Multicomponent synthesis has attracted the attention of organic chemists for assembling libraries of compounds in medicinal, combinatorial and heterocyclic chemistry. Many descriptive tags attached to MCRs are atom economy, automatable procedure, quick and simple implementation, high bond forming index, convergent, high selectivity, inherent exploratory power, time and energy saving and minimisation of chemical waste [1]. These features make MCRs well suited for the design and synthesis of diversified functional groups and heterocyclic scaffolds from readily available simple starting materials [2].
α-Amino acyl amides are important functional groups bearing excellent biological activities. For example, a wide variety of α-amino acyl amide derivatives has been used as agrochemical, pharmaceutical, biological agents. A large number of compounds bearing this group have entered preclinical and clinical trials for longer time. The pharmaceutical applications are represented by (Fig. 1) penicillin [3] (treatment for ear infection), cephalosporin [4] (treatment for skin and soft tissue infection), bicyclomycin [5] (treatment for diarrhoea), YH239-EE [6] (leukaemia treatment), nutlin-3a [7] (treatment for paediatric tumours and for haematological malignancies), TDP665759 [8] (cancer treatment) and xylocaine [9] (treatment for pain from scrapes, minor burns and insect bites). α-Amino acyl amide derivatives exhibit wide range of broad spectrum of biological activities such as anticancer [10, 11], anaesthetics [12, 13], antibiotics [14] and antibacterial [15] drugs. In the past few decades, many methods have been developed [16, 17] for the synthesis of α-amino acyl amide derivatives. Among them, the classic approach involves: (ⅰ) from aldehyde, amine, isocyanide and phenol [18], (ⅱ) from aldehyde, secondary amine, and isocyanide in aminoborane mediated and in the absence of acid [19], (ⅲ) from aldehyde, amino acid and isocyanide [20, 21], (ⅳ) from N-aryl-1, 2, 3, 4-tetrahydroisoquinoline, carboxylic acid and isonitrile [22], (ⅴ) from γ-keto acid, amine, and isocyanide [23], (ⅵ) from aldehyde, amine, isocyanide and carboxylic acid [24, 25].

|
Download:
|
| Fig. 1. Structure of some pharmacologically relevant α-amino acyl amide scaffolds. | |
The simplest and straight forward protocol was reported by Ugi involves the one pot four component condensation of aldehyde, amine, carboxylic acid and isocyanide. Unfortunately, this original procedure suffers from the long reaction duration, tedious conditions and frequently low yields. This has led to the development of new improved synthetic methodologies for the Ugi reaction, involving the application of a numerous catalysts such as TiCl4 [26], Pd(OAc)2 [27], Pd(dba)2 [28], borox [29] phosphoric acid [30] and phenyl phosphinic acid [31]. However, these methods also suffer from some draw backs such as the use of excess amounts of expensive catalysts, product diversity, corrosive acid, lack of convergence and poor or inconsistent yields. For this reason, the development of highly efficient synthetic methodology for useful α-amino acyl amide functional group containing building blocks is still an enormous challenge for chemists.
Nano structured spinel type Co3O4 has been used as a catalyst in various organic transformations such as aerobic oxidation of alkenes [32], total oxidations of several volatile organic compounds [33], oxidation of toluene [34] and oxidation of alcohols [35]. This catalyst can be easily removed from the reaction mixture by centrifugation or filtration [36]. The use of Co3O4 nano particle as a catalyst is not only cost effective and environmentally benign, but also experimentally simple and easy to handle.
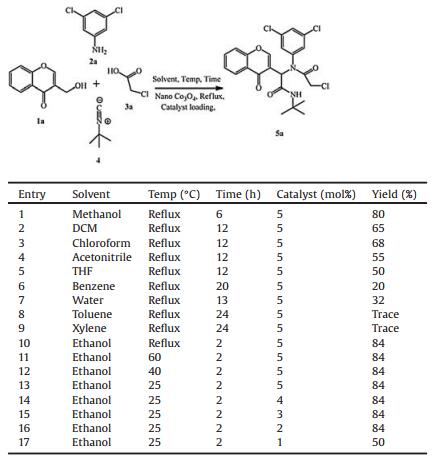
2. Results and discussionInitial exploratory studies among 3-(hydroxymethyl)-4H-chromen-4-one (1a), 3, 5-dichloroaniline (2a), chloroacetic acid (3a) and tert-butyl isocyanide (4) in acetonitrile under reflux condition without catalyst did not produce the desired Ugi adduct. When the reaction was performed in the presence of T3P, under the same reaction conditions, 31% of the desired Ugi adduct (5a) is obtained. However, we failed to obtain noticeable yields when the reaction was carried out in the presence of HIO3, Chloramine-T with ZnBr2, dimethyl dioxirane, and sodium hypochlorite. Later, we turned our attention to the metal catalysts and among the metal catalysts tried, Bi2WO6 and 5 mol% nano Co3O4 resulted in 10% and 55% of 5a, respectively.
Various solvents were screened for the synthesis of 5a in the presence of nano Co3O4 catalyst. It was found that ethanol was the most suitable solvent for this four-component reaction in terms of yield (84%) and reaction time (2h) (Table 1, entry 13). The same model reaction was tested in the presence of various amounts of nano Co3O4 and among them 2% of nano Co3O4 was found to be optimum amount to carry out this four component reaction (Table 1, entry 16).
|
|
Table 1 Optimisation studies for the synthesis of 5a. |
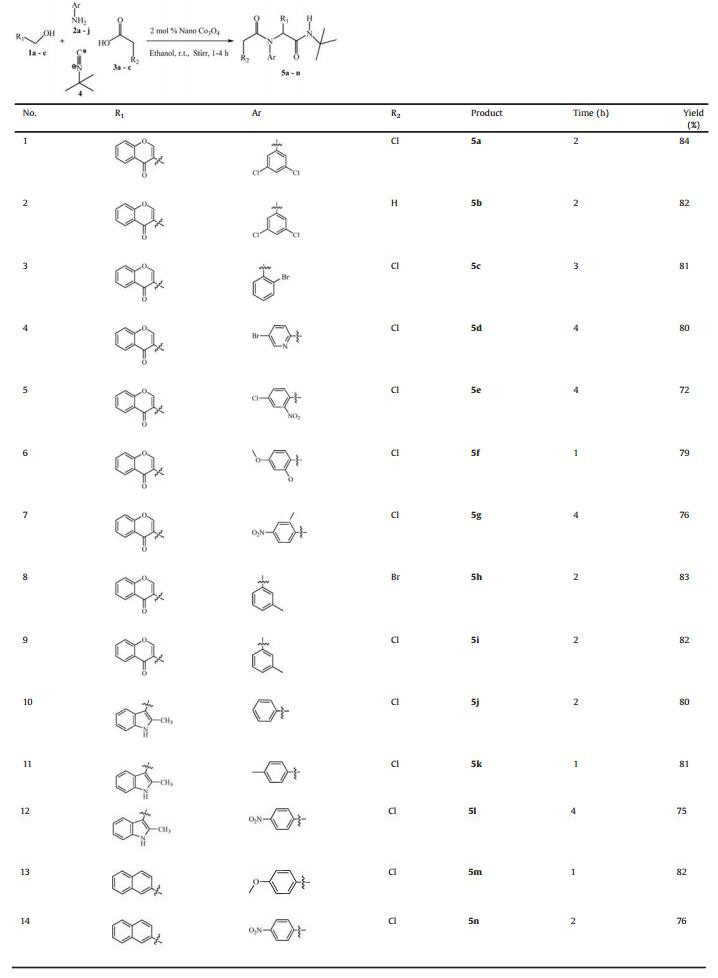
Then, the various molar ratios of the reactants were also examined. The best result was obtained when the molar ratio of 1a/2a/3a/4 is 1:1:1:1. Increasing the molar ratio of any of the components resulted in reduced yields. When a mixture of 3-(hydroxymethyl)-4H-chromen-4-one, 3, 5-dichloroaniline, chloroacetic acid and tert-butyl isocyanide in ethanol was stirred in the presence of 2 mol% of nano Co3O4 at room temperature for 2 h, the product, N-tert-butyl-2-(2-chloro-N-(3, 5-dichlorophenyl)acetamido)-2-(4-oxo-4H-chromen-3-yl)acetamide (5a) was obtained in good yield (84%). Having the optimised conditions in hand, we designed, synthesised and characterised a library of α-amino acyl amide derivatives involving aryl alcohols, amines, carboxylic acids and tert-butyl isocyanide. All the reactions proceeded smoothly to give the corresponding Ugi adducts in 72%-84% yields. The time taken to achieve complete conversions (monitored by TLC) and the isolated yields are recorded in Table 2.
|
|
Table 2 Synthesis of Ugi adducts 5a-5n. |
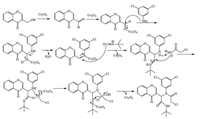
On the basis of the aforementioned experimental results, we propose that this Ugi reaction proceeds by the mechanism [37] shown in Scheme 1. In the first step, nano Co3O4 can serve as an oxidising agent for the in situ oxidation of aryl alcohol into the aryl aldehyde. The latter compound, in the second step, undergoes condensation reaction with aniline to give the corresponding imine. The imine undergoes nucleophilic addition with tert-butyl isocyanide followed by the addition of carboxylate ion to give an intermediate, which rearranges to form the α-amino acyl amide functional group via an acyl transfer. Nano Co3O4 is likely to enhance the rate of this four-component reaction.
|
Download:
|
| Scheme 1. A plausible mechanism for the formation of Ugi adduct (5a). | |
3. Conclusion
The present work describes a facile one pot protocol for the synthesis of new series of α-amino acyl amide derivatives via Ugi four-component reaction. These reactions occur smoothly under nano structured spinel Co3O4 catalyst affording a high yield.
4. Experimental 4.1. General informationMelting points were determined on an electric melting point apparatus. Infrared spectra were obtained on an Agilent Cary 630 FT-IR spectrophotometer. 1H NMR (400 MHz) spectra were recorded on a Bruker WH-200 spectrometer and 13C NMR (100 MHz) on Agilent VNRMS spectrometer using CDCl3 as solvent and TMS as an internal standard. Chemical shifts were expressed in parts per million (ppm) and coupling constant (J) in hertz (Hz). Mass spectra were recorded on an Agilent LC-MS. Elemental analysis was performed on an Elemental Vario Micro Cube rapid analyzer.
4.2. Typical experimental procedure for the synthesis of (5a)To a stirred solution of 3-(hydroxymethyl)-4H-chromen-4-one (0.45 g, 2.55 mmol) in ethanol (5 mL) was added nano Co3O4 (0.01 g, 2 mol%) and the reaction mixture was stirred at 60 ℃ for 30 min. After cooling to room temperature, 3, 5-dichloroaniline (0.41 g, 2.55 mmol) was added and stirred for another 15 min. To this reaction mixture, chloroacetic acid (0.24 g, 2.55 mmol) and tert-butyl isocyanide (0.21 g, 2.55 mmol) were added at 10 ℃ and stirring was continued at room temperature until the completion of the reaction monitored by TLC. The separated solid was dissolved in ethyl acetate and centrifuged for recovering the catalyst. The reaction mixture was extracted with ethyl acetate (3 × 50 mL). The extract was washed with water, and finally with brine. The organic solution was dried with anhydrous Na2SO4 and concentrated by rotary evaporator. Finally, the residue was purified by recrystallization from ethanol.
Physical and spectroscopic characterization data of compounds 5a-5n were given in Supporting information.
AcknowledgmentsWe gratefully acknowledged the Department of Science and Technology -Science and Engineering Research Board, (DST-SERB), India, for economic support under Fast Track Young Scientist Scheme (No. SB/FT/CS-028/2013 dated: 09.06.2014, 24.09.2015 and 12.09.2016).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.015.
| [1] | M. Haji, Beilstein J. Org. Chem. 12(2016) 1269-1301. |
| [2] | A. Domiling, W. Wei, W. Kan. Chemistry and biology of multicomponent reactions. Chem. Rev. 112 (2012) 3083–3135. DOI:10.1021/cr100233r |
| [3] | Y. Zhou, A. Antignac, S.W. Wu, A. Tomasz. Penicillin-binding proteins and cell wall composition in β-lactam-sensitive and resistant strains of Staphylococcus sciuri. J. Bacteriol. 190 (2008) 508–514. DOI:10.1128/JB.01549-07 |
| [4] | J.A. García-Rodríguez, J.L. Muñoz Bellido, J.E. García Sánchez. Oral cephalosporins: current perspectives. Int. J. Antimicrob. Agents 5 (1995) 231–243. DOI:10.1016/0924-8579(95)00015-Z |
| [5] | A. Magyar, X. Zhang, F. Abdi, H. Kohn, W.R. Widger. Identifying the bicyclomycin binding domain through biochemical analysis of antibioticresistant rho proteins. J. Biol. Chem. 274 (1999) 7316–7324. DOI:10.1074/jbc.274.11.7316 |
| [6] | Y. Huang, S. Wolf, B. Beck, et al., Discovery of highly potent p53-MDM2 antagonists and structural basis for anti-acute myeloid leukemia activities. ACS Chem. Biol. 9 (2014) 802–811. DOI:10.1021/cb400728e |
| [7] | P. Secchiero, R. Bosco, C. Celeghini, G. Zauli. Recent advances in the therapeutic perspectives of Nutlin-3. Curr. Pharm. Des. 17 (2011) 569–577. DOI:10.2174/138161211795222586 |
| [8] | H.K. Koblish, S. Zhao, C.F. Franks, et al., Benzodiazepinedione inhibitors of the Hdm2:p53 complex suppress human tumor cell proliferation in vitro and sensitize tumors to doxorubicin in vivo. Mol. Cancer Ther. 5 (2006) 160–169. DOI:10.1158/1535-7163.MCT-05-0199 |
| [9] | D. Jong, H. Rudolph. Last round for a heavyweight?. Anesth. Analg. 78 (1994) 3–4. |
| [10] | H.O. Ohnstad, E.B. Paulsen, P. Noordhuis, et al., MDM2 antagonist Nutlin-3a potentiates antitumour activity of cytotoxic drugs in sarcoma cell lines. BMC Cancer 11 (2011) 211–239. DOI:10.1186/1471-2407-11-211 |
| [11] | H. Shen, C.G. Maki. Persistent p21 expression after Nutlin-3a removal is associated with senescence-like arrest in 4N cells. J. Biol. Chem. 285 (2010) 23105–23114. DOI:10.1074/jbc.M110.124990 |
| [12] | M. Lambrechts, M.J. O'Brien, F.H. Savoie, Z. You. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer. Adher. 7 (2013) 885–890. |
| [13] | A. Serradell, R. Herrero, J.A. Villanueva, et al., Comparison of three different volumes of mepivacaine in axillary plexus block using multiple nerve stimulation. Br. J. Anaesth. 91 (2003) 519–524. DOI:10.1093/bja/aeg215 |
| [14] | J. Shepherd, M. Ibba. Direction of aminoacylated transfer RNAs into antibiotic synthesis and peptidoglycan-mediated antibiotic resistance. FEBS Lett. 587 (2013) 2895–2904. DOI:10.1016/j.febslet.2013.07.036 |
| [15] | M.J. Genin, D.A. Allwine, D.J. Anderson, et al., Substituent effects on the antibacterial activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms haemophilus influenzae and moraxella catarrhalis. J. Med. Chem. 43 (2000) 953–970. DOI:10.1021/jm990373e |
| [16] | A. Endo, A. Yanagisawa, M. Abe, et al., Total synthesis of ecteinascidin 743. J. Am. Chem. Soc. 124 (2002) 6552–6554. DOI:10.1021/ja026216d |
| [17] | A. Dömling, I. Ugi. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 39 (2000) 3168–3210. DOI:10.1002/(ISSN)1521-3773 |
| [18] | L. ElKaim, L. Grimaud, J. Oble. Phenol Ugi-smiles systems: strategies for the multicomponent N-arylation of primary amines with isocyanides, aldehydes, and phenols. Angew. Chem. Int. Ed. 44 (2005) 7961–7964. DOI:10.1002/(ISSN)1521-3773 |
| [19] | Y. Tanaka, T. Hasui, M. Suginome. Acid-free, aminoborane-mediated Ugi-type reaction leading to general utilization of secondary amines. Org. Lett. 9 (2007) 4407–4410. DOI:10.1021/ol701570c |
| [20] | D. Portlock, R. Ostaszewski, D. Naskar, L. West. A tandem petasis—Ugi multi component condensation reaction: solution phase synthesis of six dimensional libraries. Tetrahedron Lett. 44 (2003) 603–605. DOI:10.1016/S0040-4039(02)02619-9 |
| [21] | D. Bhattacharya, S. Mitra, P. Chattopadhyay. A rapid one-pot Ugi reaction based route to novel imidazole-fused benzodiazepinones. Synthesis 47 (2015) 2294–2298. DOI:10.1055/s-00000084 |
| [22] | Y. Chen, G. Feng. Visible light mediated sp3 C-H bond functionalization of Naryl-1, 2, 3, 4-tetrahydroisoquinolines via Ugi-type three-component reaction. Org. Biomol. Chem. 13 (2015) 4260–4265. DOI:10.1039/C5OB00201J |
| [23] | H. Tye, M. Whittaker. Use of a design of experiments approach for the optimisation of a microwave assisted Ugi reaction. Org. Biomol. Chem. 2 (2004) 813–815. DOI:10.1039/b400298a |
| [24] | X. Zuo, N. Mi, Z. Fan, et al., Synthesis of 4-methyl-1, 2, 3-thiadiazole derivatives via Ugi reaction and their biological activities. J. Agric. Food Chem. 58 (2010) 2755–2762. DOI:10.1021/jf902863z |
| [25] | I. Kanizsai, Z. Szakonyi, R. Sillanpaa, F. Fulop. A comparative study of the multicomponent Ugi reactions of an oxabicycloheptene-based β-amino acid in water and in methanol. Tetrahedron Lett. 47 (2006) 9113–9116. DOI:10.1016/j.tetlet.2006.10.069 |
| [26] | T. Godet, Y. Bonvin, G. Vincent, et al., Titanium catalysis in the Ugi reaction of α-amino acids with aromatic aldehydes. Org. Lett. 6 (2004) 3281–3284. DOI:10.1021/ol048850x |
| [27] | Z. Ma, Z. Xiang, T. Luo, et al., Synthesis of functionalized quinolines via Ugi and Pd-catalyzed intramolecular arylation reactions. J. Comb. Chem. 8 (2006) 696–704. DOI:10.1021/cc060066b |
| [28] | F. Bonnaterre, M.B. Choussy, J. Zhu. Rapid access to oxindoles by the combined use of an Ugi four-component reaction and a microwave-assisted intramolecular buchwald-hartwig amidation reaction. Org. Lett. 8 (2006) 4351–4354. DOI:10.1021/ol061755z |
| [29] | W. Zhao, L. Huang, Y. Guan, W.D. Wulff. Three-component asymmetric catalytic Ugi reaction—concinnity from diversity by substrate-mediated catalyst assembly. Angew. Chem. Int. Ed. 53 (2014) 3436–3441. DOI:10.1002/anie.201310491 |
| [30] | Y. Zhang, Y.F. Ao, Z.T. Huang, et al., Chiral phosphoric acid catalyzed asymmetric Ugi reaction by dynamic kinetic resolution of the primary multicomponent adduct. Angew. Chem. Int. Ed. 55 (2016) 5282–5285. DOI:10.1002/anie.201600751 |
| [31] | S.C. Pan, B. List. Catalytic three-component Ugi reaction. Angew. Chem. Int. Ed. 47 (2008) 3622–3625. DOI:10.1002/(ISSN)1521-3773 |
| [32] | Y. Zheng, W. Wang, D. Jiang, et al., Ultrathin mesoporous Co3O4 nanosheets with excellent photo-/thermo-catalytic activity. J. Mater. Chem. A. 4 (2016) 105–112. DOI:10.1039/C5TA07617J |
| [33] | Z. Ma. Cobalt Oxide catalysts for environmental remediation. Curr. Catal. 3 (2014) 15–26. DOI:10.2174/22115447113029990017 |
| [34] | S. Imamura, K. Fukuda, T. Nishida, T. Inui. Effect of Sm on the catalytic activity of Co3O4 in the oxidation of toluene. J. Catal. 93 (1985) 186–191. DOI:10.1016/0021-9517(85)90162-9 |
| [35] | R.V. Jagadeesh, T. Stemmler, A.E. Surkus, et al., Cobalt-based nanocatalysts for green oxidation and hydrogenation processes. Nat. Protoc. 10 (2015) 916–926. DOI:10.1038/nprot.2015.049 |
| [36] | V.R. Mate, M. Shirai, C.V. Rode. Heterogeneous Co3O4 catalyst for selective oxidation of aqueous veratryl alcohol using molecular oxygen. Catal. Commun. 33 (2013) 66–69. DOI:10.1016/j.catcom.2012.12.015 |
| [37] | S. Iacobucci, J.F. Reale, F. Gal. Insight into the mechanisms of the multicomponent Ugi and Ugi-Smiles reactions by ESI-MS(/MS). Eur. J. Org. Chem. 32 (2014) 7087–7090. |
 2017, Vol. 28
2017, Vol. 28