b BNLMS, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
On-surface preparation of low-dimensional nanostructures by in-situ assemblies [1-6] and reactions [7-12] of organic building blocks is a promising approach to fabricating high-performance functional nanomaterials [13-16]. Driven by the demands for the diverse and high-quality nanostructures, people have made great effort to design proper organic building blocks and explore their assemblies and reactions on surfaces. For example, halides and their halogen-bond-induced self-assemblies [17-22] and Ullmann couplings [8, 10, 11, 23-29], boronic esters and their dehydration condensations [30], alkanes and their dehydrogenative couplings [31], porphyrins and their organometallic reactions with metal atoms [32-34], and so on and so forth have been investigated in detail. Among various organic building blocks, terminal alkynes (TAs), a family of precursors suitable for on-surface assemblies and reactions, have received widespread attention in the past decade, mainly due to their potential application in fabricating carbon based scaffolds [35, 36] containing both sp2 and sp carbon atoms such as graphyne [37, 38] and graphdiyne [39-41], which are promising candidates as molecular electronic devices and energy storage materials. In addition to the reactions leading to graphyne and graphdiyne, TAs were also involved in non-bonding interactions [42-47], organometallic reactions [48-51], and other types of covalent reactions [50, 52-54]. Compared with other organic precursors, their diversified linking strategies make TAs flexible building blocks for producing various nanostructures. However, efficient controlling methodologies of their on-surface linkages are prerequisite to achieve high selectivity and quality of TA-based nanoarchitectures.
This review overviews the studies of on-surface linking strategies of TAs, including new assembling modes and reaction types and their tuning methods as well, aiming at fabricating lowdimensional nanostructures. These summarized studies were mainly performed by scanning tunneling microscopy (STM) which could scrutinize morphological transformations of TAs on surfaces at molecular and submolecular levels. The review includes two main parts. One describes three on-surface linking strategies for TAs, i.e. non-bonding interactions, organometallic bonds and covalent bonds according to previous reports. The other deals with several controlling methods for TA assemblies and reactions, including designing the structures of the TA building blocks, employing different substrates, and changing the activation modes with specific examples.
2. On-surface linking strategies for TAsFormation of non-bonding interactions, organometallic bonds and covalent bonds are three main linking strategies frequently employed in the bottom-up approach to building low-dimensional nanostructures on surfaces by using functional organic molecules including TAs. The hitherto described on-surface linking types of TAs are briefly summarized and classified into three categories, as illustrated in Scheme 1. The on-surface formations of non-bonding interactions and organometallic bonds involving TAs are in-depth reviewed with examples in the following. The covalent reactions of TAs on surfaces have been recently summarized by Klappenberger et al. [55], and therefore are not discussed in detail in this review.
|
Download:
|
| Scheme1. Schematic illustration of on-surface linking strategies for TAs. (The resulted non-bonding interactions and bonds are highlighted with dashed and solid red lines, respectively). | |
2.1. TA-involved non-bonding interactions
With acidic protons as electron acceptors, and electron-rich π-orbitals as electron donors, TAs are considered to be proper building block candidates for CH/π-type hydrogen bonding networks [56, 57], which has been confirmed by structural analyses of several TA crystals [58-61]. Similarly, TA molecules confined on surfaces should also possess the capability to form low-dimensional hydrogen bonding networks through intermolecular CH/π interactions (linking type 1, as shown in Scheme 1).
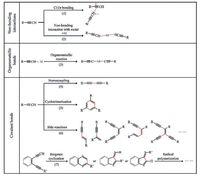
The first STM study on the nanostructure formed by CH/ π-bonded TA molecules was reported by Li et al. [43]. Phenylacetylene monomers could assemble into isolated hexamers on Au (111) at low coverage (Fig. 1a-ⅰ) by intermolecular CH/π interactions. The hexamers consist of three central molecules forming a pinwheel-like aggregate and three corner ones being nearly perpendicular to their central neighbors. The formation of CH/π interactions was theoretically confirmed by natural bond orbital (NBO) analysis (Fig. 1a-ⅱ).
|
Download:
|
| Fig. 1. Ordered low-dimensional nanostructures formed by TA molecules through non-bonding interactions. (a) STM image of an isolated hexamer formed by phenylacetylene on Au(111) (ⅰ) and its molecular model (ⅱ) with values of stabilization energy (in kcal/mol) marked by numbers and directions of electron transfer marked by arrows. The chirality of three central molecules in the hexamer is highlighted with a red arrow in (ⅱ). Adapted with permission from Ref. [43]. Copyright 2012 American Chemical Society. (b) STM image of the 2D network formed by TEB on Ag(111) (ⅰ) and the calculated electron charge difference plot (ⅱ). The chirality of the six-membered ring is highlighted with a red arrow in (ⅱ). Adapted with permission from Ref. [44]. Copyright 2013 American Chemical Society. (c) STM images of four co-assembled structures formed by DEDM molecules and Ag adatoms on Ag(111) (ⅰ-ⅳ) with molecular models superimposed and unit cells highlighted with blue parallelograms. Adapted with permission from Ref. [47]. Copyright 2016 The Royal Society of Chemistry. | |
Beside the isolated molecular clusters, two-dimensional (2D) self-assembled networks were also achieved by using TAs as building blocks. By depositing 1, 3, 5-triethynylbenzene (TEB) and 1, 3, 5-tris-(4-ethynylphenyl)benzene (Ext-TEB) onto Ag(111), Kepčija et al. [44] achieved the formation of highly-ordered 2D networks. The triangular TEB molecules took a chiral arrangement and formed six-membered rings with a central cavity (Fig. 1b-ⅰ). Electron charge difference analysis indicated that the CH/π bonds between TA molecules (shown by the green dotted ellipse in Fig. 1b-ⅱ) were the dominant interactions which stabilized the 2D networks. Follow-up studies [62] with scanning tunneling spectroscopy (STS) and density functional theory (DFT) revealed the existence of localized nanopore orbitals centered in the cavities surrounded by six CH/π-bonded TA molecules.
Similar assembling patterns of the TA molecules can be identified in both phenylacetylene-hexamers and TEB-networks: Several (specifically, three in the phenylacetylene-hexamers and six in the TEB-networks) TA molecules arrange in a ring with the terminal alkynyl proton in each molecule pointing to the π-system of the terminal alkynyl in an adjacent molecule, leading to the formation of a chiral subunit (i.e. three pinwheel-like central molecules in phenylacetylene-hexamers and six-membered rings in TEB-networks whose chiralities are marked by red arrows in Fig. 1a-ⅱ and b-ⅱ). Such a special arrangement ensures a relatively short distance between the terminal alkynyl proton (electron acceptor) and the π-system (electron donor), which is necessary for the formation of the CH/π interactions. Recently, this featured assembling pattern was also experimentally observed in other selfassembled structures formed by TAs [47].
In addition to the intermolecular CH/π bonding, our recent work [47] demonstrated the formation of non-bonding interactions between TAs and metal atoms (linking type 2 in Scheme 1). A series of ordered 2D co-assembled structures formed by 1, 4-diethynyl-2, 5-dimethylbenzene (DEDM) and Ag adatoms (Fig. 1c) were prepared by depositing DEDM onto Ag(111), followed by thermal annealing processes. The Ag adatoms in the co-assembled structures arranged into highly-ordered arrays with their density tunable by varying the preparation conditions. As supported by DFT calculations, the Ag adatoms are stabilized by their weak nonbonding interactions with DEDM molecules which originate from the substrate-mediated electron localization between the Ag adatoms and terminal alkynyls in DEDM.
As demonstrated by above examples, the functionalized sites, either cavities with special electronic states in the TEB-networks [62] or metal atoms in DEDM-Ag co-assemblies [47], are stabilized by TA-involved non-bonding interactions. These experimental discoveries may register potential applications of the TA-based weakly-bonded nanoarchitectures as hosts of functional surface sites.
2.2. Organometallic bondsOrganometallic species formed by deprotonated terminal alkynyl groups in TAs and metal atoms (linking type 3 in Scheme 1) used to be taken as one of the byproducts or intermediates of the on-surface covalent reactions of TAs in previous studies [49, 50]. Experimental observations of organometallic species formed on surfaces were rarely reported except for some cases where structures consist of both TAs and metal atoms were occasionally captured by STM [49, 50]. However, the successful syntheses of a variety of bulk metal-acetylides [63-65] suggest the possibility of isolating the organometallic species as main on-surface reaction products of the TA precursors.
By depositing TA molecules onto Ag(110) and Ag(100) and subsequent annealing the samples to activate on-surface TA reactions, we realized for the first time the highly-selective formation of metal-acetylides on these metal surfaces [48]. 2, 5-Diethynyl-1, 4-bis(phenylethynyl)benzene (DEBPB) monomers took part in the reaction with Ag adatoms on Ag(110) to form highly-oriented organometallic chains with a productivity of 88% after annealing the sample at 350 K (Fig. 2a-ⅰ and a-ⅱ). Silveracetylides formed by deprotonated terminal alkynyls in DEBPB and silver atoms (chemical structure being shown in Fig. 2a-ⅱ) were confirmed by both experimental measurements and theoretical calculations.

|
Download:
|
| Fig. 2. Organometallic products formed by on-surface TA reactions. (a) STM images of highly-oriented organometallic chains formed by DEBPB molecules and Ag atoms on Ag(110) (ⅰ and ⅱ) and optimized molecular models (ⅲ). The chemical structure of the organometallic product is superimposed in (ⅱ) and the matching relationship between organometallic chains and the substrate lattice is highlighted with dashed lines in (ⅱ) and (ⅲ). Adapted with permission from Ref. [48]. Copyright 2015 American Chemical Society. (b) STM images of metalated carbine chains formed by ethyne and Cu atoms on Cu(110) (ⅰ and ⅱ) and the optimized molecular model (ⅲ). The chemical structure of the metalated carbine chain is superimposed in (ⅱ) and the [110] direction of the substrate lattice is highlighted with dashed arrows in (ⅱ) and (ⅲ). Adapted with permission from Ref. [51]. Copyright 2016 American Chemical Society. | |
Recently, Sun et al. [51] reported another example of bottom-up synthesis of TA-based organometallic species. By depositing ethyne, the simplest TA molecule, on Cu(110) held at about 450 K, one-dimensional (1D) molecular chains were achieved lying along [110] direction of the substrate (Fig. 2b-ⅰ and b-ⅱ). Combined STM, atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS) and DFT investigations enabled them to assign the 1D chains as metalated carbine, a linear material consisting of unsaturated carbon atoms and carbon bonded metal atoms.
It is worthy to point out the difference between the organometallic species obtained by on-surface reactions of aryl halides and those resulted from the conversions of TAs. In the former case, the organometallic complexes formed by the chemical-bonded dehalogenated molecules and metal atoms are usually the intermediates of the Ullmann coupling reactions, i.e. the organometallic species would convert into covalent-coupled products by further annealing [66-69]. However, in both examples described above, the TA-based organometallic species were not involved in subsequent reactions producing homocoupling products at higher annealing temperatures [48, 51]. Instead, the molecular desorption or formation of byproducts was observed [48, 51], which was possibly due to the spectacular stability of the organometallic products on the substrates. Therefore, it is more rational to consider the achieved TA-based organometallic species as the on-surface reaction products of the TA precursors rather than the intermediates of the covalent reactions.
3. Controlling methodologies for on-surface linkage of TAsGiven the complexity of the on-surface assemblies and reactions of TAs as described in Part 2, efficient controlling methods are indispensable for the highly-selective preparations of TA-based low-dimensional nanostructures. The measures towards controllable on-surface linkage of TAs are mainly taken in three aspects: designing the structures of the TA building blocks, employing different substrates, and changing the activation modes.
3.1. Effect of the precursor structureGao et al. [70] revealed the crucial role of ortho substituents next to the terminal alkynyl groups in aromatic TAs in on-surface reactions by comparing the productivity of homocoupling reaction (linking type 4 in Scheme 1) of the TA precursors with and without ortho substituents on Ag(111). By employing 1, 4-diethynyl-2, 5-dihexylbenzene, a TA with ortho substituents, as the reaction precursor, the homocoupling productivity as high as about 96% on Ag(111) was achieved, much higher than that (about 64%) for the reaction of 4, 4'-((2, 5-dihexyl-1, 4-phenylene)bis(ethyne-2, 1-diyl)) bis(ethynylbenzene), a TA without ortho substituents, under similar conditions. This phenomenon can be interpreted as a steric hindrance effect introduced by the ortho substituents which suppresses the side reactions involving three or more TA molecules. It's noteworthy that alkynyl might not be a good choice as ortho substituents to increase the selectivity of the homocoupling reaction, because Bergman cyclization (linking type 7 in Scheme 1) is likely to take place between the substituted alkynyl and the terminal one [54, 71].
Recently, Sun et al. [72] reported another novel structural design of the TA precursors which could effectively promote the anticipated homocoupling reaction. The authors employed terminal alkynyl halides (R-C≡C-X, X for halogen atoms) instead of terminal alkynes (R-C≡C-H) as the precursors to introduce dehalogenative homocoupling reaction (Fig. 3a) in order to achieve polydiyne products with a high selectivity. This reaction scheme was successfully realized on Au(111), resulting in diyne dimers (Fig. 3b), 1D chains (Fig. 3c) and 2D networks (Fig. 3d-ⅱ) by using terminal alkynyl halides with different backbone structures (Fig. 3a). The conversion of terminal alkynyl halide monomers into covalent coupling products on the surface involves the formation of organometallic species (Fig. 3a and d-ⅰ) as intermediates, which is similar to the process of on-surface Ullmann coupling reactions [66-69].

|
Download:
|
| Fig. 3. (a) Schematic illustrations of reaction processes of BEBP, bBEBP and tBEP (from top to bottom). (b) STM image of covalent dimers resulted from the onsurface reaction of BEBP. (c) STM image of 1D covalent chains resulted from the onsurface reaction of bBEBP. (d) STM images of organometallic intermediates (ⅰ) and 2D covalent networks (ⅱ) resulted from the on-surface reaction of tBEP. Adapted with permission from Ref. [72]. Copyright 2016 American Chemical Society. | |
Apart from the influence of the ortho substituents or precursors with halogenated terminal alkynyls, the comparisons on the hitherto reported results about the on-surface reactions of TAs also suggest the effect of geometric symmetries of the TA backbones on the reaction selectivity: under similar conditions, the TA precursors with linear backbones are more likely to react into homocoupling products [70] while D3h precursors prefer to adopt multi-molecular reactions, e.g. cyclotrimerization (linking type 5 in Scheme 1) [53].
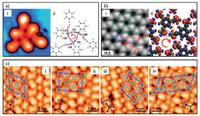
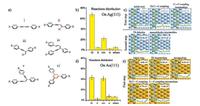
3.2. Effect of the substrateA variety of experimental observations have shown distinct behaviors of the TAs on different metal substrates. Take the reactions of Ext-TEB on Ag(111) and Au(111) as examples, different main products were obtained by varying the metal substrate employed: on Ag(111), the reaction mainly resulted in homocoupling products [73] while on Au(111), the reaction led to 75% cyclotrimerization products [53]. Gao et al. [49, 70] conducted systematical investigations on the effect of the metal substrates by comparing the TA reactions on Au(111), Ag(111) and Cu(111). The statistical analysis (Fig. 4b and d) of the products (Fig. 4a) on these substrates shows the best performance of the Ag(111) substrate in the highly-selective homocoupling reaction. Theoretical studies (Fig. 4c and e) indicate that the preference for homocoupling on Ag (111) stems from the moderate interaction between the terminal alkynyls and the substrate Ag atoms. On Au(111), Au atoms bind more strongly with alkynyl groups, leading to reduced mobility of the TA molecules and longer residence time of the intermediate dimers, which further augments the probability for multimolecular side reactions. On Cu(111), an even stronger interaction between the substrate and TAs impedes the coupling reaction at relatively low activation temperatures due to the poor mobility of the TA molecules [49], yet leads to versatile byproducts involving more than two molecules at higher temperatures [52].
|
Download:
|
| Fig. 4. (a) Different covalent products resulted from the on-surface reactions of TA precursors. (b) Statistical distribution of different reaction products of TAs on Ag(111). (c) Theoretically calculated reaction mechanism of TAs on Ag(111). (d) Statistical distribution of different reaction products of TAs on Au(111). (e) Theoretically calculated reaction mechanism of TAs on Au(111). Adapted with permission from Ref. [49]. Copyright 2013 American Chemical Society. | |
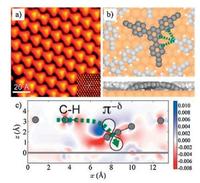
Furthermore, the composition of the metal substrate may also influence on-surface self-assemblies of TAs. For example, intact Ext-TEB molecules assemble into ordered 2D networks (Fig. 1b) mainly through intermolecular CH/π interactions on Ag(111) [44]. However, on Cu(111), the same molecules first undergo deprotonation due to the high dehydrogenating capability of the copper substrate, and the deprotonated molecules then form 2D structures (Fig. 5a) via the C-H…π-δ-type ionic hydrogen bonds (Fig. 5b and c) [46]. Such a comparison demonstrates that different substrate metals interact with the TA molecules to different extents, resulting in totally different non-bonding interaction modes.
|
Download:
|
| Fig. 5. (a) STM image of Ext-TEB molecular assembly on Cu(111). (b) Top and side views of theoretically optimized molecular models of deprotonated Ext-TEB network on Cu(111). The C-H…π-δ-type ionic hydrogen bonds are marked by green dashed lines. (c) Electron density difference plot of deprotonated Ext-TEB network on Cu(111). Blue stands for electron accumulation while red stands for electron depletion. The unit of color bar is e/Å3. The C-H…π-δ interaction is marked by the green dashed arrow. Adapted with permission from Ref. [46]. Copyright 2015 American Chemical Society. | |
The lattice plane of the substrate is another significant structural feature that can also play a key role in TA reactions. The distinct reaction selectivities of the same TA precursor, DEBPB, on different lattice planes of the Ag substrate were reported by Liu et al. [48]: On Ag(111), DEBPB monomers mainly underwent homocoupling reaction, while on Ag(110) and Ag(100), most molecules reacted with the metal atoms to yield organometallic products. Highly-resolved STM images and geometric analysis by the so-called "K-map" [74] indicated that the highly-selective formation of organometallic species on Ag(110) and Ag(100) stemmed from the high stability of the organometallic products on both substrates due to a perfect match between the periodicity of the organometallic chains and the substrate lattice constant along the specific directions (Fig. 2a-ⅱ and a-iii). A good match was also observed between metalated carbine resulted from the on-surface reaction of the ethyne and Cu adatoms and the substrate lattice of Cu(110) (Fig. 2b-ⅱ and b-iii) [51], which obviously plays a significant role in stabilizing the organometallic products on Cu (110).
Vicinal surfaces with highly dense step-edges were also utilized as reaction substrates. Cirera et al. [75] reported a significant enhancement in the selectivity of the homocoupling reaction on Ag (877) vicinal surface (Fig. 6c and d) compared with that on Ag(111) (Fig. 6b). The highly-oriented step-edges on Ag(877) acted as the templates where the linear TA precursors (Fig. 6a) preferentially adsorbed and diffused along so that the side reactions forming branched byproducts were suppressed.

|
Download:
|
| Fig. 6. (a) Chemical structure of the linear TA precursor used. (b) STM image of reaction products of the TA precursor on Ag(111). (c) STM image of 1D covalent chains formed by the homocoupling of the TA precursor on the vicinal Ag(877). (d) High-resolution STM image of a covalent chain lying along the edge of Ag(877) substrate and its chemical structure. Adapted with permission from Ref. [75]. Copyright 2014 American Chemical Society. | |
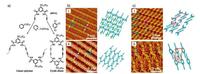
Although the effect of the metal substrate on the assemblies and reactions of TAs varies by the metal composition and its exposed facet, a general phenomenon on different metal surfaces is that the relatively strong interactions between the metal substrates and TA molecules frequently leads to the formation of unwanted byproducts. In order to reduce the productivity of the byproducts, highly oriented pyrolytic graphite (HOPG) has been employed as a nonmetallic substrate for the TA reactions [76, 77]. By either UV-irradiation [76] or catalysis [77], the TA homocoupling was realized at the solvent-HOPG interface. The solvents were proved as an important influence factor in these reaction systems. For instance, Zhang et al. [77] reported the homocoupling products from the reactions of 5-(dodecyloxy)-N1, N3-di(prop-2-ynyl)isophthalamide (DPIA) on HOPG in different solvents (Fig. 7a): At the 1-phenyloctane/HOPG interface, DPIA reacted into linear polymers (Fig. 7b) while at the 1, 2, 4-trichlorobenzene/ HOPG interface, the product was cyclic dimers (Fig. 7c).
|
Download:
|
| Fig. 7. (a) Schematic illustrations of two homocoupling models of DPIA at 1-phenyloctane/HOPG interface (left) and 1, 2, 4-trichlorobenzene/HOPG interface (right). (b) STM image of self-assembled DPIA monomers at 1-phenyloctane/HOPG interface (left) and the corresponding molecular model (right) (ⅰ). STM image of linear polymers at 1-phenyloctane/HOPG interface resulted from the homocoupling of DPIA after adding the catalysts (left) and the corresponding molecular model (right) (ⅱ). (c) STM image of self-assembled DPIA monomers at 1, 2, 4-trichlorobenzene/HOPG interface (left) and the corresponding molecular model (right) (ⅰ). STM image of cyclic dimers at 1, 2, 4-trichlorobenzene/HOPG interface resulted from the homocoupling of DPIA after adding the catalysts (left) and the corresponding molecular model (right) (ⅱ). Adapted with permission from Ref. [77]. Copyright 2014 Nature Publishing Group. | |
3.3. Activation modes
Owing to the active chemical properties and diversified reaction types of TAs, the thermal activation of the TA precursors often triggers uncontrollable side reactions. Therefore, novel activation modes other than thermal annealing were explored as a possible solution. Gao et al. [78] reported for the first time the light-induced homocoupling of TAs on metal surfaces. However, due to the low TA mobility on metal surfaces, only short oligomers were obtained by the photochemical homocouplings of the TA precursors on Ag (111), leaving a large proportion of the terminal alkynyl groups in the TA molecules unreacted. In order to improve the productivity of the photochemical homocoupling reactions, Colazzo et al. [76] conducted the light-induced TA reaction (Fig. 8a) on HOPG rather than metal surfaces. As a result, a nearly complete conversion of 4-ethynylbenzoic (para-ethynylbenzoic) acid (PEBA) (Fig. 8b) into homocoupled dimers (Fig. 8c) was achieved by UV-irradiation.

|
Download:
|
| Fig. 8. (a) Schematic illustration of the photochemical homocoupling of PEBA. (b) STM image of the self-assembly formed by PEBA monomers on HOPG. Inset: Highresolution STM image of the assembly structure. (c) STM image of the assembly formed by homocoupled dimers after UV-irradiation. Inset: High-resolution STM image of PEBA dimers. Adapted with permission from Ref. [76]. Copyright 2016 American Chemical Society. | |
Because the TA homocoupling in homogeneous synthesis can be catalyzed by metallic salts [55], Zhang et al. [77] employed the pyridine solution of Cu(OAc)2-H2O as the catalyst to initiate the TA reactions at the solvent-HOPG interfaces. The successful preparation of homocoupling products at the solvent-HOPG interfaces (Fig. 7) indicated that the addition of catalysts could be an alternative nonthermal activation mode available for the TA reactions at liquid-solid interfaces.
4. Conclusions and perspectiveThree on-surface linking strategies for the TA building blocks, i.e. non-bonding interactions, organometallic bonds and covalent bonds, have been summarized in this review. Examples are presented to show that low-dimensional nanostructures can be successfully constructed by the TA molecules through nonbonding interactions and organometallic bonds. Several controlling methods for the on-surface linkage of TAs have also been overviewed. Firstly, appropriate structural design of the TA precursors, including the introduction of ortho substituents, halogenation of the terminal alkynyls, and proper geometric symmetries of their backbones, has a great influence on the reaction selectivity of the TAs. Secondly, both the composition and the exposed lattice plane of the substrate play key roles in the productivity and selectivity of the TA reactions. Finally, lightirradiation and catalyst-introduction are two promising activation modes besides the thermal annealing one, which can be utilized to reduce unwanted side reactions. In a word, by variations of the precursor structure, substrate and activation mode, the on-surface linkage of the TAs can be effectively tuned.
Despite of the successful case studies, more have yet to be done towards the fabrication of practicable nanomaterials based on the on-surface linkage of the TAs. The following aspects might be helpful in the development of TA-based nanoarchitectures. (1) Exploring new types of the TA reactions, especially with other functionalized molecules such as Sonogashira reactions between TAs and halides and click reactions between TAs and azides. Though these two mentioned reactions have been tried on surfaces [79-83], no extended nanostructures have been practically achieved. (2) Developing new controlling methods. The methods for tuning the surface reactions frequently used in heterogeneous catalysis, for example, changing the electronic properties of the metal surfaces (e.g. work function, d-band center, etc.), might be good references for the control of the on-surface TA reactions. (3) Performing subtle characterizations and measurements of the functional properties of the TA-based low-dimensional nanostructures, such as the conductivities of graphyne and graphdiyne, the electronic properties and catalytic performance of metalacetylides, etc. Overall, with the developed on-surface linking strategies and the versatile controlling methodologies, the TA molecules seem to be very promising building blocks for bottomup fabrications of designed and anticipated functional nanomaterials.
AcknowledgmentThis work is jointly supported by National Natural Science Foundation of China (NSFC) (Nos. 91527303, 21333001).
| [1] | Y. Ye, W. Sun, Y. Wang, et al., A unified model:self-assembly of trimesic acid on gold. J. Phys. Chem. C 111 (2007) 10138–10141. DOI:10.1021/jp072726o |
| [2] | H. Liang, Y. He, Y. Ye, et al., Two-dimensional molecular porous networks constructed by surface assembling. Coord. Chem. Rev. 253 (2009) 2959–2979. DOI:10.1016/j.ccr.2009.07.028 |
| [3] | H. Liang, W. Sun, X. Jin, et al., Two-dimensional molecular porous networks formed by trimesic acid and 4, 4'-bis(4-pyridyl)biphenyl on Au(111) through hierarchical hydrogen bonds:structural systematics and control of nanopore size and shape. Angew. Chem. 50 (2011) 7562–7566. DOI:10.1002/anie.201101477 |
| [4] | J. Xu, Q.-D. Zeng. Construction of two-dimensional (2D) H-bonded supramolecular nanostructures studied by STM. Chin. Chem. Lett. 24 (2013) 177–182. DOI:10.1016/j.cclet.2013.02.005 |
| [5] | X. Zhang, N. Li, G.-C. Gu, et al., Controlling molecular growth between fractals and crystals on surfaces. ACS Nano 9 (2015) 11909–11915. DOI:10.1021/acsnano.5b04427 |
| [6] | C. Xie, Q.-M. Wu, R.-N. Li, et al., Isolated supramolecules on surfaces studied with scanning tunneling microscopy. Chin. Chem. Lett. 27 (2016) 807–812. DOI:10.1016/j.cclet.2016.03.022 |
| [7] | N.R. Champness. Building with molecules. Nat. Nanotechnol 2 (2007) 671–672. DOI:10.1038/nnano.2007.355 |
| [8] | L. Grill, M. Dyer, L. Lafferentz, et al., Nano-architectures bycovalent assemblyof molecular building blocks. Nat. Nanotechnol. 2 (2007) 687–691. DOI:10.1038/nnano.2007.346 |
| [9] | A. Gourdon. On-surface covalent coupling in ultrahigh vacuum. Angew. Chem. Int. Ed. 47 (2008) 6950–6953. DOI:10.1002/anie.v47:37 |
| [10] | N.R. Champness. Surface chemistry:making the right connections. Nat. Chem. 4 (2012) 149–150. DOI:10.1038/nchem.1276 |
| [11] | L. Lafferentz, V. Eberhardt, C. Dri, et al., Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 4 (2012) 215–220. DOI:10.1038/nchem.1242 |
| [12] | J.W. Colson, W.R. Dichtel. Rationally synthesized two-dimensional polymers. Nat. Chem. 5 (2013) 453–465. DOI:10.1038/nchem.1628 |
| [13] | J.A. Elemans, S. Lei, S. De Feyter. Molecular and supramolecular networks on surfaces:from two-dimensional crystal engineering to reactivity. Angew. Chem. Int. Ed. 48 (2009) 7298–7332. DOI:10.1002/anie.v48:40 |
| [14] | D.F. Perepichka, F. Rosei. Extending polymer conjugation into the second dimension. Science 323 (2009) 216–217. DOI:10.1126/science.1165429 |
| [15] | J. Sakamoto, J. van Heijst, O. Lukin, A.D. Schluter. Two-dimensional polymers:just a dream of synthetic chemists?. Angew. Chem. Int. Ed 48 (2009) 1030–1069. DOI:10.1002/anie.v48:6 |
| [16] | C.-A. Palma, P. Samorì. Blueprinting macromolecular electronics. Nat. Chem. 3 (2011) 431–436. DOI:10.1038/nchem.1043 |
| [17] | Y. Lu, J. Zou, H. Wang, et al., Triangular halogen trimers. A DFT study of the structure cooperativity, and vibrational properties. J. Phys. Chem. A 109 (2005) 11956–11961. DOI:10.1021/jp0547360 |
| [18] | F. Silly. Selecting two-dimensional halogen-halogen bonded self-assembled 1, 3, 5-tris(4-iodophenyl)benzene porous nanoarchitectures at the solid-liquid interface. J. Phys. Chem. C 117 (2013) 20244–20249. DOI:10.1021/jp4057626 |
| [19] | H. Walch, R. Gutzler, T. Sirtl, G. Eder, M. Lackinger. Material-and orientation-dependent reactivity for heterogeneously catalyzed carbon-bromine bond homolysis. J. Phys. Chem. C 114 (2010) 12604–12609. DOI:10.1021/jp102704q |
| [20] | J.K. Yoon, W.-J. Son, K.-H. Chung, et al., Visualizing halogen bonds in planar supramolecular systems. J. Phys. Chem. C 115 (2011) 2297–2301. |
| [21] | J. Shang, Y. Wang, M. Chen, et al., Assembling molecular Sierpinski triangle fractals. Nat. Chem. 7 (2015) 389–393. DOI:10.1038/nchem.2211 |
| [22] | A.C. Legon. The halogen bond:an interim perspective. Phys. Chem. Chem. Phys. 12 (2010) 7736–7747. DOI:10.1039/c002129f |
| [23] | S.-W. Hla, L. Bartels, G. Meyer, K.-H. Rieder. Inducing all steps of a chemical reactionwith the scanning tunneling microscope tip:towards single molecule engineering. Phys. Rev. Lett. 85 (2000) 2777–2780. DOI:10.1103/PhysRevLett.85.2777 |
| [24] | M. Bieri, M. Treier, J. Cai, et al., Porous graphenes:two-dimensional polymer synthesis with atomic precision. Chem. Commun (2009) 6919–6921. |
| [25] | L. Lafferentz, F. Ample, H. Yu, et al., Conductance of a single conjugated polymer as a continuous function of its length. Science 323 (2009) 1193–1197. DOI:10.1126/science.1168255 |
| [26] | M. Bieri, M.-T. Nguyen, O. Gröning, et al., Two-dimensional polymer formation on surfaces insight into the roles of precursor mobility and reactivity. J. Am. Chem. Soc. 132 (2010) 16669–16676. DOI:10.1021/ja107947z |
| [27] | J. Cai, P. Ruffieux, R. Jaafar, et al., Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466 (2010) 470–473. DOI:10.1038/nature09211 |
| [28] | Q. Shen, J.H. He, J.L. Zhang, et al., Self-assembled two-dimensional nanoporous molecular arrays and photoinduced polymerization of 4-bromo-4'-hydroxybiphenyl on Ag(111). J. Chem. Phys 142 (2015) 101902. DOI:10.1063/1.4906116 |
| [29] | X. Zhou, F. Bebensee, Q. Shen, et al., On-surface synthesis approach to preparing one-dimensional organometallic and poly-π-πhenylene chains. Mater. Chem. Front. 1 (2017) 119–127. DOI:10.1039/C6QM00142D |
| [30] | N.A.A. Zwaneveld, R. Pawlak, M. Abel, et al., Organized formation of 2D extended covalent organic frameworks at surfaces. J. Am. Chem. Soc. 130 (2008) 6678–6679. DOI:10.1021/ja800906f |
| [31] | D. Zhong, J.H. Franke, S.K. Podiyanachari, et al., Linear alkane polymerization on a gold surface. Science 334 (2011) 213–216. DOI:10.1126/science.1211836 |
| [32] | F. Hanke, S. Haq, R. Raval, M. Persson. Heat-to-connect surface commensurability directs organometallic one-dimensional self-assembly. ACS Nano 5 (2011) 9093–9103. DOI:10.1021/nn203337v |
| [33] | S. Haq, F. Hanke, M.S. Dyer, et al., Clean coupling of unfunctionalized porphyrins at surfaces to give highly oriented organometallic oligomers. J. Am. Chem. Soc. 133 (2011) 12031–21039. DOI:10.1021/ja201389u |
| [34] | S. Haq, F. Hank, J. Sharp, et al., Versatile bottom-up construction of diverse macromolecules on a surface observed by scanning tunneling microscopy. ACS Nano 8 (2014) 8856–8870. DOI:10.1021/nn502388u |
| [35] | F. Diederich. Carbon scaffolding:building acetylenic all-carbon and carbonrich compounds. Nature 369 (1994) 199–207. DOI:10.1038/369199a0 |
| [36] | F. Diederich, M. Kivala. All-carbon scaffolds by rational design. Adv. Mater. 22 (2010) 803–812. DOI:10.1002/adma.v22:7 |
| [37] | R.H. Baughman, H. Eckhardt, M. Kertesz. Structure-property predictions for new planar forms of carbon:layered phases containing sp2 and sp atoms. J. Chem. Phys. 87 (1987) 6687. DOI:10.1063/1.453405 |
| [38] | N. Narita, S. Nagai, S. Suzuki, K. Nakao. Optimized geometries and electronic structures of graphyne and its family. Phy. Rev. B 58 (1998) 11009–11014. DOI:10.1103/PhysRevB.58.11009 |
| [39] | M.M. Haley, S.C. Brand, J.J. Pak. Carbon networks based on dehydrobenzoannulenes:synthesis of graphdiyne substructures. Angew. Chem. Int. Ed. Engl. 36 (1997) 835–838. |
| [40] | W.B. Wan, S.C. Brand, J.J. Pak, M.M. Haley. Synthesis of expanded graphdiyne substructures. Chem. Eur. J. 6 (2000) 2044–2052. DOI:10.1002/(ISSN)1521-3765 |
| [41] | G. Li, Y. Li, H. Liu, et al., Architecture of graphdiyne nanoscale films. Chem. Commun. 46 (2010) 3256–3258. DOI:10.1039/b922733d |
| [42] | Q. Li, C. Han, M. Fuentes, - Cabrera, et al., Electronic control over attachment and self-assembly of alkyne groups on gold. ACS Nano 6 (2012) 9267–9275. DOI:10.1021/nn303734r |
| [43] | Q. Li, C. Han, S.R. Horton, et al., Supramolecular self-assembly of p-conjugated hydrocarbons via 2D cooperative CH/π interaction. ACS Nano 6 (2012) 566–572. DOI:10.1021/nn203952e |
| [44] | N. Kepčija, Y.-Q. Zhang, M. Kleinschrodt, et al., Steering on-surface self-9 assemblyof high-quality hydrocarbon networks with terminalalkynes. J. Phys. Chem. C 117 (2013) 3987. DOI:10.1021/jp310606r |
| [45] | Q. Tan, Q. Sun, L. Cai, J. Wang, Y. Ding. Exploring the self-assembly behaviors of an organic molecule functionalized by terminal alkyne and aldehyde groups on Au(111). J. Phys. Chem. C 119 (2015) 12935–12940. |
| [46] | Y.-Q. Zhang, J. Björk, P. Weber, et al., Unusual deprotonated alkynyl hydrogen bonding in metal-supported hydrocarbon assembly. J. Phys. Chem. C 119 (2015) 9669–9679. DOI:10.1021/acs.jpcc.5b02955 |
| [47] | J. Liu, X. Fu, Q. Chen, et al., Stabilizing surface Ag adatoms into tunable single atom arrays by terminal alkyne assembly. Chem. Commun. 52 (2016) 12944–12947. DOI:10.1039/C6CC06444B |
| [48] | J. Liu, Q. Chen, L. Xiao, et al., Lattice-directed formation of covalent and organometallic molecular wires by terminal alkynes on Ag surfaces. ACS Nano 9 (2015) 6305. DOI:10.1021/acsnano.5b01803 |
| [49] | H.-Y. Gao, J.-H. Franke, H. Wagner, et al., Effect of metal surfaces in on-surface Glaser coupling. J. Phys. Chem. C 117 (2013) 18595–18602. DOI:10.1021/jp406858p |
| [50] | H. Zhou, J. Liu, S. Du, et al., Direct visualization of surface-assisted twodimensional diyne polycyclotrimerization. J. Am. Chem. Soc. 136 (2014) 5567–5570. DOI:10.1021/ja501308s |
| [51] | Q. Sun, L. Cai, S. Wang, et al., Bottom-up synthesis of metalated carbyne. J. Am. Chem. Soc. 138 (2016) 1106–1109. DOI:10.1021/jacs.5b10725 |
| [52] | J. Eichhorn, W.M. Heckl, M. Lackinger. On-surface polymerization of 1, 4-diethynylbenzene on Cu(111). Chem. Commun. 49 (2013) 2900–2902. DOI:10.1039/c3cc40444g |
| [53] | J. Liu, P. Ruffieux, X. Feng, K. Müllen, R. Fasel. Cyclotrimerization of arylalkynes on Au(111). Chem. Commun. 50 (2014) 11200–11203. DOI:10.1039/C4CC02859G |
| [54] | A. Riss, S. Wickenburg, P. Gorman, et al., Local electronic and chemical structure of oligo-acetylene derivatives formed through radical cyclizations at a surface. Nano Lett. 14 (2014) 2251–2255. DOI:10.1021/nl403791q |
| [55] | F. Klappenberger, Y.Q. Zhang, J. Björk, et al., On-surface synthesis of carbonbased scaffolds and nanomaterials using terminal alkynes. Acc. Chem. Res. 48 (2015) 2140–2150. DOI:10.1021/acs.accounts.5b00174 |
| [56] | M. Nishio. CH/π hydrogen bonds in crystals. Cryst. Eng. Comm. 6 (2004) 130–158. DOI:10.1039/b313104a |
| [57] | M. Nishio. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 13 (2011) 13873–13900. DOI:10.1039/c1cp20404a |
| [58] | T. Steiner. Cooperative C≡C-H…C≡C) interactions:crystal structure of DLprop-2-ynylglycine and database study of terminal alkynes. J. Chem. Soc. Chem. Commun (1995) 95–96. |
| [59] | T. Steiner, E.B. Starikov, A.M. Amado, J.J.C. Teixeira-Dias. Weak hydrogen bonding. Part 2. The hydrogen bonding nature of short C-H…π contacts:crystallographic, spectroscopic and quantum mechanical studies of some terminal alkynes. J. Chem. Soc. Perkin Trans. 2 (1995) 1321–1326. |
| [60] | J.M.A. Robinson, B.M. Kariuki, R.J. Gough, K.D.M. Harris, D. Philp. Preferential formation of C≡C-H…π(C≡C) interactions in the solid state. J. Solid State Chem. 134 (1997) 203–206. DOI:10.1006/jssc.1997.7646 |
| [61] | H.-C. Weiss, D. Bläser, R. Boese, B.M. Doughanb, M.M. Haley. C-H…π interactions in ethynylbenzenes:the crystal structures of ethynylbenzene and 1, 3, 5-triethynylbenzene, and a redetermination of the structure of 1, 4-diethynylbenzene. Chem. Commun (1997) 1703–1704. |
| [62] | Y.Q. Zhang, J. Björk, J.V. Barth, F. Klappenberger. Intermolecular hybridization creating nanopore orbital in a supramolecular hydrocarbon sheet. Nano Lett. 16 (2016) 4274–4281. DOI:10.1021/acs.nanolett.6b01324 |
| [63] | V.W.-W. Yam, K.K.-W. Lo, K.M.-C. Wong. Luminescent polynuclear metal acetylides. J. Org. Chem. 578 (1999) 3–30. DOI:10.1016/S0022-328X(98)01106-1 |
| [64] | C.E. Powell, M.G. Humphrey. Nonlinear optical properties of transition metal acetylides and their derivatives. Coord. Chem. Rev. 248 (2004) 725–756. DOI:10.1016/j.ccr.2004.03.009 |
| [65] | G. Fanga, X. Bi. Silver-catalysed reactions of alkynes:recent advances. Chem. Soc. Rev. 44 (2015) 8124–8173. DOI:10.1039/C5CS00027K |
| [66] | Q. Fan, C. Wang, Y. Han, et al., Surface-assisted formation, assembly, and dynamics of planar organometallic macrocycles and zigzag shaped polymer chains with C-Cu-C bonds. ACS Nano 8 (2014) 709–718. DOI:10.1021/nn405370s |
| [67] | M.D. Giovannantonio, M.E. Garah, J. Lipton, - Duffin, et al., Insight into organometallic intermediate and its evolution to covalent bonding in surface-confined Ullmann polymerization. ACS Nano 7 (2013) 8190–8198. DOI:10.1021/nn4035684 |
| [68] | J. Eichhorn, T. Strunskus, A. Rastgoo, - Lahrood, et al., On-surface Ullmann polymerization via intermediate organometallic networks on Ag(111). Chem. Commun. 50 (2014) 7680–7682. DOI:10.1039/C4CC02757D |
| [69] | W. Wang, X. Shi, S. Wang, M.A. Van Hove, N. Lin. Single-molecule resolution of an organometallic intermediate in a surface-supported Ullmann coupling reaction. J. Am. Chem. Soc. 133 (2011) 13264–13267. DOI:10.1021/ja204956b |
| [70] | H.Y. Gao, H. Wagner, D. Zhong, et al., Glaser coupling at metal surfaces. Angew. Chem. Int. Ed. 52 (2013) 4024–4028. DOI:10.1002/anie.v52.14 |
| [71] | Q. Sun, C. Zhang, Z. Li, et al., On-surface formation of one-dimensional polyphenylene through Bergman cyclization. J. Am. Chem. Soc. 135 (2013) 8448–8451. DOI:10.1021/ja404039t |
| [72] | Q. Sun, L. Cai, H. Ma, C. Yuan, W. Xu. Dehalogenative homocoupling of terminal alkynyl bromides on Au(111):incorporation of acetylenic scaffolding into surface nanostructures. ACS Nano 10 (2016) 7023–7030. DOI:10.1021/acsnano.6b03048 |
| [73] | Y.Q. Zhang, N. Kepcija, M. Kleinschrodt, et al., Homo-coupling of terminal alkynes on a noble metal Surface. Nat. Commun 3 (2012) 1286. DOI:10.1038/ncomms2291 |
| [74] | G. Schull, R. Berndt. rientationally ordered (7×7) superstructure of C60 on Au (111). Phys. Rev. Lett 99 (2007) 226105. DOI:10.1103/PhysRevLett.99.226105 |
| [75] | B. Cirera, Y.Q. Zhang, J. Björk, et al., Synthesis of extended graphdiyne wires by vicinal surface templating. Nano Lett. 14 (2014) 1891–1897. DOI:10.1021/nl4046747 |
| [76] | L. Colazzo, F. Sedona, A. Moretto, M. Casarin, M. Sambi. Metal-free on-surface photochemical homocoupling of terminal alkynes. J. Am. Chem. Soc. 138 (2016) 10151–10156. DOI:10.1021/jacs.6b03589 |
| [77] | X. Zhang, L. Liao, S. Wang, et al., Polymerization or cyclic dimerization:solvent dependent homo-coupling of terminal alkynes at HOPG surface. Sci. Rep 4 (2014) 3899. |
| [78] | H.-Y. Gao, D. Zhong, H. Mönig, et al., Photochemical Glaser coupling at metal surfaces. J. Phys. Chem. C 118 (2014) 6272–6277. |
| [79] | V.K. Kanuru, G. Kyriakou, S.K. Beaumont, et al., Sonogashira coupling on an extended gold surface in vacuo reaction of phenylacetylene with iodobenzene on Au(111). J. Am. Chem. Soc. 132 (2010) 8081–8086. DOI:10.1021/ja1011542 |
| [80] | C. Sanchez, - Sanchez, N. Orozco, J.P. Holgado, et al., Sonogashira cross-coupling and homo-coupling on a silver surface:chlorobenzene and phenylacetylene on Ag(100). J. Am. Chem. Soc. 137 (2014) 940–947. |
| [81] | C. Sánchez-Sánchez, F. Yubero, A.R. González-Elipe, et al., The flexible surface revisited:adsorbate-induced reconstruction, homocoupling, and Sonogashira cross-coupling on the Au(100) Surface. J. Phys. Chem. C 118 (2014) 11677–11684. |
| [82] | O.D. Arado, H. Mönig, H. Wagner, et al., On-surface azide alkyne cycloaddition on Au(111). ACS Nano 7 (2013) 8509–8515. DOI:10.1021/nn4022789 |
| [83] | F. Bebensee, C. Bombis, S.-R. Vadapoo, et al., On-surface azide-alkyne cycloaddition on Cu(111):does it "click" in ultrahigh vacuum?. J. Am. Chem. Soc 135 (2013) 2136–2139. DOI:10.1021/ja312303a |
 2017, Vol. 28
2017, Vol. 28 


