The synthesis of amides has gained considerable interest because it is the key structural skeletal framework of many natural products and pharmaceuticals [1]. Amides are also the important synthetic intermediates in organic synthesis [2]. The traditional method for the synthesis of amides is based on the condensation of amine and activated carboxylic acids [3]. Although a number of modifications on the synthesis of amides were developed, for example, Cu [4], Au [5], Ru [6], Fe [7], La [8], etc. [9], and have been used to catalyze those reactions, the drawbacks are the use of an inert atmosphere, harsh reaction conditions and anhydrous organic solvents. Versatile and practical methods for the synthesis of amides are still desirable.
With the concept of 'green chemistry' and 'sustainable development', the synthesis of amides from the condensation of easy availability aldehyde or alcohols with amines is of great interest. Using CuI as catalyst, Huang and co-workers [10] realized the synthesis of N-pyridinamides in DMF at 80 ℃. In 2014, Rh(Ⅲ) catalyzed [11] synthesis of N-pyridinamides was described by Luo groups. In the presence of Cu(OTf)2 as a catalyst and molecular iodine as an oxidant, N-pyridinamides could be efficiently synthesized by Yadav et al. [12]. However, some drawbacks such as harsh reaction conditions and use of hazardous solvents still exist in some of those methods. Additionally, the substrates of those methods are only limited to aldehydes and aminopyridines. In line with our previous studies on developing improved methodologies for organic reactions, herein, we wish to report an efficient and eco-friendly method for the synthesis of amide. It is interesting to note that besides aromatic amines, alkyl amines are also well compatible in our system and afford the corresponding products in good to excellent yields.
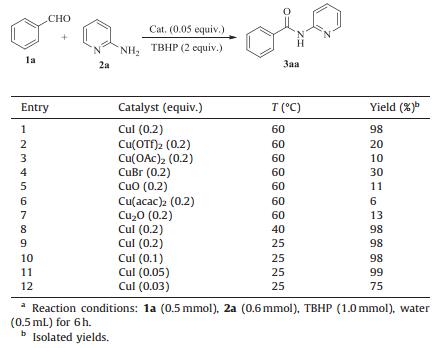
2. Results and discussionAt the beginning of our investigation, the reaction of benzaldehyde and 2-aminopyridine was chosen as a model reaction for the optimization of reaction conditions. Firstly, we examined the effect of copper source on the reaction. It could be seen from Table 1 that CuI was the best catalyst for the reaction, which could afford the desired product in 98% yield (Table 1, entry 1). Other copper catalyst such as Cu(OTf)2, CuO and CuBr could also catalyze the reaction, but the yields were very low (entries 2-7). We next turned our attention to investigate the effect of temperature on the reaction. To our delight, there was no evident difference on the yield of the product when the reaction temperature was decreased (entries 8, 9). A high yield (98%) of the desired product was obtained when the reaction was conducted at room temperature (entry 9). Further study indicated that almost the same yield could be obtained when CuI loading was changed from 0.1 equiv. to 0.05 equiv. (entries 10, 11). But the yield of product was significantly decreased when 0.03 equiv. Cu/I was used (entry 12). The optimized reaction conditions for the model reaction were CuI (0.05 equiv.), TBHP (2.0 equiv.) at room temperature for 6 h under aqueous conditions.
|
|
Table 1 Optimization of the conditions.a |
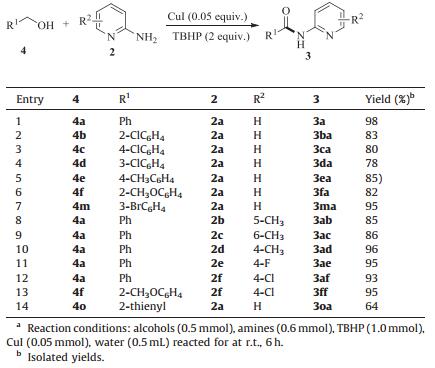
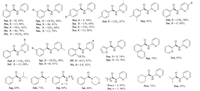
To examine the versatility of this protocol, a variety of aldehydes and aminopyridines were investigated under the optimized reaction conditions. It was found that, in most cases, the reactions afforded the corresponding products in good to excellent yields (Fig. 1). For example, aldehydes bearing electron-donating (CH3, OCH3) or electron-withdrawing (F, Cl, Br, CN and NO2) groups were well tolerated and provided the corresponding products in moderate to excellent yields (3aa-3ma). Disubstituted benzaldehyde, such as 3, 4-dimethylbenzaldehyde, also underwent the reaction with 2-aminopyridine under the present reaction conditions and afforded the corresponding product in 85% yield (3na). The present reaction conditions were also suitable for heterocyclic aldehydes, such as 2-pyridinecarboxaldehyde, 1-naphthaldehyde, furfural, 2-thenaldehyde and the corresponding products were isolated in 74%-95% yields (3oa-3ra). To our delight, high yields were also obtained when aliphatic aldehydes were tested in this system (3ta-3wa).
|
Download:
|
| Fig. 1. Synthesis of amides from aldehydes and amines under the optimized conditions. | |
Next, the effect of substituents on the 2-aminopyridine was also investigated. We were pleased to observe that the substituents (H, CH3, Cl and F) were all tolerated (3ab-3af, 3ed, 3fe, 3gc, 3ff, Fig. 1) and moderate yields of the products were obtained. It is worth noting that aliphatic amines were also good substrates in this reaction. When butylamine, isopropylamine, dimethylamine, morpholine were used under optimized reaction conditions, the desired products can be isolated in high yields (3ag-3aj, Fig. 1).
We speculated that benzyl alcohol may also be tolerated in this reaction because of the oxidative properties of the reaction condition. To our delight, high yield was also obtained when benzyl alcohol was subjected to this reaction directly (3aa, Table 2). As can be seen from Table 2, other substituted benzyl alcohols were also compatible with this catalytic system and delivered the desired products with 82%-98% yields. Moderate yield was obtained when heterocyclic 2-thiophenemethanol was subjected to this system.
|
|
Table 2 Copper-catalyzed synthesis of amides from alcohols.a |
To understand the mechanism further, the reaction of benzaldehyde and 2-aminopyridine was tested in the presence of 1 equiv. of 2, 2, 6, 6-tetramethylpiperidinyl-1-oxy (TEMPO), a well-known radical inhibitor, as a radical scavenger. The corresponding product was also isolated in high yield (95%). Thereby, a radical mechanism maybe excluded for this reaction. When CuI was not added in this reaction, very low yield (15%) of the product was obtained. Moreover, reaction did not occur if TBHP (tert-butyl hydroperoxide) was not added. These results indicate that TBHP and CuI play an important role in this reaction.
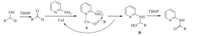
According to the previous literature and our control experiment, a plausible mechanism was proposed in Scheme 1. Alcohols could be oxidized to aldehyde in the presence of TBHP. With the help of CuI, the intermediated A was formed. Then the nucleophilic attach addition of 2-aminopyridine to aldehyde occurred to produce the hemiaminal intermediate B. Finally, the desired amide product was obtained from the oxidation of intermediate B by TBHP.
|
Download:
|
| Scheme 1. Proposed reaction mechanism. | |
3. Conclusions
In conclusion, a highly efficient synthetic method for the preparation of amides has been developed. In the presence of CuI and tert-butyl hydroperoxide (TBHP), the oxidative coupling reactions of aldehydes or alcohols with amines proceeded smoothly at room temperature under aqueous conditions to generate the corresponding products in good to excellent yields. Remarkable observation was that besides aromatic amines, alkyl amines were also well tolerated in this catalytic system. Further investigation on the reaction mechanism and application of this kind of oxidative system are currently underway in our laboratory.
4. ExperimentalAll major chemicals were obtained from commercial sources and used without further purification. All reactions were monitored by TLC. Column chromatography was performed on silica gel.
General procedure: A sealable reaction tube equipped with a magnetic stirrer bar was charged with aldehydes (1.0 mmol), amines (1.2 mmol), CuI (0.1 mmol), tert-butyl hydroperoxide (TBHP, 2.0 mmol), water (1 mL). The reaction vessel was carried out at room temperature. After stirring the mixture for 6 h, it was diluted with ethyl acetate, washed with water and brine, dried with Mg2SO4. After the solvent was removed under reduced pressure, the residue was purified by column chromatography on silica gel using petroleum ether/ethyl acetate as eluent to afford the corresponding product.
AcknowledgmentsThe National Natural Science Foundation of China (No. 21402103), the China Postdoctoral Science Foundation (No. 150030), the Scientific Research Foundation of Shandong Province Outstanding Young Scientist Award (No. BS2013YY024) and the research fund of Qingdao Agricultural University's High-level Person (No. 631303) were gratefully acknowledged.
| [1] |
(a) J. S. Carey, D. Laffan, C. Thomson, M. T. Williams, Analysis of the reactions used for the preparation of drug candidate molecules, Org. Biomol. Chem. 4(2006) 2337-2347; (b) E. Valeur, M. Bradley, Amide bond formation: beyond the myth of coupling reagents, Chem. Soc. Rev. 38(2009) 606-631; (c) K. Ekoue-Kovi, C. Wolf, One-pot oxidative esterification and amidation of aldehydes, Chem. Eur. J. 14(2008) 6302-6315; (d) J. M. Humphrey, A. R. Chamberlin, Chemical synthesis of natural product peptides: coupling methods for the incorporation of noncoded amino acids into peptides, Chem. Rev. 97(1997) 2243-2266. |
| [2] |
(a) B. Shen, D. M. Makley, J. N. Johnston, Umpolung reactivity in amide and peptide synthesis, Nature 465(2010) 1027-1032; (b) H. A. Soliman, A. Y. Mubarak, S. S. Elmorsy, An efficient synthesis of bis(indolyl) methanes and N, N'-alkylidene bisamides by silzic under solvent free conditions, Chin. Chem. Lett. 27(2016) 353-356; (c) W. Tang, J. Guo, X. Gui, D. Han, J. Li, An efficient synthesis of imidodicarbonic diamides from 1, 3-thiazetidin-2-ones with NH2OH HCl via ring-opening reaction, Chin. Chem. Lett. 26(2015) 85-88; (d) H. Liu, J. Liu, Y. Zhang, C. Shao, J. Yu, Copper-catalyzed amide bond formation from formamides and carboxylic acids, Chin. Chem. Lett. 26(2015) 11-14. |
| [3] | E. Valeur, M. Bradley. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 38 (2009) 606–631. DOI:10.1039/B701677H |
| [4] |
(a) S. H. Cho, E. J. Yoo, I. Bae, S. Chang, Copper-catalyzed hydrative amide synthesis with terminal alkyne, sulfonyl azide, and water, J. Am. Chem. Soc. 127(2005) 16046-16047; (b) M. P. Cassidy, J. Raushel, V. V. Fokin, Practical synthesis of amides from in situ generated copper(I) acetylides and sulfonyl azides, Angew. Chem. Int. Ed. 45(2006) 3154-3157; (c) S. C. Ghosh, J. S. Y. Ngiam, A. M. Seayad, et al. , Copper-catalyzed oxidative amidation of aldehydes with amine salts: synthesis of primary, secondary, and tertiary amides, J. Org. Chem. 77(2012) 8007-8015. |
| [5] | G.L. Li, K.K.Y. Kung, M.K. Wong. Gold-catalyzed amide synthesis from aldehydes and amines in aqueous medium. Chem. Commun. 48 (2012) 4112–4114. DOI:10.1039/c2cc17689k |
| [6] | B. Gnanaprakasam, D. Milstein. Synthesis of amides from esters and amines with liberation of H2 under neutral conditions. J. Am. Chem. Soc. 133 (2011) 1682–1685. DOI:10.1021/ja109944n |
| [7] |
(a) Y. Li, L. Ma, F. Jia, Z. Li, Amide bond formation through iron-catalyzed oxidative amidation of tertiary amines with anhydrides, J. Org. Chem. 78(2013) 5638-5646; (b) A. Porcheddu, L. D. Luca, Iron-catalyzed amidation of aldehydes with Nchloroamines, Adv. Synth. Catal. 354(2012) 2949-2953. |
| [8] | H. Morimoto, R. Fujiwara, Y. Shimizu, K. Morisaki, T. Ohshima. Lanthanum(Ⅲ) triflate catalyzed direct amidation of esters. Org. Lett. 16 (2014) 2018–2021. DOI:10.1021/ol500593v |
| [9] |
(a) X. Liu, K. F. Jensen, Multistep synthesis of amides from alcohols and amines in continuous flow microreactor systems using oxygen and urea hydrogen peroxide as oxidants, Green Chem. 15(2013) 1538-1541; (b) B. Du, B. Jin, P. Sun, The syntheses of (-ketoamides via nBu4NI-catalyzed multiple sp3C-H bond oxidation of ethylarenes and sequential coupling with dialkylformamides, Org. Biomol. Chem. 12(2014) 4586-4589; (c) W. Mai, H. Wang, Z. Li, et al. , nBu4NI-catalyzed direct synthesis of (-ketoamides from aryl methyl ketones with dialkylformamides in water using TBHP as oxidant, Chem. Commun. 48(2012) 10117-10119. |
| [10] | S. Yang, H. Yan, X. Ren, et al., Copper-catalyzed dehydrogenative reaction: synthesis of amide from aldehydes and aminopyridine. Tetrahedron 69 (2013) 6431–6435. DOI:10.1016/j.tet.2013.05.072 |
| [11] | W. Dai, Y. Liu, T. Tong, X. Li, F. Luo. Rh(Ⅲ)-catalyzed oxidative amidation of aldehydes: an efficient route to N-pyridinamides and imides. Chin. J. Catal. 35 (2014) 1012–1016. DOI:10.1016/S1872-2067(14)60141-8 |
| [12] | O.P.S. Patel, D. Anand, R.K. Maurya, P.P. Yadav. Copper-catalyzed highly efficient oxidative amidation of aldehydes with 2-aminopyridines in an aqueous micellar system. Green Chem. 17 (2015) 3728–3732. DOI:10.1039/C5GC00628G |
 2017, Vol. 28
2017, Vol. 28