b School of Life Science, Beijing Institute of Technology, Beijing 100081, China
In the field of analytical chemistry, especially in bioanalysis, the complex sample system requests an effective separation technique. Compared with gas and liquid chromatography, capillary electrophoresis (CE) is increasingly used in routine analysis due to its rapid separation speed, high resolving power, small sample volume consumption and flexibility in choosing separation modes. However, the limitations of CE are also obvious such as its low sensitivity caused by the nanoliter sample introduction. Coupling CE with mass spectrometry (MS) will significantly improve the limitation of detection. Furthermore, MS can provide the mass to charge ratio and structural information of an ion.
Several issues need to be overcome when combining CE with MS, specifically the interfacing techniques and the selection of a proper separation methodology. A series of review articles on similar topics were given by Kleparnik [1, 2]. Commercially available interfaces, such as electrospray ionization (ESI) and inductively coupled plasma (ICP) interfaces, are widely used in CEMS systems nowadays. The matrix assisted laser desorption ionization (MALDI) interface usually combines CE-MS with an off-line mode, and some other ambient ionization interfaces have also been reported in recent years. The use of microfluidic devices makes a breakthrough as an interface of CE-MS, and a number of CE separation modes have been successfully combined with various mass spectrometers, such as capillary zone electrophoresis (CZE), capillary gel electrophoresis (CGE), capillary isotachophoresis (CITP), capillary isoeletric focusing (CIEF), capillary electronchro-matography (CEC), micellar electrokinetic chromatography (MEKC), nonaqueous capillary electrophoresis (NACE), etc. With unique features (such as mixture, reaction, and separation), the procedure of sample injection, treatment, separation, and ionization can be integrated on a chip. Another trend is the improvement of sampling and injection method, which is closely related with analysis objectives. An efficient, micro-volume, robotized sample injection method is suitable for single cell analysis, while a high throughput and repeatability sample injection method is necessary for the bio-omics analysis and rapid detection. Online sample treatment and enrichment, coating technologies are increasingly applied in CE-MS systems. Applications of CE-MS have been ranging from small inorganic ions to complex biomolecules. A considerable amount of reviews were published on bio-omics research [3-5] (such as proteomics [6] and metabolomics [7-10]), biomarker [11, 12] and clinical [12-15] applications. Thus applications of CE-MS will not be emphasized in this paper.
This paper focuses on the recent instrumentation and methodology developments of CE-MS systems. More than 1000 hits were retrieved by the keyword "CE-MS" in Web of Science in years between 2013 and 2017; and papers were selected; which covers the topic of injection modes; ionization interfaces and CE separation modes. The latest analytical applications were briefly introduced and classified based on the usage of different mass spectrometers.
2. Development of capillary electrophoresis-mass spectrometry interfacing techniques 2.1. Sampling/injection interfacesAn efficient and reproducible sample injection interface is the precondition ensuring the performance of CE separation. Two main driving modes of CE injection are electrokinetic and hydrodynamic injection. In order to improve the quantitation accuracy, sensitivity and resolution of CE separation, a number of injection methods were applied in CE-MS systems and some new injection interfaces were also developed.
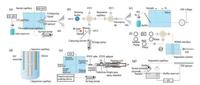
Electrokinetic sample injection is one of the most widely used injection modes in CE-MS. Both electrophoretic migration of charged sample ions and electroosmotic flow of the sample solution provide the injection driving force while the sample charge discrimination is inevitable. Device improvements are usually focusing on target analyte enrichment. Wang et al. proposed a preconcentration approach for CE-MS using the counterflow-assisted electrokinetic injection [16]. The interface assembled by a polydimethylsiloxane (PDMS) microfluidic device, which provided liquid-film electrical conduction as shown in Fig. 1a. A hydrodynamic counterflow was introduced into the separation capillary to retard the stacking boundary and achieve a long injection time. Using the dynamic coated technique, system sensitivity was improved by 750-1480 for peptide samples. D'Ulivo et al. expanded the applicability of pressure-assisted electrokinetic injection (PAEKI) to online pre-concentrated positively charged analytes [17]. L-Arginine, L-lysine, and imidazole were analyzed by CZE-Q-TOF and a limit of detection (LOD) of 18-28 pg/mL was obtained. Park et al. developed another sampling and injection interface, which couples ambient pulsed mid-IR laser into the CE-ESI-MS system (as shown in Fig. 1b) [18]. Samples were ablated by the IR laser and captured instantly in the sampling solvent, then injected into a separation capillary by electrokinetic injection. A mixture of peptide and protein standards was separated and analyzed by this system. The author suggested that the interface has potential applications in MS imaging as well as rapid sample analysis.
|
Download:
|
| Fig. 1. Injection interface designs. (a) the counterflow-assisted electrokinetic injection device schematic diagram reported in ref [16]; (b) ambient laser ablation sampling device schematic diagram reported in ref [18]; (c) pneumatic microvalve-based hydrodynamic injection device schematic diagram reported in ref [20]; (d) capillary batch injection device schematic diagram reported in ref [24]; (e) field-amplified sample injection microchip electrophoresis device schematic diagram reported in ref [26]; (f) field-amplified sample-stacking device schematic diagram reported in ref [28]; (g) electrochemically assisted injection device schematic diagram reported in ref [32]. | |
Hydrodynamic sample injection could be classified by the driving force, such as gravity flow, pressure and vacuum injection. With hydrodynamic sample injection, sample charge discrimination could be eliminated. Under automatic operations, sample injection volume can be accurately controlled with high repeatability. Wang et al. reported a hydrodynamic flow assisted double junction CE-MS interface to alleviate signal suppression resulting from nonvolatile positive ion additives [19]. A 20-fold signal enhancement for triazines was obtained. Kelly et al. introduced a pneumatic microvalve-based hydrodynamic sample injection system to achieve high-throughput and quantitative [20]. A three-layer PDMS microchip was used to achieve high injection repeatability (Fig. 1c), and a mixture of peptide standards was tested by the CE-nano-ESI-QqQ-MS. Similar effects have been achieved by Kuehnbaum and his coworkers, in which they proposed a multi-segment injection (MSI) technique based on the hydrodynamic injection [21, 22]. Injection of discrete sample segments in a series can be achieved within a single capillary. Detected by TOF-MS, high throughput metabolic analysis was performed. To minimize the sample injection volume, Matysik et al. demonstrated the concept of capillary batch injection (CBI) [23]. Consisting of a microcontroller, a micropump and microsyringes, the improved CBI cell (as illustrated in Fig. 1d) could remove an ultra-small sample volume (about 500 pL) into the separation capillaries with an efficiency of ~100% [24]. The cyclic nucleotide (cGMP) in human urine samples was detected by the CBI-CE-TOF-MS.
Field amplified sample injection (FASI) is a traditional on-line preconcentration technique in CE field. When applying a high voltage, a large amount of sample ions in the lower ionic strength buffer will transfer into the higher ionic strength buffer filled in the separation capillary. Martinez-Villalba et al. used the FASI-CZE-ITMS equipment to analyze the amprolium residues in egg samples and a LOD of 75 μg/kg was achieved with optimized parameters [25]. Cheng et al. developed a microchip electrophoresis-inductively coupled plasma-mass spectrometry (MCE-ICP-MS) system coupled with the FASI technique to analyze the bromine speciation in bread samples (as shown in Fig. 1e) [26]. A three-step experimental procedure was optimized to achieve sample enrichment (more than 12 times) and rapid separation (35 s). Similar techniques such as field-amplified stacking, field-amplified sweeping, and transient isotachophoresis (tITP) can enhance sample loading volume as well as detection sensitivity. He et al. reported field-amplified sample-stacking (FASS) method combined with CE-ESI-MS to on-line enrich and detect four beta(2)-agonists in human urine samples [27]. Hung et al. proposed a strategy based on FASS-CE-ESI-MS/MS to analyze haloacetic acids (HAAs) in tap water samples [28], as shown in Fig. 1f. Four HAAs were detected at ppb level with 300-to 1400-fold signal improvements. Ito et al. utilized the high-sensitivity CE-ESI-QTOF to characterize four kinds of pyridylaminated (PA) oligosaccharides [29]. A LOD of 25 amol/μL was obtained by using the head-column field amplified sample stacking (HC-FASS), which is a sheathless ionization interface and narrow mass range repeated high-speed switching technique. Wuethrich et al. reported an approach of field-enhanced sample injection-micelle-to-solvent stacking to improve sensitivity of CZE-ESI-MS, and eight penicillins and sulfonamides were detected with LODs from 0.11 to 0.55 ng/mL [30].
Electrochemically assisted injection (EAI) is a new sample injection concept for CE, which enables neutral analytes being charged by an electrochemical conversion during the injection process. Matysik's group developed a fully automated EAI injection device to enhance the performance of CE-MS, and applied to study the reduction of 4-nitrotoluene (4-NT) [31]. Three different carbon-based screen-printed electrodes (SPEs) were compared. In a later research, the fully automated EAI-CE-MS system was used to simulate the oxidative stress and DNA mutationsand (Fig. 1g) [32]. The oxidation products of targets were generated electrochemically, separated by CE, and detected by ESI-TOF-MS.
2.2. Ionization interfacesAnalytes were first transferred from liquid phase to gas phase and then ionized by an ionization interface. Some commercial interfaces are available in hybrid CE-MS instruments, such as ESI and ICP interfaces. On the other hand, new ionization interfaces are being developed for methodology improvement. Two main ionization interfaces were sheath-flow and sheathless devices. Sheath-flow interface can be divided into coaxial sheath flow and liquid junction. The performance of a CE-MS system depends largely on a robust and efficient ionization interface. Lindenburg et al. introduced the developments in interface designs for CE-MS and the applications in proteomics and metabolomics [4]. Jarvas et al. reviewed the computer modeling and simulation technologies for CE-ESI-MS interface designs [33].
As one of the most widely used CE-MS interface, the advances of (nano-)ESI were published in many articles. However, the compatibility of CE electrolytes with ESI solvent restrains the applications of ESI interfaces. Flow rate compatibility is another problem of ESI interface. Bonvin et al. reviewed the fundamental concepts and technical developments of CE-ESI-MS interfaces [34]. Some improvements in engineering also facilitate the use of CE-MS interface. Krenkova et al. reported a construction of selfaligning subatmospheric hybrid liquid junction electrospray interface for CE, which helps to eliminate the manual adjustment and improve the ion transport [35].
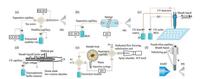
Sheath-flow interfaces have been popular since the early years of CE-MS applications. The eletrokinetically pumped nano-ESI devices reported by Dovichi's group was a typical automated sheath-flow interface. (see Section 3.1) Lindenburg et al. investigated the performance of the flow-through microvial (MV) assisted CE-MS interface for cationic metabolomics (Fig. 2a) [36]. A mixed sample comprising 45 cationic metabolites was tested. A threetime sensitivity improvement and five-time LOD reduction were obtained. Linhardt et al. demonstrated an electrokinetic pumpbased sheath-flow ESI interface [37, 38]. Reverse polarity CE separation was used to analysis of heparin oligosaccharides. Choi et al. reported a tapered-tip CE-nanoESI interface for MS [39]. A 260-zmol lower limit of detection for angiotensin Ⅱ was obtained, and 217 different protein groups were detected using 1 ng mouse cortex digest protein.
|
Download:
|
| Fig. 2. Ionization interface designs. (a) the flow-through microvial assisted interface schematic diagram reported in ref [36]; (b) the sheathless ESI interface schematic diagram reported in ref [40]; (c) MALDI interface schematic diagram reported in ref [47]; (d) ICP interface schematic diagram reported in ref [53]; (e) flow focusing nebulization ICP interface schematic diagram reported in ref [55]; (f) DART interface schematic diagram reported in ref [72]. | |
With higher sensitivity, sheathless interfaces regain popularity in CE-MS. The porous tip sheathless interfaces were commercially available now. (see Section 3.1 Jeong et al. described a high durability sheathless electrospray ionization interface based on an ionophore membrane-packed electro-conduction channel (Fig. 2b) [40]. Coupled with QqQ-MS, the interface was applied for the rapid determination of underivatized amino acids, phenylalanine and tyrosine extracted from a dried blood spot (DBS). Her's group developed a series of sheathless ESI interfaces for CE-MS [16, 41, 42]. Huang et al. constructed a two column sheathless CE-MS interface using a PDMS microdevice [41]. In a subsequent paper, Wang et al. developed a sheathless CE-MS interface using a robust PDMS membrane emitter and liquid-film electric conduction [42]. The utility of interfaces was demonstrated by the analysis of peptide mixtures. Tycova et al. explored an "interface-free" approach, where the CE-MS analysis was performed in narrow bore electrospray capillary [43, 44]. The separation capillary and electrospray tip formed one entity and shared the same high voltage. Cytochrome C digest was analyzed by the system. Kammeijer et al. used dopant enriched nitrogen gas combined with sheathless ESI for glycopeptide analysis, and 25-fold higher sensitivities were obtained [45].
MALDI interfaces have better tolerance for separation background electrolyte (BGE) and frequently coupled with CE in an offline mode. In this way, mass spectra acquisition is not limited by the instrument scan rate. Zhong et al. reviewed the recent advances in CE coupling to ESI-and MALDI-MS [46]. Investigation trends are the automatic process control and separation/ionization compatible buffer. Biacchi et al. reported the instrumental development of the automated off-line CE-UV/MALDI-MS/MS with a homemade delivery matrix system (Fig. 2c) [47]. Five intact proteins and a tryptic digest mixture were tested to evaluate the method performance. Tomalova et al. developed an method, which couples CE separation to both MALDI and substrate-assisted laser desorption inductively coupled plasma (SALD ICP) mass spectrometry by using a liquid junction and subatmospheric deposition chamber [48]. CE fractions were extracted from a separation capillary and collected on a custom-built polyethylene terephthalate glycol (PETG) target plate coated with a 10-nm gold layer. A mixture of rabbit liver metallothionein isoforms was analyzed by the system. Springer et al. presented an approach for the determination of ciprofloxacin, norfloxacin and ofloxacin in milk samples by CE-MADLI-TOF-MS [49]. Chen et al. introduced a CE-MALDI-MS system with an interface consisting of a robot to drive the separation capillary and MALDI-MS target [50]. A compatible buffer was investigated and tested on protein and peptide samples.
CE interfaced with ICP-MS has been widely used in elemental analysis and commercial CE-ICP-MS interfaces were available. Timerbaev et al. reviewed the advances of speciation analysis by CE-MALDI-MS and CE-ICP-MS [51]. Device innovations are committed to improve nebulization and transport efficiency of the interface including nebulizer and spray chamber. Jiang's group reported an interface of CE-ICP-MS for the determination of ten arsenic compounds under optimized conditions [52]. In a following research, an improved interface with a novel directinjection high-efficiency nebulizer (DIHEN) chamber was developed by Liu and coworkers (Fig. 2d) [53]. Six arsenic species and five selenium species were separated and determined within 9 min. Another method for the identification and accurate size characterization of nanoparticles (NPs) in complex media based on CE-ICP-MS was developed by the same group [54]. Kovachev et al. assembled a system for CE-ICP sample introduction with a dedicated flow focusing based nebulizer (Fig. 2e) [55]. The system was coupled to an inductively coupled plasma-optical emission spectrometer (ICP-OES) and a ICP-MS for Cr(Ⅲ) and Cr(Ⅳ) speciation. The interfaces of CE-ESI-MS and CE-ICP-MS were compared for the speciation analysis of free aluminum and aluminum fluoride complexes by Nakamoto et al. [56].
Sample pretreatment methods were also developed to improve the sensitivity and specificity of a CE-MS system. Qu et al. reported the development and optimization of CE-ICP-MS system for speciation and characterization of metallic nanoparticles in a dietary supplement [57]. In the next paper, they introduced a method for the quantification of common arsenic species in rice and rice cereal using CE-ICP-MS [58]. An enzyme-assisted waterphase microwave extraction procedure was used to increase the injection volume and enhance the signal response. Chen et al. utilized the ultrasonic-assisted extraction for the detection of trace Cr (Ⅳ), Cr(Ⅲ), and chromium (Ⅲ) picolinate (CrPic) in foods and the microwave-assisted extraction for the analysis of Pb2+, trimethyl lead chloride (TML), and triethyl lead chloride (TEL) using CE-ICP-MS [59, 60]. Yang et al. described a method for the quantification of labeled lysozyme based on the CE-ICP-MS [61]. Furthermore, several applications using CE-ICP-MS were published in the investigation for metal complexation behavior [62-64], metallomics for drug development [65-68], food safety [69, 70], etc.
To improve the compatibility of CE separation modes, some ambient ionization techniques were applied in CE-MS. Cheng et al. used the atmospheric pressure chemical ionization (APCI) interface to connect the online pre-concentration CEC and MS [71]. This interface has high compatibility with non-volatile BGEs, and is more suitable for ionizing less polar compounds. Chang et al. developed a coaxial tip interface to realize the online coupling of CE with ambient direct analysis in real time mass spectrometry (DART-MS) as shown in Fig. 2f [72]. The setup can tolerate higher concentrations of detergents and salts. CZE and MEKC modes were achieved to the separation a mixture of 4-aminoantipyrine, zolmitriptan and quinine. The detection of endogenous caffeine in Chinese white tea was demonstrated using the system. Zhang et al. developed a CE-MS interface with dielectric barrier discharge ionization (DBDI), the mixture of metronidazole and acetaminophen solutions were separated as a proof-of-concept experiment [73].
3. Instrumental improvement to match capillary electrophoresis modesThe wide applications of capillary electrophoresis could be attributed to its various separation modes. Most CE modes are interchangeable by changing the composition of the background electrolyte and experimental procedures. However, the CE buffers in different modes may not be compatible with MS, due to the suppression of analyte ionization. Therefore, it is necessary to develop novel instrumentations to couple different separation methods with the different mass spectrometers.
3.1. Capillary zone electrophoresis (CZE)CZE is the most commonly used separation mode in CE due to its simple operation, although only charged species can be separated in this mode. A single buffer solution is used in CZE, and the separation mechanism is based on the different electrophoretic mobilities of analytes which depend on their charge/mass ratios. Both porous tip interfaces and microfluidic devices based liquid junction interfaces are frequently used to couple CZE with MS. Pejchinovski et al. reviewed the clinical proteomics studies using CZE-MS [74].
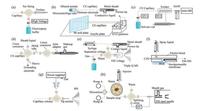
Dovichi's group reported plenty of work on online CZE-MS analytical techniques [75-77], especially in the applications of proteomics research [78-86]. A series of electrokinetically pumped sheath flow nanoelectrospray interfaces to CZE-MS were developed by Dovichi's group, as depicted in Fig. 3a [80, 82, 85, 87, 88]. A review on CZE-ESI coupled with Orbitrap Velos and linear Q-trap mass spectrometers was given by Sun et al. [80]. Moini et al. introduced an ultrafast capillary electrophoresis (UFCE) mass spectrometry method [89]. An in-house portable CE apparatus with porous tip interface was built (Fig. 3b), and a peptide mixture and protein digests were analyzed within 1 min. In the following paper, Moini et al. utilized a low-flow rate ESI to reduce the suppression effect of cyclodextrins salts. Cathinone derivatives and their optical isomers were separated and detected [90]. Xu et al. developed a mini CE-MS system to improve the performance of portable IT-MS and the design was showed in Fig. 3c [91]. The isobaric peptides were separated by CZE, and charge competition effects in nano-ESI interface was relieved. Another portable battery operated CE/ESI source was introduced by Moini et al. [92]. Detected by LTQ Velos, amino acids and their optical isomers were separated in ~1 min. Bergstrom et al. used CZE-MS to separate small native/deamidated peptide pairs and identify the potential sites for deamidation [93]. Kohl et al. introduced a heart-cut 2D-CE separation system using ESI-MS detection. 2D analysis of BSA tryptic digest sample was performed by the system using a nonvolatile background electrolyte in the first dimension [94].
|
Download:
|
| Fig. 3. Instrumental improvement to match CE modes. (a) electrokinetically pumped sheath flow interface schematic diagram reported in ref [82]; (b) UFCE-MS system schematic diagram reported in ref [89]; (c) miniature CE-MS system schematic diagram reported in ref [91]; (d) CGE-ICP-MS system schematic diagram reported in ref [106]; (e) CITP/CZE-QqQ-MS system schematic diagram reported in ref [107]; (f) CITP/CZE-MS/MS system schematic diagram reported in ref [112]; (g) CEC-MS system schematic diagram reported in ref [132]; (h) pressurized CEC-MS system schematic diagram reported in ref [133]. | |
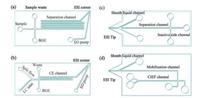
More methods based on microfluidic devices and capillary modification technology were reported in CE-MS systems to achieve special features. Nordman et al. reported the fabrication of shape-anchored porous polymer monoliths (PPMs) for on-chip SPE prior to online microchip electrophoresis (ME) separation and onchip ESI-MS [95]. 15-to 23-fold enrichment factors were obtained in a 25-s loading time. More applications using microchip electrophoresis ESI-MS were reported by the same research group [96, 97]. Mellors et al. built a hybrid multidimensional separation system, which integrates capillary liquid chromatography in a microfluidic device (Fig. 4a) [98]. A mixture of peptides yielding an approximately peak capacity of 1400 was analyzed in 50 min. Black et al. demonstrated the utility of the same microchip CE-ESI device for hydrogen exchange (HX) MS [99]. A bovine hemoglobin pepsin digestion was performed in 1 min with a peak capacity of 62. Redman et al. developed the online CE-ESI-MS device for the separation of intact monoclonal antibody charge variants [100, 101]. Batz et al. described a chemical vapor deposition (CVD) method for the surface modified of microfluidic devices by aminopropyl silane reagents [102]. The microfluidic chip design was showed in Fig. 4b, and peptides sample yielding a peak capacity of 64 was separated in less than 90 s. Mikuma et al. used the chemically modified capillary containing sulfonated groups to identified 8 amphetamine-type stimulants (ATS) enantiomers by chiral CE-MS/MS [103]. Li et al. reported a microchip electrophoresis-mass spectrometric platform for single cell analysis [104, 105].
|
Download:
|
| Fig. 4. Microfluidic chip to couple CE-MS. (a) microfluidic device design reported in ref [98]; (b) microfluidic chip design reported in ref [102]; (c) (d) isoelectric focusing microchip designs reported in ref [128]. | |
3.2. Capillary gel electrophoresis (CGE)
CGE is a separation mode combining the effective resolution of gel electrophoresis with the simple CZE devices. In CGE, the capillary is filled with gel as a molecular sieve. The separation mechanism is based on the different mobilities of the components via the gel due to their molecular size. Small size components migrate faster and will be detected earlier than the larger one. The solution system compatibility is the decisive factor when coupled to MS.
Fujii et al. developed a CGE-ICP-MS system to detect the double-stranded (ds) DNA [106]. An interface was designed to seamless connect the CGE and ICP-MS with a high performance concentric nebulizer (HPCN) (Fig. 3d). Fragments of dsDNA were separated and analyzed by measuring 31P+, and the LOD and absolute detection limit of P were 3.7 μg/kg and 0.6 pg, respectively.
3.3. Capillary isotachophoresis (CITP)The CITP mode is based on the use of a discontinuous buffer system without EOF. Analytes are injected between the terminating electrolyte with a lower electrophoretic than the sample and the leading electrolyte with a higher electrophoretic mobility than the sample. When applying electric field, the analytes are distributed in adjacent zones and move within the capillary at the same velocity between the buffers of different mobility. A twodimensional separation combining CITP and CZE was the typical pattern in the CE-MS system.
Tang et al. demonstrated a sheathless CITP/CZE-QqQ-MS interface to achieve large sample loading capacity and stable nano-ESI operation [107-109]. A schematic plot of the setup was showed in Fig. 3e. Leu-enkephalin and angiotensin Ⅱ, spiked in a BSA tryptic digest matrix were analyzed under the optimized parameters. A picomolar LOQ with measurement reproducibility of the CV less than 22% was obtained. Kler et al. presented a modular two-dimensional electrophoretic separation (ITP/CE) coupled to MS by using a glass microfluidic chip [110]. Four different human angiotensin peptides were pre-concentrated, separated, detected and identified by the system. After that, a nonaqueous ITP (NAITP) method using dimethylsulfoxide (DMSO) as solvent was applied in the similar system to analyze 20 proteinogenic amino acids [111]. Piestansky et al. developed an approach based on two-dimensional column coupled CE (ITP/CZE) hyphenated with tandem mass spectrometry (QqQ) as shown in Fig. 3f [112-114]. The optimized method was applied for the direct identification and ultratrace (pg/mL) determination of varenicline, pheniramine (PHM), phenylephrine (PHE), paracetamol (PCM) and their potential metabolic products in untreated/diluted human urine.
Different modes of ITP were applied to CE-MS system as methodology improvements. Marak et al. investigated the impact of buffer salts/matrix effects on the signal in direct injection MS with an electrospray interface (DI-ESI-MS) following pITP fractionation [115]. Mala et al. demonstrated a model of extended ITP and its applicability on the thiabendazole (Tbz) analysis in orange juice by full-format ITP-ESI-MS [116]. A LOD of 10-10 mol/L (20 ng/L) was obtained. Gahoual et al. reported the complete characterization of the primary structure of a multimeric glycoprotein by CE-MS [117]. Transient isotachophoresis (tITP) was introduced as a preconcentration technique in this work.
Capillary electrokinetic fractionation (CEkF) is investigated as an approach for semi-preparative and analytical method based on pKa-dependant pH-driven electrophoretic mobility. CITP is principally the closest technique to CEkF. He et al. proposed a method to fractionate constituents based on their pKa values by coupling CEkF to FT-ICR-MS [118]. Strong acids and phenols from two wine samples were tested by this technique.
3.4. Capillary isoeletric focusing (CIEF)The separation of CIEF is based on the pI differences of solutes. CIEF enables the separation of amino acids, peptides, proteins and other substances of amphoteric character. A large sample loading volume can be achieved in the CIEF mode. When an electric field is applied, the pH gradient is established within the capillary filled with the ampholytes solution, and sample components will migrate until the solution environment pH value equals to their pI. On the other hand, the ampholytes may lead to many problems when coupling with a MS, such as degraded sample ionization and polluted instruments. The focused sample zone may be broadened when transferring to MS.
An overview of capillary isoelectric focusing-mass spectrometry coupling strategies was given by Huhner [119]. Then Huhner et al. combined CIEF with CZE-ESI-MS by a multiple heart-cut approach [120]. Model proteins were analyzed on intact level. Dovichi's group did a series of investigation in online CIEF-MS methodology for protein and peptides analysis [121-123]. Zhu et al. reported the application of CIEF coupled with Orbitrap Velos MS for quantitative analysis of a complex proteome [121]. A set of amino acids were used as the ampholytes, and this approach identified 835 protein groups and produced 2329 unique peptides IDs. Li et al. presented a strategy of IEF-HPLC-MS-ELISA for hemoglobin (Hb) detection [124]. The proposed GSS-HbA(1c) was separated and purified by micro-preparative CIEF and the target fractions were pooled and identified by ESI-MS. Przybylski et al. demonstrated a non-denaturing detection mode by on-line hyphenation of CIEF with ESI-MS [125]. 40% glycerol-water medium was used as anti-convective agent in CE capillary. The highly basic cytokine human interferon-gamma (IFN-γ) was characterized as a non-covalent homodimer and its pI under the experimental conditions was determined with a value of 9.95. Horka et al. introduced a method, in which CIEF in tapered fusedsilica capillary was coupled with MALDI-TOF-MS for unambiguous identification of probiotic bacteria in real sample [126, 127]. CIEF analysis of both cultivated bacteria and bacteria in milk was optimized, and pIs of the examined bacteria were determined. In another article, eight strains of bacteria from plant-tissuecontaining samples were identified by the same method. Nordman et al. developed an interface of microchip CIEF with online MS detection via a fully integrated on-chip sheath flow ESI emitter [128]. The two-dimensional separation chip for two-step CIEF-tITP was used for peptide analysis, and schematic views can be found in Fig. 4c and d. Zhang et al. reported a design of immobilized pH gradient (IPG) based on monolithic column CIEF-MALDI-MS platform for the complex neuropeptides analysis [129]. The separation time of peptide mixtures was less than 10 min, while the MS signal was enhanced.
3.5. Capillary electrochromatography (CEC)The advantages of HPLC (high selectivity) and CE (high efficiency) are combined in CEC mode. Charged solutes are separated by the partition between stationary phase and mobile phase as well as by the differences in electrophoretic mobility. The incompatibility of CZE-MS with many separation buffers can also be overcome by using CEC.
Cheng et al. introduced a method about online pre-concentration CEC coupled with atmospheric pressure chemical ionization mass spectrometry (APCI-MS) [71]. Poly(stearyl methacrylate-divinylbenzene) (poly(SMA-DVB)) monolith was used as the separation column and 16 PAHs were analyzed. An LOD of 10 ng/g for PAH residues in seafood samples was obtained. Tiala et al. reported an on-line open-tubular CEC-MS method [130]. The model steroids were separated within coated fused silica capillaries under the optimized CEC conditions. Bragg et al. presented an approach for high throughput enantiomeric separations and detection of chiral analytes [131]. A short 7 cm CEC column packed with cellulose was used for CEC coupled to a singlequadrupole MS.
D'Orazio et al. developed a nano-liquid-junction interface for coupling both CEC or nano-liquid chromatography (nano-LC) with MS (Fig. 3g) [132]. The sample of organophosphorus pesticides (OPPs) and some acidic drugs were separated by CEC-MS, and the LOD ranged between 0.03 and 6.80 μg/mL. Wu et al. introduced a pressurized CEC (pCEC) method coupled to Q-TOF-MS (Fig. 3h) [133]. Three interfaces were compared and a sheathless interface was selected. The method was applied to lung cancer metabolomics under the optimized conditions. Simpson et al. developed an interface for the hyphenation of CEC with a hybrid IT-TOF-MS [134]. IR laser desorption and UV photoionization occurred within a quadrupole ion trap in order to simplify instrument. Trace analysis of naphthalene was achieved with an LOD of 500 nmol/L.
3.6. Micellar electrokinetic chromatography (MEKC)In MEKC, surfactants were added in the buffer solution to form micelles moving at a different velocity from the EOF. The neutral analytes can be separated by their distributions between the aqueous and the micellar phases. Ammonium salt of perfluorooctanoic acid, as MS friendly surfactant, is frequently used as BGE in MECK coupled to MS.
D'Orazio et al. evaluated a methodology based on dispersive liquid-liquid microextraction (DLLME)-MEKC-MS/MS. The aqueous solution of perfluorooctanoic acid (PFOA) and ammonium PFO (APFO) were used as BGE to separate and determine 12 estrogenic compounds [135]. Furthermore, the method was applied for the analysis of four endoestrogens and their major metabolites from milk and yogurt samples [136]. LODs in the low mg/L range were attained. Twelve new designer drugs of synthetic cathinones were identified by Svidrnoch et al. using the MEKC-MS/MS [137]. Akamatsu et al. described a method for the simultaneous determination of 12synthetic cannabinoids by MEKC-MS/MS, and LODs were 6.5-76.5 μg/g [138]. Moreno-Gonzalez et al. included a MEKC-ESI-MS method for the analysis of amino acids (AAs) in human urine [139]. LODs ranging from 9 to 26 ng/mL. Wang et al. used polysodium N-undecenoyl-L, L-leucylvalinate (poly-L, L-SULV) as a chiral pseudophase in MEKC-MS/MS baseline separation of warfarin (WAR) metabolites [140]. The LOD and quantitation limit were at levels of 2 and 5 ng/mL, respectively. Franze et al. used MEKC-ICP-MS for separation, size characterization, and speciation of gold and silver nanoparticles [141]. LODs were in the sub-microgram per liter range.
3.7. Nonaqueous capillary electrophoresis (NACE)NACE is the CE mode using the nonaqueous solvents as electrolytic solutions. The nonaqueous electrolyte can charge the analytes or interact with them, so that a selectivity separation can be achieved in NACE. Many natural products and drug-like small molecules can be separated and identified by the NACE-MS method.
Rodriguez et al. determined sunitinib, N-desethyl sunitinib and pregabalin in human urine using the NACE-TOF-MS, and LODs were 0.07 mg/L, 0.15 mg/L, 0.03 g/mL, respectively [142, 143]. Zhang et al. developed a NACE-ESI-IT-MS method for separation, identification, and quantification of the Amaryllidaceae alkaloids [144]. The LOD was lower than 240 ng/mL, and the fragmentation pathways of main fragment ions were studied by tandem mass spectrometry. Matrine and oxymatrine were determined by the same group [145], with LODs of 37.5 ng/mL and 50.0 ng/mL, respectively. Chen et al. identified tetrandrine (TET), fangchinoline (FAN), and sinomenine (SIN) using NACE-IT-MS with nano-ESI interface [146]. The LODs of TET, FAN, and SIN were 0.05, 0.08, and 0.15 mg/mL, respectively. Bonvin et al. compared the aqueous CZE and the NACE coupled to negative ESI-MS, the separation performance and sensitivity of several pharmaceutical acidic compounds were evaluated [147]. Tho Chau Minh Vinh et al. applied the NACE-MS to analyze the alkaloids in Nelumbo nucifera leaves [148]. Montealegre et al. investigated the glycerophospholipid fraction in olive fruit and olive oil samples by NACE method with electrospray-mass spectrometric detection [149]. Roscher et al. used NACE in metabolism studies of harmane, and 26 products were detected in NACE-MS analysis [150]. Malik et al. reported an approach for the determination and speciation of organotin compounds with NACE hyphenated to TOF-MS [151].
4. Analytical applications with different mass spectrometric analyzersMass analyzers provide a sensitive detection solution for the analytes separated by CE, especially in unknown components identification. The characteristics of different MS analyzers (such as resolution, scan speed, quantitation, cost and so on) should be taken into consideration under different situations. Rodriguez Robledo and Smyth reviewed the performance of CE-MS platforms from this perspective [5]. In recent years, almost all kinds of MS analyzers have been applied to CE detection, such as quadrupole and triple quadrupole mass spectrometer (Q/QqQ), ion trap mass spectrometer (IT), time-of-flight mass spectrometer (TOF), Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR) and Orbitrap mass spectrometer.
Less expensive but with relatively low-resolution, the quadrupole mass analyzer is one of the earliest mass analyzers coupled with CE. Bonvin et al. developed a sheath liquid interface with a make-up liquid, and intact proteins were analyzed by the CE-Q-MS [152]. More applications using quadrupole analyzer were reported in the analysis of nucleosides [153, 154], food [155], and drugs [27].
QqQ analyzers can be considered as a linear combination of three quadrupoles and show an improved resolution and more accurate quantification. Furthermore, collision induced dissociation (CID) process can be achieve in QqQ, so that ion structural information can be inferred from the ion fragments. Some papers about CE-QqQ-MS were published by Wang [107, 108], Piestansky [112, 113], Marakova [156, 157] and their coworkers. More applications in the analysis of glycan [158], toxins [159] and food [160, 161] using triple quadrupole analyzer were reported.
IT analyzers with varied geometrical structures can store and analyze sample ions. MSn and several gas phase ion reactions can be performed in an IT, and molecule structural information can be obtained by bioinformatics technique, especially in biological molecules such as proteins and peptides. Portable IT-MS coupled with CE is suitable for the on-site detection [91]. Ortiz-Villanueva et al. prepared and evaluated of open tubular C18-silica monolithic micro-cartridges for pre-concentration of five neuropeptides analyzing by CE-IT-MS [162]. Dong et al. reported a pH-mediated stacking CE coupled with ESI-MS/MS method to determine the phosphorylation sites of three synthetic phosphopeptides containing structural isomers [163]. More applications using IT analyzer were reported in the analysis of peptides [164], natural products [165, 166], soil [167, 168], therapeutic albumin [169], and organic dyes [170].
The TOF analyzer is the most popular mass analyzer in CE-MS system. With high resolution and scan rate, TOF analyzer is especially suitable for the omics analysis such as proteinomics and metabolomics. Causon et al. demonstrated a concept in using two types of sheath-flow reactions for CE coupled with Q-TOF-MS detection [171]. Medina-Casanellas et al. prepared an immunoaffinity (IA) sorbent with antibody fragments for the analysis of opioid peptides by on-line immunoaffinity solid-phase extraction CE-TOF-MS [172]. A variety of CE-TOF-MS applications were reported in the analysis of peptides and proteins [172-183], nucleic acids [184], glycans [185, 186], proteomics [187], metabolomics [188-202], drugs [49, 203-206], toxicologic [207-209], natural products [210, 211], single cell [212], organics [151, 213-216] and so on.
High resolution mass spectrometric (HRMS) analyzers provide accurate mass information in element level. Fourier transform mass spectrometers, such as FT-ICR analyzer, have a highresolution but expensive cost, and it rarely used for CE-MS at present. He et al. identified wines by CEkF-FT-ICR-MS, and addressed the existence of different species responding to flavor or color [118]. Michalke et al. investigated the relevant Mn-carrier species which are responsible for transport across neural barriers (NB) by FR-ICR-MS [217]. Yassine et al. analyzed acidic constituents of atmospheric organic aerosol an approach combining of CE-MS and FT-ICR-MS [218]. The Orbitrap mass analyzer is another fast developing HRMS and commonly used in proteomics research. Dovichi's group did a lot of work coupling CE with the Orbitrap MS [78, 80, 82, 84, 121, 219, 220].
5. ConclusionCapillary electrophoresis is a conventional separation method with high separation efficiency, short separation time and low sample consumption. Among many analyzers, mass spectrometer is one of the most rapid development analytical techniques with high resolution, sensitivity and the capacity of obtaining unknown molecule structure information. The combination of CE and MS shows a broader outlook in applications of analytical chemistry. A large amount of literatures published in past years give a powerful evidence for it. Nevertheless, many problems remain to be solved for the system. This review introduced the latest advances of the instrumentation and methodology in CE-MS.
An ionization process between CE and MS is necessary, which limits the applications of some CE separation modes. Only a few specific combinations are frequently used at present, such as CZEESI-MS, CIEF-MALDI-MS, CZE/CITP-ICP-MS. The interfacing techniques play an important role in promoting the combination of CEMS. Several ESI based interfaces were developed, such as the electrokinetically pumped sheath flow nanoelectrospray interfaces and the porous tips based interfaces. Furthermore, increased microfluidic devices were designed to couple CE-MS for special functions, such as 2D separation. A targeted design of injection interface can reduce components charge discrimination and achieve sample preconcentration.
There are two main trends in the development of instruments, high performance or portability. CE-MS shows a great potential in both two aspects. As a complementary technology for HPLC-MS, commercial sheathless CE-ESI-MS instrument was introduced in 2015, and more applications were reported. With higher sensitivity and analysis speed, an ultra-trace (single cell analysis) and highthroughput analysis (omics analysis) could be carryied out using CE-MS. It is possible to study the structure of biological molecules combining native MS method and novel CE buffer system, especially in obtaining time-dependent information, such as interaction of enzyme and substrate. On the other hand, portable CE-MS system facilitate the rapid detection. With improved quantitative detection performance, complex sample analysis could be achieved in a short time.
AcknowledgementsThis work was supported byMinistry of Science and Technology (MOST) instrumentation program of China (No. 2012YQ040140-07), National Natural Science Foundation of China (NSF) (No. 21475010), Beijing Natural Science Foundation (BNSF) (No. 16L00065) and State Key Laboratory Explosion Science and Technology (No. YBKT16-17).
| [1] | K. Kleparnik. Recent advances in combination of capillary electrophoresis with mass spectrometry:methodology and theory. Electrophoresis 36 (2015) 159–178. DOI:10.1002/elps.v36.1 |
| [2] | K. Kleparnik. Recent advances in the combination of capillary electrophoresis with mass spectrometry:from element to single-cell analysis. Electrophoresis 34 (2013) 70–85. DOI:10.1002/elps.v34.1 |
| [3] | C. Ibanez, C. Simo, V. Garcia-Canas, A. Cifuentes, M. Castro-Puyana. Metabolomics, peptidomics and proteomics applications of capillary electrophoresis-mass spectrometry in Foodomics:a review. Anal. Chim. Acta 802 (2013) 1–13. DOI:10.1016/j.aca.2013.07.042 |
| [4] | P.W. Lindenburg, R. Haselberg, G. Rozing, R. Ramautar. Developments in interfacing designs for CE-MS:towards enabling tools for proteomics and metabolomics. Chromatographia 78 (2015) 367–377. DOI:10.1007/s10337-014-2795-5 |
| [5] | V. Rodriguez Robledo, W.F. Smyth. Review of the CE-MS platform as a powerful alternative to conventional couplings in bio-omics and targetbased applications. Electrophoresis 35 (2014) 2292–2308. DOI:10.1002/elps.v35.16 |
| [6] | A. Stalmach, A. Albalat, W. Mullen, H. Mischak. Recent advances in capillary electrophoresis coupled to mass spectrometry for clinical proteomic applications. Electrophoresis 34 (2013) 1452–1464. DOI:10.1002/elps.v34.11 |
| [7] | A. Hirayama, M. Wakayama, T. Soga. Metabolome analysis based on capillary electrophoresis-mass spectrometry. Trac-Trends in Anal. Chem. 61 (2014) 215–222. DOI:10.1016/j.trac.2014.05.005 |
| [8] | R. Ramautar, G.W. Somsen, G.J. de Jong. CE-MS for metabolomics:developments and applications in the period 2010-2012. Electrophoresis 34 (2013) 86–98. DOI:10.1002/elps.v34.1 |
| [9] | R. Ramautar, G.W. Somsen, G.J. de Jong. CE-MS for metabolomics:developments and applications in the period 2012-2014. Electrophoresis 36 (2015) 212–224. DOI:10.1002/elps.v36.1 |
| [10] | X. Wang, K. Li, E. Adams, A. Van Schepdael. Capillary electrophoresis-mass spectrometry in metabolomics:the potential for driving drug discovery and development. Curr. Drug. Metab. 14 (2013) 807–813. DOI:10.2174/13892002113149990101 |
| [11] | A. Albalat, H. Husi, J. Siwy, et al., Capillary electrophoresis interfaced with a mass spectrometer (CE-MS):technical considerations and applicability for biomarker studies in animals. Curr. Protein Pept. Sci. 15 (2014) 23–35. DOI:10.2174/1389203715666140221123920 |
| [12] | C. Pontillo, S. Filip, D.M. Borras, et al., CE-MS-based proteomics in biomarker discovery and clinical application. Proteomics Clin. Appl. 9 (2015) 322–334. DOI:10.1002/prca.201400115 |
| [13] | A. Albalat, H. Husi, A. Stalmach, J.P. Schanstra, H. Mischak. Classical MALDIMS versus CE-based ESI-MS proteomic profiling in urine for clinical applications. Bioanalysis 6 (2014) 247–266. DOI:10.4155/bio.13.313 |
| [14] | A. Latosinska, M. Frantzi, A. Vlahou, H. Mischak. Clinical applications of capillary electrophoresis coupled to mass spectrometry in biomarker discovery:focus on bladder cancer. Proteomics Clin. Appl. 7 (2013) 779–793. DOI:10.1002/prca.v7.11-12 |
| [15] | H. Mischak, A. Vlahou, J.P.A. Ioannidis. Technical aspects and inter-laboratory variability in native peptide profiling:the CE-MS experience. Clin. Biochem. 46 (2013) 432–443. DOI:10.1016/j.clinbiochem.2012.09.025 |
| [16] | C.-W. Wang, G.-R. Her. The development of a counterflow-assisted preconcentration technique in capillary electrophoresis electrospray-ionization mass spectrometry. Electrophoresis 35 (2014) 1251–1258. DOI:10.1002/elps.v35.9 |
| [17] | L. D' Ulivo, Y.-L. Feng. Expanding the scope of pressure-assisted electrokinetic injection for online concentration of positively charged analytes in capillary electrophoresis-mass spectrometry. Electrophoresis 36 (2015) 1024–1027. DOI:10.1002/elps.v36.7-8 |
| [18] | S.-G. Park, K.K. Murray. Ambient laser ablation sampling for capillary electrophoresis mass spectrometry. Rapid Commun. Mass Spectrom. 27 (2013) 1673–1680. DOI:10.1002/rcm.6618 |
| [19] | N.H. Wang, G.R. Her. The development of a hydrodynamic flow assisted double junction interface for signal improvement in capillary electrophoresis-mass spectrometry using positively charged nonvolatile additives. J. Chromatog. A 1379 (2015) 106–111. DOI:10.1016/j.chroma.2014.12.046 |
| [20] | R.T. Kelly, C. Wang, S.J. Rausch, C.S. Lee, K. Tang. Pneumatic microvalve-based hydrodynamic sample injection for high-throughput, quantitative zone electrophoresis in capillaries. Anal. Chem. 86 (2014) 6723–6729. DOI:10.1021/ac501910p |
| [21] | N.L. Kuehnbaum, J.B. Gillen, A. Kormendi, et al., Multiplexed separations for biomarker discovery in metabolomics:elucidating adaptive responses to exercise training. Electrophoresis 36 (2015) 2226–2236. DOI:10.1002/elps.v36.18 |
| [22] | N.L. Kuehnbaum, A. Kormendi, P. Britz-McKibbin. Multisegment injectioncapillary electrophoresis-mass spectrometry:a high-throughput platform for metabolomics with high data fidelity. Anal. Chem. 85 (2013) 10664–10669. DOI:10.1021/ac403171u |
| [23] | M. Grundmann, F.-M. Matysik. Analyzing small samples with high efficiency:capillary batch injection-capillary electrophoresis-mass spectrometry. Anal. Bioanal. Chem. 404 (2012) 1713–1721. DOI:10.1007/s00216-012-6282-2 |
| [24] | J.J.P. Mark, A. Beutner, M. Cindric, F.-M. Matysik. Microanalytical study of subnanoliter samples by capillary electrophoresis-mass spectrometry with 100% injection efficiency. Microchim. Acta 182 (2015) 351–359. DOI:10.1007/s00604-014-1339-x |
| [25] | A. Martinez-Villalba, O. Nunez, E. Moyano, M. Teresa Galceran. Field amplified sample injection-capillary zone electrophoresis for the analysis of amprolium in eggs. Electrophoresis 34 (2013) 870–876. DOI:10.1002/elps.201200579 |
| [26] | H. Cheng, C. Han, Z. Xu, J. Liu, Y. Wang. Sensitivity enhancement by fieldamplified sample injection in interfacing microchip electrophoresis with inductively coupled plasma mass spectrometry for bromine speciation in bread. Food Anal. Method. 7 (2014) 2153–2162. DOI:10.1007/s12161-014-9848-0 |
| [27] | Y. He, X. Li, P. Tong, et al., An online field-amplification sample stacking method for the determination of beta(2)-agonists in human urine by CE-ESI/MS. Talanta 104 (2013) 97–102. DOI:10.1016/j.talanta.2012.11.041 |
| [28] | S.-H. Hung, G.-R. Her. A convenient and sensitive method for haloacetic acid analysis in tap water by on-line field-amplified sample-stacking CE-ESI-MS. J. Sep. Sci. 36 (2013) 3635–3643. DOI:10.1002/jssc.v36.21-22 |
| [29] | E. Ito, K. Nakajima, H. Waki, et al., Structural characterization of pyridylaminated oligosaccharides derived from neutral glycosphingolipids by high-sensitivity capillary electrophoresis-mass spectrometry. Anal. Chem. 85 (2013) 7859–7865. DOI:10.1021/ac401460f |
| [30] | A. Wuethrich, P.R. Haddad, J.P. Quirino. Field-enhanced sample injection micelle-to-solvent stacking capillary zone electrophoresis-electrospray ionization mass spectrometry of antibiotics in seawater after solid-phase extraction. Electrophoresis 37 (2016) 1139–1142. DOI:10.1002/elps.v37.9 |
| [31] | P. Palatzky, A. Zoepfl, T. Hirsch, F.-M. Matysik. Electrochemically assisted injection in combination with capillary electrophoresis-mass spectrometry (EAI-CE-MS)-mechanistic and quantitative studies of the reduction of 4-nitrotoluene at various carbon-based screen-printed electrodes. Electroanalysis 25 (2013) 117–122. DOI:10.1002/elan.201200393 |
| [32] | R. Scholz, P. Palatzky, F.-M. Matysik. Simulation of oxidative stress of guanosine and 8-oxo-7, 8-dihydroguanosine by electrochemically assisted injection-capillary electrophoresis-mass spectrometry. Anal. Bioanal. Chem. 406 (2014) 687–694. DOI:10.1007/s00216-013-7500-2 |
| [33] | G. Jarvas, A. Guttman, F. Foret. Numerical modeling of capillary electrophoresis-electrospray mass spectrometry interface design. Mass Spectrom. Rev. 34 (2015) 558–569. DOI:10.1002/mas.v34.5 |
| [34] | G. Bonvin, J. Schappler, S. Rudaz. Capillary electrophoresis-electrospray ionization-mass spectrometry interfaces:fundamental concepts and technical developments. J. Chromatogr. A 1267 (2012) 17–31. DOI:10.1016/j.chroma.2012.07.019 |
| [35] | J. Krenkova, K. Kleparnik, J. Grym, J. Luksch, F. Foret. Self-aligning subatmospheric hybrid liquid junction electrospray interface for capillary electrophoresis. Electrophoresis 37 (2016) 414–417. DOI:10.1002/elps.v37.3 |
| [36] | P.W. Lindenburg, R. Ramautar, R.G. Jayo, D.D.Y. Chen, T. Hankemeier. Capillary electrophoresis-mass spectrometry using a flow-through microvial interface for cationic metabolome analysis. Electrophoresis 35 (2014) 1308–1314. DOI:10.1002/elps.v35.9 |
| [37] | L. Lin, X.Y. Liu, F.M. Zhang, et al., Analysis of heparin oligosaccharides by capillary electrophoresis-negative-ion electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 409 (2017) 411–420. DOI:10.1007/s00216-016-9662-1 |
| [38] | X.J. Sun, L. Lin, X.Y. Liu, et al., Capillary electrophoresis-mass spectrometry for the analysis of heparin oligosaccharides and low molecular weight heparin. Anal. Chem. 88 (2016) 1937–1943. DOI:10.1021/acs.analchem.5b04405 |
| [39] | S.B. Choi, M. Zamarbide, M.C. Manzini, P. Nemes. Tapered-tip capillary electrophoresis nano-electrospray ionization mass spectrometry for ultrasensitive proteomics:the mouse cortex. J. Am. Soc. Mass Spectrom. 28 (2017) 597–607. DOI:10.1007/s13361-016-1532-8 |
| [40] | J.-S. Jeong, S.-K. Kim, S.-R. Park. Amino acid analysis of dried blood spots for diagnosis of phenylketonuria using capillary electrophoresis-mass spectrometry equipped with a sheathless electrospray ionization interface. Anal. Bioanal. Chem. 405 (2013) 8063–8072. DOI:10.1007/s00216-013-6999-6 |
| [41] | J.-L. Huang, R.-Y. Hsu, G.-R. Her. The development of a sheathless capillary electrophoresis electrospray ionization-mass spectrometry interface based on thin conducting liquid film. J.Chromatogr. A 1267 (2012) 131–137. DOI:10.1016/j.chroma.2012.08.081 |
| [42] | C.-W. Wang, G.-R. Her. Sheathless capillary electrophoresis electrospray ionization-mass spectrometry interface based on poly(dimethylsiloxane) membrane emitterand thin conducting liquid film. Electrophoresis 34 (2013) 2538–2545. DOI:10.1002/elps.201300069 |
| [43] | A. Tycova, F. Foret. Capillary electrophoresis in an extended nanospray tipelectrosprayas an electrophoretic column. J. Chromatogr. A 1388 (2015) 274–279. DOI:10.1016/j.chroma.2015.02.042 |
| [44] | A. Tycova, M. Vido, P. Kovarikova, F. Foret. Interface-free capillary electrophoresis-mass spectrometry system with nanospray ionization Analysis of dexrazoxane in blood plasma. J. Chromatogr. A 1466 (2016) 173–179. DOI:10.1016/j.chroma.2016.08.042 |
| [45] | G.S.M. Kammeijer, I. Kohler, B.C. Jansen, et al., Dopant enriched nitrogen gas combined with sheathless capillary electrophoresis-electrospray ionizationmass spectrometryfor improved sensitivity and repeatability in glycopeptide analysis. Anal. Chem. 88 (2016) 5849–5856. DOI:10.1021/acs.analchem.6b00479 |
| [46] | X. Zhong, Z. Zhang, S. Jiang, L. Li. Recent advances in coupling capillary electrophoresis-based separation techniques to ESI and MALDI-MS. Electrophoresis 35 (2014) 1214–1225. DOI:10.1002/elps.v35.9 |
| [47] | M. Biacchi, R. Bhajun, N. Said, et al., Analysis of monoclonal antibody by a novel CE-UV/MALDI-MS interface. Electrophoresis 35 (2014) 2986–2995. DOI:10.1002/elps.201400276 |
| [48] | I. Tomalova, P. Foltynova, V. Kanicky, J. Preisler. MALDI MS and ICP MS detection of a single CE separation record:a tool for metalloproteomics. Anal. Chem. 86 (2014) 647–654. DOI:10.1021/ac402941e |
| [49] | V. Springer, J. Jacksen, P. Ek, A.G. Lista, A. Emmer. Capillary electrophoretic determination of fluoroquinolones in bovine milk followed by off-line malditof-ms analysis. Chromatographia 78 (2015) 285–290. DOI:10.1007/s10337-014-2823-5 |
| [50] | H.-X. Chen, J.-M. Busnel, L. Qiao, et al., Compatible buffer for capillary electrophoresis and matrix-assisted laser desorption/ionization mass spectrometry. Anal Method 5 (2013) 4258–4262. DOI:10.1039/c3ay40397a |
| [51] | A.R. Timerbaev, K. Pawlak, S.S. Aleksenko, et al., Advances of CE-ICP-MS in speciation analysis related to metalloproteomics of anticancer drugs. Talanta 102 (2012) 164–170. DOI:10.1016/j.talanta.2012.07.031 |
| [52] | L. Liu, B. He, Z. Yun, J. Sun, G. Jiang. Speciation analysis of arsenic compounds by capillary electrophoresis on-line coupled with inductivelycoupled plasma mass spectrometry using a novel interface. J. Chromatogr. A 1304 (2013) 227–233. DOI:10.1016/j.chroma.2013.07.034 |
| [53] | L. Liu, Z. Yun, B. He, G. Jiang. Efficient interface for online coupling of capillary electrophoresis with inductively coupled plasma-mass spectrometry and its application in simultaneous speciation analysis of arsenic and selenium. Anal. Chem. 86 (2014) 8167–8175. DOI:10.1021/ac501347d |
| [54] | L. Liu, B. He, Q. Liu, et al., Identification and accurate size characterization of nanoparticles in complex media. Angew. Chem. In. Edit. 53 (2014) 14476–14479. DOI:10.1002/anie.201408927 |
| [55] | N. Kovachev, M. Angel Aguirre, M. Hidalgo, et al., Elemental speciation by capillary electrophoresis with inductively coupled plasma spectrometry:a new approach by Flow Focusing (R) nebulization. Microchem. J. 117 (2014) 27–33. DOI:10.1016/j.microc.2014.06.005 |
| [56] | D. Nakamoto, M. Tanaka. Speciation of aluminum by CE-ESI-MS and CE-ICPMS. Bunseki Kagaku 63 (2014) 383–390. DOI:10.2116/bunsekikagaku.63.383 |
| [57] | H. Qu, T.K. Mudalige, S.W. Linder. Capillary electrophoresis/inductivelycoupled plasma-mass spectrometry:development and optimization of a high resolution analytical tool for the size-based characterization of nanomaterials in dietary supplements. Anal. Chem. 86 (2014) 11620–11627. DOI:10.1021/ac5025655 |
| [58] | H. Qu, T.K. Mudalige, S.W. Linder. Arsenic speciation in rice by capillary electrophoresis/inductively coupled plasma mass spectrometry:enzymeassisted water-phase microwave digestion. J. Agric. Food Chem. 63 (2015) 3153–3160. DOI:10.1021/acs.jafc.5b00446 |
| [59] | Y. Chen, J. Chen, Z. Xi, et al., Simultaneous analysis of Cr(Ⅲ), Cr(Ⅳ), and chromium picolinate in foods using capillary electrophoresis-inductively coupled plasma mass spectrometry. Electrophoresis 36 (2015) 1208–1215. DOI:10.1002/elps.v36.9-10 |
| [60] | Y. Chen, L. Huang, W. Wu, et al., Speciation analysis of lead in marine animals by using capillary electrophoresis couple online with inductively coupled plasma mass spectrometry. Electrophoresis 35 (2014) 1346–1352. DOI:10.1002/elps.v35.9 |
| [61] | M. Yang, W. Wu, Y. Ruan, et al., Ultra-sensitive quantification of lysozyme based on element chelate labeling and capillary electrophoresis inductively coupled plasma mass spectrometry. Anal. Chim. Acta 812 (2014) 12–17. DOI:10.1016/j.aca.2014.01.003 |
| [62] | B. Brunel, V. Philippini, M. Mendes, J. Aupiais. Actinide oxalate complexes formation as a function of temperature by capillary electrophoresis coupled with inductively coupled plasma mass spectrometry. Radiochim. Acta 103 (2015) 27–37. |
| [63] | R. Kautenburger, C. Hein, J.M. Sander, H.P. Beck. Influence of metal loading and humic acid functional groups on the complexation behavior of trivalent lanthanides analyzed by CE-ICP-MS. Anal. Chim. Acta 816 (2014) 50–59. DOI:10.1016/j.aca.2014.01.044 |
| [64] | J.C. Stern, D.I. Foustoukos, J.E. Sonke, V.J.M. Salters. Humic acid complexation of Th, Hf and Zr in ligand competition experiments:metal loading and pH effects. Chem. Geo. 363 (2014) 241–249. DOI:10.1016/j.chemgeo.2013.11.001 |
| [65] | S.S. Aleksenko, M. Matczuk, X. Lu, et al., Metallomics for drug development:an integrated CE-ICP-MS and ICP-MS approach reveals the speciation changes for an investigational ruthenium(Ⅲ) drug bound to holo-transferrin in simulated cancer cytosol. Metallomics 5 (2013) 955–963. DOI:10.1039/c3mt00092c |
| [66] | M. Matczuk, M. Przadka, S.S. Aleksenko, et al., Metallomics for drug development:a further insight into intracellular activation chemistry of a ruthenium(iii)-based anticancer drug gained using a multidimensional analytical approach. Metallomics 6 (2014) 147–153. DOI:10.1039/C3MT00252G |
| [67] | T.T.T.N. Nguyen, J. Ostergaard, S. Sturup, B. Gammelgaard. Determination of platinum drug releaseand liposome stability inhumanplasma by CE-ICP-MS. Int. J. Pharm. 449 (2013) 95–102. DOI:10.1016/j.ijpharm.2013.03.055 |
| [68] | T.T.T.N. Nguyen, J. Ostergaard, S. Sturup, B. Gammelgaard. Metallomics in drug development:characterization of a liposomal cisplatin drug formulation in human plasma by CE-ICP-MS. Anal. Bioanal. Chem. 405 (2013) 1845–1854. DOI:10.1007/s00216-012-6355-2 |
| [69] | F. Chen, L. Zheng, L. Han, et al., Analysis of arsenic species in dry seafood products by capillary electrophoresis-inductively coupled plasma mass spectrometry. Sci. Tech. Food Indus. 35 (2014) 304–307. |
| [70] | V. Vacchina, C. Ionescu, S. Oguey, R. Lobinski. Determination of Zn-, Cu-and Mn-glycinate complexes in feed samples and in-vitro and in-vivo assays to assess their bioaccessibility in feed samples. Talanta 113 (2013) 14–18. DOI:10.1016/j.talanta.2013.03.083 |
| [71] | Y.-J. Cheng, S.-H. Huang, J.-Y. Chiu, W.-L. Liu, H.-Y. Huang. Analyses of polycyclic aromatic hydrocarbons in seafood by capillary electrochromatography-atmospheric pressure chemical ionization/mass spectrometry. J. Chromatogr. A 1313 (2013) 132–138. DOI:10.1016/j.chroma.2013.08.035 |
| [72] | C. Chang, G. Xu, Y. Bai, et al., Online coupling of capillary electrophoresis with direct analysis in real time mass spectrometry. Anal. Chem. 85 (2013) 170–176. DOI:10.1021/ac303450v |
| [73] | Y.D. Zhang, W.P. Ai, Y. Bai, et al., An interface for online coupling capillary electrophoresis to dielectric barrier discharge ionization mass spectrometry. Anal. Bioanal. Chem. 408 (2016) 8655–8661. DOI:10.1007/s00216-016-9822-3 |
| [74] | M. Pejchinovski, D. Hrnjez, A. Ramirez-Torres, et al., Capillary zone electrophoresis on-line coupled to mass spectrometry:a perspective application for clinical proteomics. Proteom. Clin. Appl. 9 (2015) 453–468. DOI:10.1002/prca.v9.5-6 |
| [75] | L. Sun, M.D. Knierman, G. Zhu, N.J. Dovichi. Fast top-down intact protein characterization with capillary zone electrophoresis-electrospray ionization tandem mass spectrometry. Anal. Chem. 85 (2013) 5989–5995. DOI:10.1021/ac4008122 |
| [76] | L. Sun, G. Zhu, N.J. Dovichi. Integrated capillary zone electrophoresis-electrospray ionization tandem mass spectrometry system with an immobilized trypsin microreactor for online digestion and analysis of picogram amounts of RAW 264.7 cell lysate. Anal. Chem 85 (2013) 4187–4194. DOI:10.1021/ac400523x |
| [77] | Y. Zhao, L. Sun, M.M. Champion, M.D. Knierman, N.J. Dovichi. Capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry for topdown characterization of the mycobacterium marinum secretome. Anal. Chem. 86 (2014) 4873–4878. DOI:10.1021/ac500092q |
| [78] | L. Sun, A.S. Hebert, X. Yan, et al., Over 10000 peptide identifications from the hela proteome by using single-shot capillary zone electrophoresis combined with tandem mass spectrometry. Angew. Chem. Int. Edit. 53 (2014) 13931–13933. DOI:10.1002/anie.201409075 |
| [79] | L. Sun, G. Zhu, S. Mou, et al., Capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry for quantitative parallel reaction monitoring of peptide abundance and single-shot proteomic analysis of a human cell line. J. Chromatogr. A 1359 (2014) 303–308. DOI:10.1016/j.chroma.2014.07.024 |
| [80] | L. Sun, G. Zhu, X. Yan, M.M. Champion, N.J. Dovichi. Capillary zone electrophoresis for analysis of complex proteomes using an electrokinetically pumped sheath flow nanospray interface. Proteomics 14 (2014) 622–628. DOI:10.1002/pmic.v14.4-5 |
| [81] | L. Sun, G. Zhu, X. Yan, N.J. Dovichi. High sensitivity capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry for the rapid analysis of complex proteomes. Curr. Opin. Chem. Bio. 17 (2013) 795–800. DOI:10.1016/j.cbpa.2013.07.018 |
| [82] | L. Sun, G. Zhu, Z. Zhang, S. Mou, N.J. Dovichi. Third-Generation electrokinetically pumped sheath-flow nanospray interface with improved stability and sensitivity for automated capillary zone electrophoresis-mass spectrometry analysis of complex proteome digests. J. Proteom. Res. 14 (2015) 2312–2321. DOI:10.1021/acs.jproteome.5b00100 |
| [83] | Z. Zhang, L. Sun, G. Zhu, X. Yan, N.J. Dovichi. Integrated strong cationexchange hybrid monolith coupled with capillary zone electrophoresis and simultaneous dynamic pH junction for large-volume proteomic analysis by mass spectrometry. Talanta 138 (2015) 117–122. DOI:10.1016/j.talanta.2015.01.040 |
| [84] | G. Zhu, L. Sun, X. Yan, N.J. Dovichi. Single-shot proteomics using capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry with production of more than 1250 escherichia coli peptide identifications in a 50min separation. Anal. Chem. 85 (2013) 2569–2573. DOI:10.1021/ac303750g |
| [85] | G. Zhu, L. Sun, X. Yan, N.J. Dovichi. Stable reproducible, and automated capillary zone electrophoresis-tandem mass spectrometry system with an electrokinetically pumped sheath-flow nanospray interface. Anal. Chim. Acta 810 (2014) 94–98. DOI:10.1016/j.aca.2013.11.057 |
| [86] | K.R. Ludwig, L.L. Sun, G.J. Zhu, N.J. Dovichi, A.B. Hummon. Over 2300 phosphorylated peptide identifications with single-shot capillary zone electrophoresis-tandem mass spectrometry in a 100min separation. Anal. Chem. 87 (2015) 9532–9537. DOI:10.1021/acs.analchem.5b02457 |
| [87] | E.H. Peuchen, G.J. Zhu, L.L. Sun, N.J. Dovichi. Evaluation of a commercial electro-kinetically pumped sheath-flow nanospray interface coupled to an automated capillary zone electrophoresis system. Anal. Bioanal. Chem. 409 (2017) 1789–1795. DOI:10.1007/s00216-016-0122-8 |
| [88] | S.A. Sarver, N.M. Schiavone, J. Arceo, et al., Capillary electrophoresis coupled to negative mode electrospray ionization mass spectrometry using an electrokinetically-pumped nanospray interface with primary amines grafted to the interior of a glass emitter. Talanta 165 (2017) 522–525. DOI:10.1016/j.talanta.2017.01.002 |
| [89] | M. Moini, B. Martinez. Ultrafast capillary electrophoresis/mass spectrometry with adjustable porous tip for a rapid analysis of protein digest in about a minute. Rapid Commun. Mass Spectrom. 28 (2014) 305–310. DOI:10.1002/rcm.6786 |
| [90] | M. Moini, C.M. Rollman. Compatibility of highly sulfated cyclodextrin with electrospray ionization at low nanoliter/minute flow rates and its application to capillary electrophoresis/electrospray ionization mass spectrometric analysis of cathinone derivatives and their optical isomers. Rapid Commun. Mass Spectrom. 29 (2015) 304–310. DOI:10.1002/rcm.7106 |
| [91] | M. He, Z. Xue, Y. Zhang, et al., Development and characterizations of a miniature capillary electrophoresis mass spectrometry system. Anal. Chem. 87 (2015) 2236–2241. DOI:10.1021/ac504868w |
| [92] | M. Moini, C.M. Rollman. Portable, battery operated capillary electrophoresis with optical isomer resolution integrated with ionization source for mass spectrometry. J. Am. Soc. Mass Spectrom. 27 (2016) 388–393. DOI:10.1007/s13361-015-1314-8 |
| [93] | T. Bergstrom, S.-A. Fredriksson, C. Nilsson, C. Astot. Deamidation in ricin studied by capillary zone electrophoresis-and liquid chromatography-mass spectrometry. J. Chromatogr. B-Anal. Tech. Biomed. Life Sci. 974 (2015) 109–117. DOI:10.1016/j.jchromb.2014.10.015 |
| [94] | F.J. Kohl, C. Montealegre, C. Neususs. On-line two-dimensional capillary electrophoresis with mass spectrometric detection using a fully electric isolated mechanical valve. Electrophoresis 37 (2016) 954–958. DOI:10.1002/elps.201500579 |
| [95] | N. Nordman, B. Barrios-Lopez, S. Lauren, et al., Shape-anchored porous polymer monoliths for integrated online solid-phase extraction-microchip electrophoresis-electrospray ionization mass spectrometry. Electrophoresis 36 (2015) 428–432. DOI:10.1002/elps.201400278 |
| [96] | E. Ollikainen, A. Bonabi, N. Nordman, et al., Rapid separation of phosphopeptides by microchip electrophoresis-electrospray ionization mass spectrometry. J. Chromatogr. A 1440 (2016) 249–254. DOI:10.1016/j.chroma.2016.02.063 |
| [97] | S.M. Tahka, A. Bonabi, V.P. Jokinen, T.M. Sikanen. Aqueous and non-aqueous microchip electrophoresis with on-chip electrospray ionization mass spectrometry on replica-molded thiol-ene microfluidic devices. J. Chromatogr. A 1496 (2017) 150–156. DOI:10.1016/j.chroma.2017.03.018 |
| [98] | J.S. Mellors, W.A. Black, A.G. Chambers, et al., Hybrid capillary/microfluidic system for comprehensive online liquid chromatography-capillary electrophoresis-electrospray ionization-mass spectrometry. Anal. Chem. 85 (2013) 4100–4106. DOI:10.1021/ac400205a |
| [99] | W.A. Black, B.B. Stocks, J.S. Mellors, J.R. Engen, J.M. Ramsey. Utilizing microchip capillary electrophoresis electrospray ionization for hydrogen exchange mass spectrometry. Anal. Chem. 87 (2015) 6280–6287. DOI:10.1021/acs.analchem.5b01179 |
| [100] | E.A. Redman, N.G. Batz, J.S. Mellors, J.M. Ramsey. Integrated microfluidic capillary electrophoresis-electrospray ionization devices with online ms detection for the separation and characterization of intact monoclonal antibody variants. Anal. Chem. 87 (2015) 2264–2272. DOI:10.1021/ac503964j |
| [101] | E.A. Redman, J.S. Mellors, J.A. Starkey, J.M. Ramsey. Characterization of intact antibody drug conjugate variants using microfluidic capillary electrophoresis-mass spectrometry. Anal. Chem. 88 (2016) 2220–2226. DOI:10.1021/acs.analchem.5b03866 |
| [102] | N.G. Batz, J.S. Mellors, J.P. Alarie, J.M. Ramsey. Chemical vapor deposition of aminopropyl si lanes in microfluidic channels for highly efficient microchip capillary electrophoresis-electrospray ionization-mass spectrometry. Anal. Chem. 86 (2014) 3493–3500. DOI:10.1021/ac404106u |
| [103] | T. Mikuma, Y.T. Iwata, H. Miyaguchi, et al., The use of a sulfonated capillary on chiral capillary electrophoresis/mass spectrometry of amphetamine-type stimulants for methamphetamine impurity profiling. Forensic Sci. Int. 249 (2015) 59–65. DOI:10.1016/j.forsciint.2015.01.015 |
| [104] | X.T. Li, H.K. Hu, S.L. Zhao, Y.M. Liu. Microfluidic platform with in-chip electrophoresis coupled tomass spectrometry for monitoring neurochemical release from nerve cells. Anal. Chem. 88 (2016) 5338–5344. DOI:10.1021/acs.analchem.6b00638 |
| [105] | X.T. Li, S.L. Zhao, H.K. Hu, Y.M. Liu. A microchip electrophoresis-mass spectrometric platform with double cell lysis nano-electrodes for automated single cell analysis. J. Chromatogr. A 1451 (2016) 156–163. DOI:10.1016/j.chroma.2016.05.015 |
| [106] | S.-i. Fujii, K. Inagaki, S.-i. Miyashita, et al., A coupling system of capillary gel electrophoresis with inductively coupled plasma-mass spectrometry for the determination of double stranded DNA fragments. Metallomics 5 (2013) 424–428. DOI:10.1039/c3mt00057e |
| [107] | C. Wang, C.S. Lee, R.D. Smith, K. Tang. Ultrasensitive sample quantitation via selected reaction monitoring using citp/cze-esi-triple quadrupole MS. Anal. Chem. 84 (2012) 10395–10403. DOI:10.1021/ac302616m |
| [108] | C. Wang, C.S. Lee, R.D. Smith, K. Tang. Capillary lsotachophoresis-nanoelectrospray ionization-selected reaction monitoring ms via a novel sheath less interface for high sensitivity sample quantification. Anal. Chem. 85 (2013) 7308–7315. DOI:10.1021/ac401202c |
| [109] | X.J. Guo, T.L. Fillmore, Y.Q. Gao, K.Q. Tang. Capillary electrophoresis-nanoelectrospray ionization-selected reaction monitoring mass spectrometry via a true sheathless metal-coated emitter interface for robust and high-sensitivity sample quantification. Anal. Chem 88 (2016) 4418–4425. DOI:10.1021/acs.analchem.5b04912 |
| [110] | P.A. Kler, T.N. Posch, M. Pattky, R.M. Tiggelaar, C. Huhn. Column coupling isotachophoresis-capillary electrophoresis with mass spectrometric detection:characterization and optimization of microfluidic interfaces. J. Chromatogr. A 1297 (2013) 204–212. DOI:10.1016/j.chroma.2013.04.046 |
| [111] | P.A. Kler, C. Huhn. Non-aqueous electrolytes for isotachophoresis of weak bases and its application to the comprehensive preconcentration of the 20 proteinogenic amino acids in column-coupling ITP/CE-MS. Anal. Bioanal. Chem. 406 (2014) 7163–7174. DOI:10.1007/s00216-014-8152-6 |
| [112] | J. Piestansky, K. Marakova, M. Koval, P. Mikus. Comparison of hydrodynamically closed isotachophoresis-capillary zone electrophoresis with hydrodynamically open capillary zone electrophoresis hyphenated with tandem mass spectrometry in drug analysis:pheniramine, its metabolite and phenylephrine in human urine. J. Chromatogr. A 1358 (2014) 285–292. DOI:10.1016/j.chroma.2014.06.083 |
| [113] | J. Piestansky, K. Marakova, L. Veizerova, J. Galba, P. Mikus. On-line column coupled isotachophoresis-capillary zone electrophoresis hyphenated with tandem mass spectrometry in drug analysis:varenicline and its metabolite in human urine. Anal. Chim. Acta 826 (2014) 84–93. DOI:10.1016/j.aca.2014.04.003 |
| [114] | J. Piest, ' ansky, K. Marakova, M. Koval, E. Havranek, P. Mikus. Enantioselective column coupled electrophoresis employing large bore capillaries hyphenated with tandem mass spectrometry for ultra-trace determination of chiral compounds in complex real samples. Electrophoresis 36 (2015) 3069–3079. DOI:10.1002/elps.201500351 |
| [115] | J. Marak, A. Stanova. Buffer salt effects in off-line coupling of capillary electrophoresis and mass spectrometry. Electrophoresis 35 (2014) 1268–1274. DOI:10.1002/elps.v35.9 |
| [116] | Z. Mala, P. Pantuckova, P. Gebauer, P. Bocek. Advanced electrolyte tuning and selectivity enhancement for highly sensitive analysis of cations by capillary ITP-ESI MS. Electrophoresis 34 (2013) 777–784. DOI:10.1002/elps.201200533 |
| [117] | R. Gahoual, J.-M. Busnel, A. Beck, Y.-N. Francois, E. Leize-Wagner. Full antibody primary structure and microvariant characterization in a single injection using transient isotachophoresis and sheathless capillary electrophoresis-tandem mass spectrometry. Anal. Chem 86 (2014) 9074–9081. DOI:10.1021/ac502378e |
| [118] | Y. He, M. Harir, G. Chen, et al., Capillary electrokinetic fractionation mass spectrometry (CEkF/MS):technology setup and application to metabolite fractionation from complex samples coupled at-line with ultrahighresolution mass spectrometry. Electrophoresis 35 (2014) 1965–1975. DOI:10.1002/elps.201400041 |
| [119] | J. Huhner, M. Lammerhofer, C. Neususs. Capillary isoelectric focusing-mass spectrometry:coupling strategies and applications. Electrophoresis 36 (2015) 2670–2686. DOI:10.1002/elps.201500185 |
| [120] | J. Huhner, K. Jooss, C. Neusubb. Interference-free mass spectrometric detection of capillary isoelectric focused proteins, including charge variants of a model monoclonal antibody. Electrophoresis 38 (2017) 914–921. DOI:10.1002/elps.201600457 |
| [121] | G. Zhu, L. Sun, R.B. Keithley, N.J. Dovichi. Capillary lsoelectric focusingtandem mass spectrometry and reversed-phase liquid chromatography-tandem mass spectrometry for quantitative proteomic analysis of differentiating pc12 cells by eight-plex isobaric tags for relative and absolute quantification. Anal. Chem. 85 (2013) 7221–7229. DOI:10.1021/ac4009868 |
| [122] | G. Zhu, L. Sun, R. Wojcik, et al., A rapid cIEF-ESI-MS/MS method for host cell protein analysis of a recombinant human monoclonal antibody. Talanta 98 (2012) 253–256. DOI:10.1016/j.talanta.2012.07.017 |
| [123] | G. Zhu, L. Sun, P. Yang, N.J. Dovichi. On-line amino acid-based capillary isoelectric focusing-ESI-MS/MS for protein digests analysis. Anal. Chim. Acta 750 (2012) 207–211. DOI:10.1016/j.aca.2012.04.026 |
| [124] | S. Li, C.-G. Guo, L. Chen, et al., Impact of glutathione-HbA(1c) on HbA(1c) measurement in diabetes diagnosis via array isoelectric focusing liquid chromatography, mass spectrometry and ELISA. Talanta 115 (2013) 323–328. DOI:10.1016/j.talanta.2013.05.040 |
| [125] | C. Przybylski, M. Mokaddem, M. Prull-Janssen, et al., On-line capillary isoelectric focusing hyphenated to native electrospray ionization mass spectrometry for the characterization of interferon-gamma and variants. Analyst 140 (2015) 543–550. DOI:10.1039/C4AN01305K |
| [126] | M. Horka, P. Karasek, J. Salplachta, et al., Capillary isoelectric focusing of probiotic bacteria from cow's milk in tapered fused silica capillary with offline matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification. Anal. Chim. Acta 788 (2013) 193–199. DOI:10.1016/j.aca.2013.05.059 |
| [127] | M. Horka, J. Salplachta, P. Karasek, et al., Combination of capillary isoelectric focusing in a tapered capillary with MALDI-TOF MS for rapid and reliable identification of dickeya species from plant samples. Anal. Chem. 85 (2013) 6806–6812. DOI:10.1021/ac4009176 |
| [128] | N. Nordman, S. Lauren, T. Kotiaho, et al., Interfacing microchip isoelectric focusing with on-chip electrospray ionization mass spectrometry. J. Chromatogr. A 1398 (2015) 121–126. DOI:10.1016/j.chroma.2015.04.031 |
| [129] | Z. Zhang, J. Wang, L. Hui, L. Li. Poly(glycidyl methacrylate-divinylbenzene) based immobilized pH gradient capillary isoelectric focusing coupling with MALDI mass spectrometry for enhanced neuropeptide analysis. Electrophoresis 33 (2012) 661–665. DOI:10.1002/elps.201100447 |
| [130] | H. Tiala, M.-L. Riekkola, S.K. Wiedmer. Study on capillaries covalently bound with phospholipid vesicles for open-tubular CEC and application to on-line open-tubular CEC-MS. Electrophoresis 34 (2013) 3180–3188. DOI:10.1002/elps.v34.22-23 |
| [131] | W. Bragg, S.A. Shamsi. High throughput analysis of chiral compounds using capillary electrochromatography (CEC) and CEC-mass spectrometry with cellulose based stationary phases. Sep. Sci. Tech. 48 (2013) 2589–2599. DOI:10.1080/01496395.2012.719984 |
| [132] | G. D' Orazio, S. Fanali. Pressurized nano-liquid-junction interface for coupling capillary electrochromatography and nano-liquid chromatography with mass spectrometry. J. Chromatogr. A 1317 (2013) 67–76. DOI:10.1016/j.chroma.2013.08.052 |
| [133] | Q. Wu, X.W. Yu, Y. Wang, et al., Pressurized CEC coupled with QTOF-MS for urinary metabolomics. Electrophoresis 35 (2014) 2470–2478. DOI:10.1002/elps.v35.17 |
| [134] | D.C. Simpson, A.J. Yates, J.H. Knox, P.R.R. Langridge-Smith. A novel two-laser interface for coupling capillary electrochromatography with ion-trap timeof-flight mass spectrometry. Int. J. Mass Spectrom. 363 (2014) 8–15. DOI:10.1016/j.ijms.2014.02.005 |
| [135] | G. D' Orazio, M. Asensio-Ramos, J. Hernandez-Borges, S. Fanali, M. Angel Rodriguez-Delgado. Estrogenic compounds determination in water samples by dispersive liquid-liquid microextraction and micellar electrokinetic chromatography coupled to mass spectrometry. J. Chromatogr. A 1344 (2014) 109–121. DOI:10.1016/j.chroma.2014.04.005 |
| [136] | G. D' Orazio, M. Asensio-Ramos, J. Hernandez-Borges, M. Angel RodriguezDelgado, S. Fanali. Evaluation of the combination of a dispersive liquid-liquid microextraction method with micellar electrokinetic chromatography coupled to mass spectrometry for the determination of estrogenic compounds in milk and yogurt. Electrophoresis 36 (2015) 615–625. DOI:10.1002/elps.v36.4 |
| [137] | M. Svidrnoch, L. Lnenickova, I. Valka, P. Ondra, V. Maier. Utilization of micellar electrokinetic chromatography-tandem mass spectrometry employed volatile micellar phase in the analysis of cathihone designer drugs. J. Chromatogr. A 1356 (2014) 258–265. DOI:10.1016/j.chroma.2014.06.058 |
| [138] | S. Akamatsu, T. Mitsuhashi. MEKC-MS/MS method using a volatile surfactant for the simultaneous determination of 12 synthetic cannabinoids. J. Sep. Sci. 37 (2014) 304–307. DOI:10.1002/jssc.v37.3 |
| [139] | D. Moreno-Gonzalez, J.S. Torano, L. Gamiz-Gracia, et al., Micellar electrokinetic chromatography-electrospray ionization mass spectrometry employing a volatile surfactant for the analysis of amino acids in human urine. Electrophoresis 34 (2013) 2615–2622. DOI:10.1002/elps.v34.18 |
| [140] | X. Wang, J. Hou, M. Jann, Y.Y. Hon, S.A. Shamsi. Development of a chiral micellar electrokinetic chromatography-tandem mass spectrometry assay for simultaneous analysis of warfarin and hydroxywarfarin metabolites: application to the analysis of patients serum samples. J. Chromatogr. A 1271 (2013) 207–216. DOI:10.1016/j.chroma.2012.11.046 |
| [141] | B. Franze, C. Engelhard. Fast separation characterization, and speciation of gold and silver nanoparticles and their ionic counterparts with micellar electrokinetic chromatography coupled to ICP-MS. Anal. Chem. 86 (2014) 5713–5720. DOI:10.1021/ac403998e |
| [142] | J. Rodriguez, G. Castaneda, L. Munoz. Direct determination of pregabalin in human urine by nonaqueous CE-TOF-MS. Electrophoresis 34 (2013) 1429–1436. DOI:10.1002/elps.v34.9-10 |
| [143] | J. Rodriguez, G. Castaneda, L. Munoz, J.C. Villa. Quantitation of sunitinib, an oral multitarget tyrosine kinase inhibitor, and its metabolite in urine samples by nonaqueous capillary electrophoresis time of flight mass spectrometry. Electrophoresis 36 (2015) 1580–1587. DOI:10.1002/elps.v36.14 |
| [144] | Y. Zhang, Z. Chen. Nonaqueous CE ESI-IT-MS analysis of amaryllidaceae alkaloids. J. Sep. Sci. 36 (2013) 1078–1084. DOI:10.1002/jssc.201201083 |
| [145] | J. Zhang, Z. Chen. Determination of matrine and oxymatrine in sophora flavescens by nonaqueous capillary electrophoresis-electrospray ionizationion trap-mass spectrometry. Analy. Lett. 46 (2013) 651–662. DOI:10.1080/00032719.2012.726684 |
| [146] | Q. Chen, J. Zhang, W. Zhang, Z. Chen. Analysis of active alkaloids in the Menispermaceae family by nonaqueous capillary electrophoresis-ion trap mass spectrometry. J. Sep. Sci. 36 (2013) 341–349. DOI:10.1002/jssc.201200678 |
| [147] | G. Bonvin, J. Schappler, S. Rudaz. Non-aqueous capillary electrophoresis for the analysis of acidic compounds using negative electrospray ionization mass spectrometry. J. Chromatogr. A 1323 (2014) 163–173. DOI:10.1016/j.chroma.2013.11.011 |
| [148] | D. Tho Chau, Minh Vinh, N. Tuan Duc, T. Hung, H. Stuppner, M. Ganzera. Analysis of alkaloids in Lotus (Nelumbo nucifera Gaertn.) leaves by nonaqueous capillary electrophoresis using ultraviolet and mass spectrometric detection. J. Chromatography A 1302 (2013) 174–180. DOI:10.1016/j.chroma.2013.06.002 |
| [149] | C. Montealegre, L. Sanchez-Hernandez, A.L. Crego, M.L. Marina. Determination and characterization of glycerophospholipids in olive fruit and oil by nonaqueous capillary electrophoresis with electrospray-mass spectrometric detection. J. Agric. Food Chem. 61 (2013) 1823–1832. DOI:10.1021/jf304357e |
| [150] | J. Roscher, H. Faber, M. Stoffels, et al., Nonaqueous capillary electrophoresis as separation technique to support metabolism studies by means of electrochemistry and mass spectrometry. Electrophoresis 35 (2014) 2386–2391. DOI:10.1002/elps.v35.16 |
| [151] | A.K. Malik, M. Grundmann, F.-M. Matysik. Development of a fast capillary electrophoresis-time-of-flight mass spectrometry method for the speciation of organotin compounds under separation conditions of high electrical field strengths. Talanta 116 (2013) 559–562. DOI:10.1016/j.talanta.2013.07.025 |
| [152] | G. Bonvin, S. Rudaz, J. Schappler. In-spray supercharging of intact proteins by capillary electrophoresis-electrospray ionization-mass spectrometry using sheath liquid interface. Anal. Chim. Acta 813 (2014) 97–105. DOI:10.1016/j.aca.2013.12.043 |
| [153] | M. Mateos-Vivas, E. Rodriguez-Gonzalo, J. Dominguez-Alvarez, et al., Analysis of free nucleotide monophosphates in human milk and effect of pasteurisation or high-pressure processing on their contents by capillary electrophoresis coupled to mass spectrometry. Food Chem 174 (2015) 348–355. DOI:10.1016/j.foodchem.2014.11.051 |
| [154] | E. Rodriguez-Gonzalo, R. Hernandez-Prieto, D. Garcia-Gomez, R. CarabiasMartinez. Capillary electrophoresis-mass spectrometry for direct determination of urinary modified nucleosides. Evaluation of synthetic urine as a surrogate matrix for quantitative analysis. J. Chromatogr. B-Anal. Tech. Biomed. Life Sci 942 (2013) 21–30. |
| [155] | M. Bustamante-Rangel, M.M. Delgado-Zamarreno, L. Perez-Martin, R. Carabias-martinez. QuEChERS method for the extraction of isoflavones from soy-based foods before determination by capillary electrophoresis-electrospray ionization-mass spectrometry. Microchem. J 108 (2013) 203–209. DOI:10.1016/j.microc.2012.10.023 |
| [156] | K. Marakova, J. Piestansky, E. Havranek, P. Mikus. Simultaneous analysis of vitamins B in pharmaceuticals and dietary supplements by capillary electrophoresis hyphenated with triple quadrupole mass spectrometry. Pharmazie 69 (2014) 663–668. |
| [157] | K. Marakova, J. Piest, ' ansky, L. Veizerova, et al., Multidrug analysis of pharmaceutical and urine matrices by on-line coupled capillary electrophoresis and triple quadrupole mass spectrometry. J. Sep. Sci. 36 (2013) 1805–1816. DOI:10.1002/jssc.v36.11 |
| [158] | R.G. Jayo, M. Thaysen-Andersen, P.W. Lindenburg, et al., Simple capillary electrophoresis-mass spectrometry method for complex glycan analysis using a flow-through microvial interface. Anal. Chem. 86 (2014) 6479–6486. DOI:10.1021/ac5010212 |
| [159] | P. Ginterova, B. Sokolova, P. Ondra, et al., Determination of mushroom toxins ibotenic acid, muscimol and muscarine by capillary electrophoresis coupled with electrospray tandem mass spectrometry. Talanta 125 (2014) 242–247. DOI:10.1016/j.talanta.2014.03.019 |
| [160] | M. Kondekova, V. Maier, P. Ginterova, J. Marak, J. Sevcik. Analysis of lysozyme in cheese samples by on-line combination of capillary zone electrophoresis and mass spectrometry. Food Chem. 153 (2014) 398–404. DOI:10.1016/j.foodchem.2013.12.078 |
| [161] | D. Daniel, V.B. dos Santos, D.T.R. Vidal, C.L. do Lago. Determination of biogenic amines in beer and wine by capillary electrophoresis-tandem mass spectrometry. J. Chromatogr. A 1416 (2015) 121–128. DOI:10.1016/j.chroma.2015.08.065 |
| [162] | E. Ortiz-Villanueva, F. Benavente, E. Gimenez, F. Yilmaz, V. Sanz-Nebot. Preparation and evaluation of open tubular C18-silica monolithic microcartridges for preconcentration of peptides by on-line solid phase extraction capillary electrophoresis. Anal. Chim. Acta 846 (2014) 51–59. DOI:10.1016/j.aca.2014.06.046 |
| [163] | Y.-M. Dong, K.-Y. Chien, J.-T. Chen, et al., Site-specific separationand detection of phosphopeptide isomers with pH-mediated stacking capillary electrophoresis-electrospray ionization-tandem mass spectrometry. J. Sep. Sci. 36 (2013) 1582–1589. DOI:10.1002/jssc.201300054 |
| [164] | A. Prior, L. Sanchez-Hernandez, J. Sastre-Torano, M.L. Marina, G.J. de Jong, G. W. Somsen. Enantioselective analysis of proteinogenic amino acids in cerebrospinal fluid by capillary electrophoresis-mass spectrometry. Electrophoresis 37 (2016) 2410–2419. DOI:10.1002/elps.v37.17-18 |
| [165] | M. Tascon, F. Benavente, V.M. Sanz-Nebot, L.G. Gagliardi. Fast determination of harmala alkaloids in edible algae by capillary electrophoresis mass spectrometry. Anal. Bioanal. Chem. 407 (2015) 3637–3645. DOI:10.1007/s00216-015-8579-4 |
| [166] | D. Moreno-Gonzalez, F.J. Lara, N. Jurgovska, L. Gamiz-Gracia, A.M. GarciaCampana. Determination of aminoglycosides in honey by capillary electrophoresis tandem mass spectrometry and extraction with molecularly imprinted polymers. Anal. Chim. Acta 891 (2015) 321–328. DOI:10.1016/j.aca.2015.08.003 |
| [167] | C.R. Warren. High diversity of small organic Nobserved in soil water. Soil Biol. Biochem. 57 (2013) 444–450. DOI:10.1016/j.soilbio.2012.09.025 |
| [168] | C.R. Warren. Response of organic N monomers in a sub-alpine soil to a drywet cycle. Soil Biol. Biochem. 77 (2014) 233–242. DOI:10.1016/j.soilbio.2014.06.028 |
| [169] | A.-L. Marie, C. Przybylski, F. Gonnet, et al., Capillary zone electrophoresis and capillary electrophoresis-mass spectrometry for analyzing qualitative and quantitative variations in therapeutic albumin. Anal. Chim. Acta 800 (2013) 103–110. DOI:10.1016/j.aca.2013.09.023 |
| [170] | A. Ma Lopez-Montes, A.-L. Dupont, B. Desmazieres, B. Lavedrine. Identification of synthetic dyes in early colour photographs using capillary electrophoresis and electrospray ionisation-mass spectrometry. Talanta 114 (2013) 217–226. DOI:10.1016/j.talanta.2013.04.020 |
| [171] | T.J. Causon, L. Maringer, W. Buchberger, C.W. Klampfl. Addition of reagents to the sheath liquid:a novel concept in capillary electrophoresis-mass spectrometry. J. Chromatogr. A 1343 (2014) 182–187. DOI:10.1016/j.chroma.2014.04.002 |
| [172] | S. Medina-Casanellas, F. Benavente, J. Barbosa, V. Sanz-Nebot. Preparation and evaluation of an immunoaffinity sorbent with Fab' antibody fragments for the analysis of opioid peptides by on-line immunoaffinity solid-phase extraction capillary electrophoresis-mass spectrometry. Anal. Chim. Acta 789 (2013) 91–99. DOI:10.1016/j.aca.2013.06.030 |
| [173] | A. Barroso, E. Gimenez, F. Benavente, J. Barbosa, V. Sanz-Nebot. Analysis of human transferrin glycopeptides by capillary electrophoresis and capillary liquid chromatography-mass spectrometry. Application to diagnosis of alcohol dependence. Anal. Chim. Acta 804 (2013) 167–175. DOI:10.1016/j.aca.2013.09.044 |
| [174] | A. Barroso, E. Gimenez, F. Benavente, J. Barbosa, V. Sanz-Nebot. Modelling the electrophoretic migration behaviour of peptides and glycopeptides from glycoprotein digests in capillary electrophoresis-mass spectrometry. Anal. Chim. Acta 854 (2015) 169–177. DOI:10.1016/j.aca.2014.10.038 |
| [175] | C. Brueckner, D. Imhof, G.K.E. Scriba. Capillary electrophoretic study of the degradation pathways and kinetics of the aspartyl model tetrapeptide GlyPhe-Asp-GlyOH in alkaline solution. J. Pharm. Biomed. Anal 76 (2013) 96–103. DOI:10.1016/j.jpba.2012.12.012 |
| [176] | S. Catala-Clariana, F. Benavente, E. Gimenez, J. Barbosa, V. Sanz-Nebot. Identification of bioactive peptides in hypoallergenic infant milk formulas by CE-TOF-MS assisted by semiempirical model of electromigration behavior. Electrophoresis 34 (2013) 1886–1894. DOI:10.1002/elps.201200547 |
| [177] | R. Haselberg, G.J. de Jong, G.W. Somsen. Low-flow sheathless capillary electrophoresis-mass spectrometry for sensitive glycoform profiling of intact pharmaceutical proteins. Anal. Chem. 85 (2013) 2289–2296. DOI:10.1021/ac303158f |
| [178] | R. Haselberg, S. Oliveira, R. van der Meel, G.W. Somsen, G.J. de Jong. Capillary electrophoresis-based assessment of nanobody affinity and purity. Anal. Chim. Acta 818 (2014) 1–6. DOI:10.1016/j.aca.2014.01.048 |
| [179] | G. Klein, J. P. Schanstra, J. Hoffmann, et al. , Proteomics as a quality control tool of pharmaceutical probiotic bacterial lysate products, PLoS One 8(2013). |
| [180] | I. Kohler, M. Augsburger, S. Rudaz, J. Schappler. New insights in carbohydrate-deficient transferrin analysis with capillary electrophoresis-mass spectrometry. Forensic Sci. In. 243 (2014) 14–22. DOI:10.1016/j.forsciint.2014.03.014 |
| [181] | L. Pont, F. Benavente, J. Barbosa, V. Sanz-Nebot. Analysis of transthyretin in human serum bycapillary zone electrophoresis electrospray ionization timeof-flight mass spectrometry. Application to familial amyloidotic polyneuropathy type I. Electrophoresis 36 (2015) 1265–1273. DOI:10.1002/elps.v36.11-12 |
| [182] | L. Bertoletti, J. Schappler, R. Colombo, et al., Evaluation of capillary electrophoresis-mass spectrometry for the analysis of the conformational heterogeneity of intact proteins using beta(2)-microglobulin as model compound. Anal. Chim. Acta 945 (2016) 102–109. DOI:10.1016/j.aca.2016.10.010 |
| [183] | M. Han, B.M. Rock, J.T. Pearson, D.A. Rock. Intact mass analysis of monoclonal antibodies by capillary electrophoresis-Mass spectrometry. J. Chromatogr. BAnal. Tech. Biomed. Life Sci. 1011 (2016) 24–32. DOI:10.1016/j.jchromb.2015.12.045 |
| [184] | N. Khan, G. Mironov, M.V. Berezovski. Direct detection of endogenous MicroRNAs and their post-transcriptional modifications in cancer serum by capillary electrophoresis-mass spectrometry. Anal. Bioanal. Chem. 408 (2016) 2891–2899. DOI:10.1007/s00216-015-9277-y |
| [185] | S.-C. Bunz, F. Cutillo, C. Neusuess. Analysis of native and APTS-labeled Nglycans by capillary electrophoresis/time-of-flight mass spectrometry. Anal. Bioanal. Chem. 405 (2013) 8277–8284. DOI:10.1007/s00216-013-7231-4 |
| [186] | S.-C. Bunz, E. Rapp, C. Neusuess. Capillary electrophoresis/mass spectrometry of apts-labeled glycans for the identification of unknown glycan species in capillary electrophoresis/laser-induced fluorescence systems. Anal. Chem. 85 (2013) 10218–10224. DOI:10.1021/ac401930j |
| [187] | E.M. Weissinger, W. Mullen, A. Albalat. Urinary proteomics employing capillary electrophoresis coupled to mass spectrometry in the monitoring of patients after stem cell transplantation. Methods Mol. Biol 1109 (2014) 293–306. DOI:10.1007/978-1-4614-9437-9 |
| [188] | P.K. Contreras-Gutierrez, E. Hurtado-Fernandez, M. Gomez-Romero, et al., Determination of changes in the metabolic profile of avocado fruits (Persea americana) by two CE-MS approaches (targeted and non-targeted). Electrophoresis 34 (2013) 2928–2942. |
| [189] | A. Garcia, S. Naz, C. Barbas. Metabolite fingerprinting by capillary electrophoresis-mass spectrometry. Methods Mol. Biol. 1198 (2014) 107–123. DOI:10.1007/978-1-4939-1258-2 |
| [190] | J. Godzien, D. Garcia-Martinez, P. Martinez-Alcazar, F.J. Ruperez, C. Barbas. Effect of a nutraceutical treatment on diabetic rats with targeted and CE-MS non-targeted approaches. Metabolomics 9 (2013) S188–S202. DOI:10.1007/s11306-011-0351-y |
| [191] | C. Ibanez, C. Simo, A. Valdes, et al., Metabolomics of adherent mammalian cells by capillary electrophoresis-mass spectrometry:HT-29 cells as case study. J. Pharm. Biomed. Anal. 110 (2015) 83–92. DOI:10.1016/j.jpba.2015.03.001 |
| [192] | K. Kami, T. Fujimori, H. Sato, et al., Metabolomic profiling of lung and prostate tumor tissues by capillary electrophoresis time-of-flight mass spectrometry. Metabolomics 9 (2013) 444–453. DOI:10.1007/s11306-012-0452-2 |
| [193] | M.G.M. Kok, M.M.A. Ruijken, J.R. Swann, et al., Anionic metabolic profiling of urine from antibiotic-treated rats by capillary electrophoresis-mass spectrometry. Anal. Bioanal Chem. 405 (2013) 2585–2594. DOI:10.1007/s00216-012-6701-4 |
| [194] | M.G.M. Kok, G.W. Somsen, G.J. de Jong. Comparison of capillary electrophoresis-mass spectrometry and hydrophilic interaction chromatography-mass spectrometry for anionic metabolic profiling of urine. Talanta 132 (2015) 1–7. DOI:10.1016/j.talanta.2014.08.047 |
| [195] | S. Kume, M. Yamato, Y. Tamura, et al., Potential biomarkers of fatigue identified by plasma metabolome analysis in rats. PLoS One 10 (2015) e0120106. DOI:10.1371/journal.pone.0120106 |
| [196] | H.J. Kwon, Y. Ohmiya. Metabolomic analysis of differential changes in metabolites during ATP oscillations in chondrogenesis. BioMed Res. Int 2013 (2013) 213972. |
| [197] | S. Muroya, M. Oe, I. Nakajima, K. Ojima, K. Chikuni. CE-TOF MS-based metabolomic profiling revealed characteristic metabolic pathways in postmortem porcine fast and slow type muscles. Meat Sci 98 (2014) 726–735. DOI:10.1016/j.meatsci.2014.07.018 |
| [198] | S. Naz, A. Garcia, M. Rusak, C. Barbas. Method development and validation for rat serum fingerprinting with CE-MS:application to ventilator-inducedlung-injury study. Anal. Bioanal. Chem. 405 (2013) 4849–4858. DOI:10.1007/s00216-013-6882-5 |
| [199] | M. Tsuruoka, J. Hara, A. Hirayama, et al., Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis 34 (2013) 2865–2872. |
| [200] | J. Zeng, H. Kuang, C. Hu, et al., Effect of bisphenol a on rat metabolic profiling studied by using capillary electrophoresis time-of-flight mass spectrometry. Environ. Sci. Tech. 47 (2013) 7457–7465. DOI:10.1021/es400490f |
| [201] | J. Zeng, P. Yin, Y. Tan, et al., Metabolomics study of hepatocellular carcinoma: discovery and validation of serum potential biomarkers by using capillary electrophoresis-mass spectrometry. J. Proteome Res 13 (2014) 3420–3431. DOI:10.1021/pr500390y |
| [202] | H. Yamamoto, K. Sasaki. Metabolomics-based approach for ranking the candidate structures of unidentified peaks in capillary electrophoresis timeof-flight mass spectrometry. Electrophoresis 38 (2016) 1053–1059. |
| [203] | T.A. Isbell, E.C. Strickland, J. Hitchcock, G. McIntire, C.L. Colyer. Capillary electrophoresis-mass spectrometry determination of morphine and its isobaric glucuronide metabolites. J. Chromatogr. B-Anal. Tech. Biomed. Life Sci. 980 (2015) 65–71. DOI:10.1016/j.jchromb.2014.11.035 |
| [204] | G. Merola, H. Fu, F. Tagliaro, T. Macchia, B.R. McCord. Chiral separation of 12 cathinone analogs bycyclodextrin-assisted capillary electrophoresis with UV and mass spectrometry detection. Electrophoresis 35 (2014) 3231–3241. DOI:10.1002/elps.v35.21-22 |
| [205] | A. Wozniakiewicz, R. Wietecha-Posluszny, M. Wozniakiewicz, E. Bryczek, P. Koscielniak. A quick method for determination of psychoactive agents in serum and hair by using capillary electrophoresis and mass spectrometry. J. Pharm. Biomed. Anal. 111 (2015) 177–185. DOI:10.1016/j.jpba.2015.03.029 |
| [206] | N. Said, R. Gahoual, L. Kuhn, et al., Structural characterization of antibody drug conjugate by a combination of intact, middle-up and bottom-up techniques using sheathless capillary electrophoresis-Tandem mass spectrometry as nanoESI infusion platform and separation method. Anal. Chim. Acta 918 (2016) 50–59. DOI:10.1016/j.aca.2016.03.006 |
| [207] | I. Kohler, J. Schappler, S. Rudaz. Highly sensitive capillary electrophoresismass spectrometry for rapid screening and accurate quantitation of drugs of abuse in urine. Anal. Chim. Acta 780 (2013) 101–109. DOI:10.1016/j.aca.2013.03.065 |
| [208] | I. Kohler, J. Schappler, T. Sierro, S. Rudaz. Dispersive liquid-liquid microextraction combined with capillary electrophoresis and time-offlight mass spectrometry for urine analysis. J. Pharm. Biomed. Anal. 73 (2013) 82–89. DOI:10.1016/j.jpba.2012.03.036 |
| [209] | A.M. van Wijk, H.A.G. Niederlander, M.D. van Ogten, G.J. de Jong. Sensitive CEMS analysis of potentially genotoxic alkylation compounds using derivatization and electrokinetic injection. Anal. Chim. Acta 874 (2015) 75–83. DOI:10.1016/j.aca.2015.02.067 |
| [210] | J. Chen, Q. Shi, Y. Wang, Z. Li, S. Wang. Dereplication of known nucleobase and nucleoside compounds in natural product extracts by capillary electrophoresis-high resolution mass spectrometry. Molecules 20 (2015) 5423–5437. DOI:10.3390/molecules20045423 |
| [211] | S. Gusenkov, C. Ackaert, H. Stutz. Separation and characterization of nitrated variants of the major birch pollen allergen by CZE-ESI-mu TOF MS. Electrophoresis 34 (2013) 2695–2704. DOI:10.1002/elps.v34.18 |
| [212] | P. Nemes, S.S. Rubakhin, J.T. Aerts, J.V. Sweedler. Qualitative and quantitative metabolomic investigation of single neurons by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Protoc. 8 (2013) 783–799. DOI:10.1038/nprot.2013.035 |
| [213] | T.J. Causon, M. Himmelsbach, W. Buchberger, C.W. Klampfl. Identification of polyimide materials using quantitative CE with UV and QTOF-MS detection. Electrophoresis 34 (2013) 944–949. DOI:10.1002/elps.201200525 |
| [214] | I. Hintersteiner, M. Himmelsbach, C. Klampfl, W.W. Buchberger. Characterization of hindered amine light stabilizers employing capillary electrophoresis coupled to quadrupole time-of-flight mass spectrometry. Electrophoresis 35 (2014) 1368–1374. DOI:10.1002/elps.v35.9 |
| [215] | A. Kula, M. Krol, R. Wietecha-Posluszny, M. Wozniakiewicz, P. Koscielniak. Application of CE-MS to examination of black inkjet printing inks for forensic purposes. Talanta 128 (2014) 92–101. DOI:10.1016/j.talanta.2014.04.004 |
| [216] | T. Nolte, T.N. Posch, C. Huhn, J.T. Andersson. Desulfurized fuels from athabasca bitumen and their polycyclic aromatic sulfur heterocycles. Analysis based on capillaryelectrophoresis coupled with TOF MS. Energ. Fuel 27 (2013) 97–107. DOI:10.1021/ef301424d |
| [217] | B. Michalke, M. Lucio, A. Berthele, B. Kanawati. Manganese speciation in paired serum and CSF samples using SEC-DRC-ICP-MS and CE-ICP-DRC-MS. Anal. Bioanal. Chem. 405 (2013) 2301–2309. DOI:10.1007/s00216-012-6662-7 |
| [218] | M.M. Yassine, E. Dabek-Zlotorzynska, M. Harir, P. Schmitt-Kopplin. Identification of weak and strong organic acids in atmospheric aerosols by capillary electrophoresis/mass spectrometry and ultra-high-resolution fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 84 (2012) 6586–6594. DOI:10.1021/ac300798g |
| [219] | X. Yan, D.C. Essaka, L. Sun, G. Zhu, N.J. Dovichi. Bottom-up proteome analysis of E. coli using capillary zone electrophoresis-tandem mass spectrometry with an electrokinetic sheath-flow electrospray interface. Proteomics 13 (2013) 2546–2551. DOI:10.1002/pmic.v13.17 |
| [220] | G. Zhu, L. Sun, X. Yan, N.J. Dovichi. Bottom-up proteomics of escherichia coli using dynamic ph junction preconcentration and capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry. Anal. Chem. 86 (2014) 6331–6336. DOI:10.1021/ac5004486 |
 2017, Vol. 28
2017, Vol. 28 


