding affinities of foldamer receptors for halide anions. On the contrary, π-π stacking interaction between the terminal substituents gives rise to an enhancement in the binding strengths. In our studied cases, the terminal substituents do not significantly influence the binding selectivity of the foldamer receptor. These results are of great significance for facilitating the future rational design and fabrication of novel aryl-triazole foldamers for anions binding.
4. ExperimentalSynthetic routes to foldamer receptors 1-4 are outlined in Scheme 1. The key issue in these approaches is Huisgen copper-catalyzed azide-alkyne cycloaddition (CuAAC) ("click" reaction) [43, 44]. Initially, the starting material 5, which was prepared according to the method we previously reported [30], was PEGlated to give 6 in 91% yield. The methyl ester group on compound 6 was hydrolyzed to provide the corresponding acid 7 in a yield of 87%. Amidation of 7 with n-butylamine, benzylamine, and 1-pyrenemethanamine afforded the corresponding amide compounds 8, 9, and 10, respectively. Finally, dialkyne compound 11 [45] was clicked with the azide compounds 6, 8, 9, and 10 to give the target oligomers 1-4 in a yield of 73%, 73%, 67%, 62%, respectively. The structures of 1-4 were fully characterized by 1H NMR and 13C NMR spectroscopy as well as the high-resolution mass spectrometry (see the Supporting information).
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21332008 and 21472015).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.06.006.
| [1] | P.D. Beer, P.A. Gale. Anion recognition and sensing:the state of the art and future perspectives. Angew. Chem. Int. Ed. 40 (2001) 486–516. DOI:10.1002/1521-3773(20010202)40:3<>1.0.CO;2-A |
| [2] | J.L. Sessler, S. Camiolo, P.A. Gale. Pyrrolic and polypyrrolic anion binding agents. Coord. Chem. Rev. 240 (2003) 17–55. DOI:10.1016/S0010-8545(03)00023-7 |
| [3] | V. Amendola, D. Esteban-Gmez, L. Fabbrizzi, M. Licchelli. What anions do to NH-containing receptors. Acc. Chem. Res. 39 (2006) 343–353. DOI:10.1021/ar050195l |
| [4] | C. Caltagirone, P.A. Gale. Anion receptor chemistry:highlights from 2007. Chem. Soc. Rev. 38 (2009) 520–563. DOI:10.1039/B806422A |
| [5] | S. Kubik. Amino acid containing anion receptors. Chem. Soc. Rev. 38 (2009) 585–605. DOI:10.1039/B810531F |
| [6] | M. Wenzel, J.R. Hiscock, P.A. Gale. Anion receptor chemistry:highlights from 2010. Chem. Soc. Rev. 41 (2012) 480–520. DOI:10.1039/C1CS15257B |
| [7] | N.H. Evans, P.D. Beer. Advances in anion supramolecular chemistry:from recognition to chemical applications. Angew. Chem. Int. Ed. 53 (2014) 11716–11754. DOI:10.1002/anie.201309937 |
| [8] | D.J. Hill, M.J. Mio, R.B. Prince, T.S. Hughes, J.S. Moore. A field guide to foldamers. Chem. Rev 101 (2001) 3893–4011. DOI:10.1021/cr990120t |
| [9] | S. Hecht, I. Huc, Foldamers: Structures, Properties, and Applications, WileyVCH, Weinheim, 2007. |
| [10] | H. Juwarker, J.M. Suk, K.S. Jeong. Foldamers with helical cavities for binding complementary guests. Chem. Soc. Rev. 38 (2009) 3316–3325. DOI:10.1039/b909034g |
| [11] | H. Juwarker, K.S. Jeong. Anion-controlled foldamers. Chem. Soc. Rev 39 (2010) 3664–3674. DOI:10.1039/b926162c |
| [12] | K.J. Chang, D. Moon, M.S. Lah, K.S. Jeong. Indole-based macrocycles as a class of receptors for anions. Angew. Chem. Int. Ed. 44 (2005) 7926–7929. DOI:10.1002/(ISSN)1521-3773 |
| [13] | K.J. Chang, B.N. Kang, M.H. Lee, K.S. Jeong. Oligoindole-based foldamers with a helical conformation induced by chloride. J. Am. Chem. Soc. 127 (2005) 12214–12215. DOI:10.1021/ja0547984 |
| [14] | H. Juwarker, J.M. Lenhardt, D.M. Pham, S.L. Craig. 1, 2, 3-Triazole CH…Cl contacts guide anion binding and concomitant folding in 1, 4-diaryl triazole oligomers. Angew. Chem. Int. Ed. 47 (2008) 3740–3743. DOI:10.1002/(ISSN)1521-3773 |
| [15] | V. Amendola, L. Fabbrizzi, L. Mosca. Anion recognition by hydrogen bonding:urea-based receptors. Chem. Soc. Rev. 39 (2010) 3889–3915. DOI:10.1039/b822552b |
| [16] | C.R. Bondy, S.J. Loeb. Amide based receptors for anions. Coord. Chem. Rev. 240 (2003) 77–99. DOI:10.1016/S0010-8545(02)00304-1 |
| [17] | D.W. Zhang, X. Zhao, J.L. Hou, Z.T. Li. Aromatic amide foldamers:structures, properties, and functions. Chem. Rev. 112 (2012) 5271–5316. DOI:10.1021/cr300116k |
| [18] | M.J. Chmielewski, M. Charon, J. Jurczak. 1, 8-Diamino-3, 6-dichlorocarbazole:a promising building block for anion receptors. Org. Lett. 6 (2004) 3501–3504. DOI:10.1021/ol048661e |
| [19] | V.S. Bryantsev, B.P. Hay. Are C-H groups significant hydrogen bonding sites in anion Receptors? Benzene complexes with Cl- NO3-, and ClO4-. J. Am. Chem. Soc. 127 (2005) 8282–8283. DOI:10.1021/ja0518272 |
| [20] | G.W. Bates, ${referAuthorVo.mingEn} Triyanti, M.E. Light, M. Albrecht, P.A. Gale. 2, 7-Functionalized indoles as receptors for anions. J. Org. Chem. 72 (2007) 8921–8927. DOI:10.1021/jo701702p |
| [21] | G.W. Bates, P.A. Gale, M.E. Light. Isophthalamides and 2, 6-dicarboxamidopyridines with pendant indole groups:a 'twisted' binding mode for selective fluoride recognition. Chem. Commun. 21 (2007) 2121–2123. |
| [22] | U.I. Kim, J.M. Suk, V.R. Naidu, K.S. Jeong. Folding and anion-binding properties of fluorescent oligoindole foldamers. Chem. Eur. J. 14 (2008) 11406–11414. DOI:10.1002/chem.200801713 |
| [23] | D.W. Zhang, X. Zhao, Z.T. Li. Aromatic amide and hydrazide foldamer-based responsive host-guest Systems. Acc. Chem. Res. 47 (2014) 1961–1970. DOI:10.1021/ar5000242 |
| [24] | S. Lee, Y. Hua, H. Park, A.H. Flood. Intramolecular hydrogen bonds preorganize an aryl-triazole receptor into a crescent for chloride binding. Org. Lett. 12 (2010) 2100–2102. DOI:10.1021/ol1005856 |
| [25] | S. Lee, Y. Hua, A.H. Flood. b-Sheet-like hydrogen bonds interlock the helical turns of a photoswitchable foldamer to enhance the binding and release of chloride. J. Org. Chem. 79 (2014) 8383–8396. DOI:10.1021/jo501595k |
| [26] | V. Haridas, S. Sahu, P.P. Praveen Kumar. Triazole-based chromogenic and nonchromogenic receptors for halides. Tetrahedron Lett. 52 (2011) 6930–6934. DOI:10.1016/j.tetlet.2011.10.066 |
| [27] | V. Haridas, S. Sahu, P. Venugopalan. Halide binding and self-assembling behavior of triazole-based acyclic and cyclic molecules. Tetrahedron 67 (2011) 727–733. DOI:10.1016/j.tet.2010.11.078 |
| [28] | Y. Wang, F. Li, Y. Han, F.Y. Wang, H. Jiang. Folding and aggregation of cationic oligo(aryl-triazole)s in aqueous solution. Chem. Eur. J. 15 (2009) 9424–9433. DOI:10.1002/chem.v15:37 |
| [29] | Y. Wang, F.S. Bie, H. Jiang. Controlling binding affinities for anions by a photoswitchable foldamer. Org. Lett. 12 (2010) 3630–3633. DOI:10.1021/ol1014043 |
| [30] | Y. Wang, J. Xiang, H. Jiang. Halide-guided oligo(aryl-triazole-amide)s foldamers:receptors for multiple halide ions. Chem. Eur. J. 17 (2011) 613–619. DOI:10.1002/chem.201001560 |
| [31] | J. Shang, N.M. Gallagher, F.S. Bie, et al., Aromatic triazole foldamers induced by C-H…X (X=F, Cl) intramolecular hydrogen bonding. J. Org. Chem. 79 (2014) 5134–5144. DOI:10.1021/jo500582c |
| [32] | J. Shang, W. Si, W. Zhao, et al., Preorganized aryltriazole foldamers as effective transmembrane transporters for chloride anion. Org. Lett. 16 (2014) 4008–4011. DOI:10.1021/ol501772v |
| [33] | W. Zhao, Y. Wang, J. Shang, Y.K. Che, H. Jiang. Acid/base-mediated uptake and release of halide anions with a preorganized aryl-triazole foldamer. Chem. Eur. J. 21 (2015) 7731–7735. DOI:10.1002/chem.v21.21 |
| [34] | W. Zhao, F. Huang, Y. Wang, et al., Aryl-triazole foldamers with ethynyl spacers as effective receptors for halides and oxyanions. Tetrahedron Lett. 15 (2016) 1691–1694. |
| [35] | J. Shang, W. Zhao, X.C. Li, Y. Wang, H. Jiang. Aryl-triazole foldamers incorporating a pyridinium motif for halide anion binding in aqueous media. Chem. Commun. 52 (2016) 4505–4508. DOI:10.1039/C5CC10422J |
| [36] | Y. Wang, W. Zhao, F.S. Bie, et al., Ruthenium(Ⅱ) complexes of aryl triazole foldamers as receptors for anions. Chem. Eur. J. 22 (2016) 5233–5242. DOI:10.1002/chem.201504910 |
| [37] | H. Juwarker, J.M. Lenhardt, D.M. Pham, S.L. Craig. 1, 23-Triazole CH…Cl contacts guide anion binding and concomitant folding in 1, 4-diaryl triazole oligomers. Angew. Chem. Int. Ed. 47 (2008) 3740–3743. DOI:10.1002/(ISSN)1521-3773 |
| [38] | I. Saraogi, A.D. Hamilton. Recent advances in the development of aryl-based foldamers. Chem. Soc. Rev. 38 (2009) 1726–1743. DOI:10.1039/b819597h |
| [39] | Y. Hua, A.H. Flood. Click chemistry generates privileged CH hydrogen-bonding triazoles:the latest addition to anion supramolecular chemistry. Chem. Soc. Rev. 39 (2010) 1262–1271. DOI:10.1039/b818033b |
| [40] | B. Qiao, A. Sengupta, Y. Liu, et al., Electrostatic and allosteric cooperativity in ion-pair binding:a quantitative and coupled experiment-theory study with aryl-triazole-ether Macrocycles. J. Am. Chem. Soc. 137 (2015) 9746–9757. DOI:10.1021/jacs.5b05839 |
| [41] | M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al. , Gaussian 03, Revision D. 02, Gaussian Inc. , Pittsburgh, PA, 2004. |
| [42] | M.J. Hynes. EQNMR:a computer program for the calculation of stability constants from nuclear magnetic resonance chemical shift data. J. Chem. Soc. Dalton Trans (1993) 311–312. |
| [43] | V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless. A stepwise Huisgen cycloaddition process:copper(Ⅰ)-catalyzed regioselective Ligation of azides and terminal alkynes. Angew. Chem. 114 (2002) 2708–2711. DOI:10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 |
| [44] | C.W. Torn, C. Christensen, M. Meldal. Peptidotriazoles on solid phase:[12, 3]-triazoles by regiospecific copper(Ⅰ)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67 (2002) 3057–3064. DOI:10.1021/jo011148j |
| [45] | Y. Li, A.H. Flood. trong, size-selective, and electronically tunable C-H… halide binding with steric control over aggregation from synthetically modular, shape-persistent [з4] triazolophanes. J. Am. Chem. Soc. 130 (2008) 12111–12122. DOI:10.1021/ja803341y |
 2017, Vol. 28
2017, Vol. 28