Pyrazole derivatives are well known for their broad range of biological properties [1-8]. Thus, it is attracting the scientists towards their preparation [9, 10]. Pyrazolones are structurally closed to pyrazoles, which are also associated with wide spectrum of biological activities [11-16]. Inspired by their importance, many research groups have reported different protocols for the synthesis of 4-pyrazolylmethylenepyrazol-5-(4H)-ones through two-component condensation of pyrazolone and pyrazole-4-carbaxaldehyde in the presence of different catalysts [17-20]. However, some of the above reported methods suffer from one or more drawbacks like use of unfavourable conditions, non-eco-friendly catalysts, solvents, prolonged reaction times, high catalyst loading and the use of expensive ionic liquids.
Literature survey reveals that, the multicomponent reactions (MCRs) have been recently discovered as a powerful tool in the field of medicinal chemistry and synthetic organic chemistry [21-24]. Moreover, MCRs are having huge applications like short reaction time, avoiding of intermediate isolation, diversity in reaction, atomeconomy, environmental benign and substantial minimization of waste.
Organic transformations under the solvent-free conditions have also gained attention particularly from the view point of green strategy [25]. Physical grinding is one of the techniques which have increased in the use of organic synthesis rather than other traditional methods [26, 27]. This method offers many advantages interims of green protocol by avoiding of hazardous solvents, cost efficiency and product yields.
Sulfamic acid has been found to be a promising solid, proton donor catalyst. It is insoluble in almost all organic solvents, relatively stable, non-volatile, non-hygroscopic, non-corrosive and economically cheaper. This catalyst could be easily recycled and reused due to its very high immiscibility with most organic solvents. It acts as a solid acid catalyst for so many organic transformations as witnessed by many reports published in the past [28-34]. Mohan et al. reported the synthesis of pyrazolone with phenyl hydrazine and ethyl acetoacetate in the presence of sulfamic acid [35]. Thus, we decided to explore the capability of sulfamic acid to perform multi component reactions.
Here, we report a simple, convenient and eco-friendly method for the synthesis of 4-(pyrazol-4-yl)methylenepyrazol-5(4H)-one 6(a-n) by using 3-diphenyl-1H-pyrazol-4-carboxaldehyde 4(a-h) and pyrazolone 9(a, b) (Scheme 1) and one-pot, three component reaction of 3-diphenyl-1H-pyrazol-4-carboxaldehyde 4(a-h), β-ketoesters 5(a, b) and phenyl hydrazine 2 in the presence of sulfamic acid and has not been reported elsewhere on its use for the synthesis of 4-(pyrazol-4-yl)methylenepyrazol-5(4H)-one.

|
Download:
|
| Scheme 1. Synthesis of 1, 3-diphenylpyrazolin-5-(4H)-4-carbaldehyde 4(a-h). | |
2. Results and discussion
The starting materials consumed in the present study, 3-diphenylpyrazol-4-carboxaldehyde 4(a-h) were prepared in accordance with literature procedure. The reaction of substituted acetophenones 1(a-h) with phenyl hydrazine (2) in ethanol containing catalytic amount of acetic acid yields acetophenone phenyl hydrazones 3(a-h). The latter undergoes Vilsmeier-Haack reaction to give 4-(1H-pyrazol-4-yl) methylenepyrazol-5(4H)-one 4(a-h) in good yield (Scheme 1).
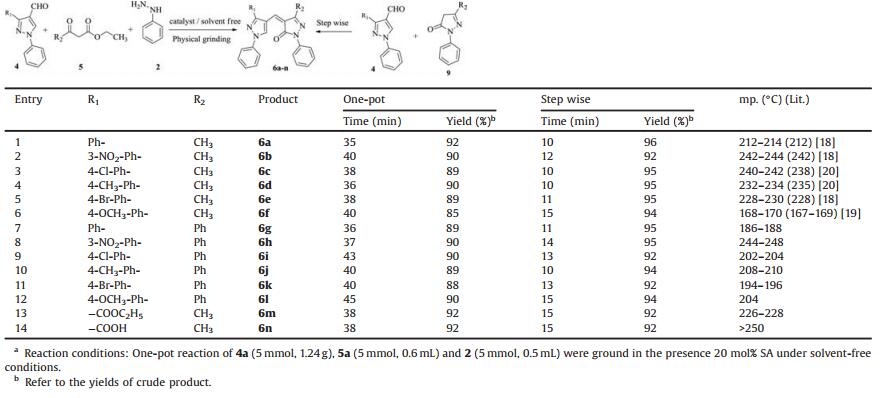
To optimise the reaction conditions, we have chosen 3-diphenyl-1H-pyrazol-4-carboxaldehyde (4a), ethyl acetoacetate (5a) and phenyl hydrazine (2) as model substrates (Scheme 2). Initially, we have focused to find out the best catalyst for the synthesis of 4-(pyrazol-4-yl) methylenepyrazol-5(4H)-one 6(a-n) by employing variety catalysts under different conditions (Table 1).

|
Download:
|
| Scheme 2. One-pot synthesis of 4-((1, 3-diphenyl-1H-pyrazol-4-yl) methylene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-ones 6a. | |
|
|
Table 1 Optimisation of reaction conditions for the synthesis of 6a.a |
It is obvious from Table 1 that the best results were obtained in the presence of 20 mol% sulfamic acid under solvent-free grinding conditions (Table 1, entry 8). The other catalysts such as L-proline, InCl3, montmorillonite-K10 and amberlyst-15 (Table 1, entries 2-5) afforded moderate to good yields. From the view of cost and performance of the catalyst SA was found as the best catalyst for the synthesis of 6a through one-pot, three component reaction. The model reaction was also checked under catalyst-free conditions in which the reaction did not proceed even after 100 min (Table 1, entry 1). From these observations, it can be confirmed the crucial role played by SA to yield the desired product 6a. The influence of various amounts of the catalyst was also examined on the model reaction. It is confirmed that, 20 mol% of sulfamic acid (Table 1, entry 8) is enough to get good yields in a shortest reaction time (35 min). Excess amount of sulfamic acid did not effect on reaction time and yields (Table 1, entry 9). Further, we thought of scrutinising with various solvents and the role played by them on the reaction time and yields. It was found that protic solvents like ethanol and methanol (Table 1, entries 11, 12) were effective than non-protic solvents (Table 1, entries 13-15). It is confirmed from Table 1, the best optimised reaction conditions are observed when the model reaction is carried out in the presence of 20 mol% sulfamic acid under solvent-free conditions.
After optimising the reaction conditions, formation of undesired side products was also examined under the similar conditions by carrying a set of reactions. Primarily, Knoevenagel condensation was carried out with 3-diphenyl-1H-pyrazol-4-carboxaldehyde (4a) and ethyl acetoacetate (5a) in the presence of sulfamic acid, under solvent-free grinding conditions. It does not yield condensation product even after 60 min (Scheme 3).

|
Download:
|
| Scheme 3. Condensation of 4a, 5a in the presence of SA under solvent-free conditions. | |
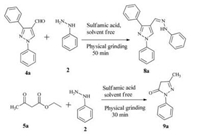
In another set of reactions, formation of hydrazones was tested by using 4a and phenyl hydrazine 2 under the above similar conditions. It results in the formation of hydrazone 8a in 50 min (Scheme 4).
|
Download:
|
| Scheme 4. Synthesis of hydrazone 8a and pyrazolones 9a. | |
Finally, pyrazolone 9a was prepared as per literature procedure [35] (Scheme 4) by the condensation of ethyl acetoacetate 5a with phenyl hydrazine 2 in the presence of sulfamic acid under solventfree conditions for 30 min.
As it is clear from the above sets of reactions, in threecomponent reaction between 4a, 5a and 2 initially, it results in the formation of the pyrazolone as intermediate which further undergoes Knoevenagel condensation with 4a. Hence, there is no side products formation in multicomponent reaction.
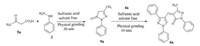
The compound 6a can also be synthesized through tandem method as well as stepwise method. In stepwise manner, 9a was prepared from 5a, 2 and sulfamic acid were ground together for 30 min under solvent-free conditions [35]. Further, the compound 9a was condensed with 4a under the same conditions for 10 min. It results in the formation of 6a in good yield (Scheme 5).
|
Download:
|
| Scheme 5. Stepwise synthesis of compound 6a. | |
To compare the capability and efficiency of our methodology with respect to the reported catalysts for the preparation of 6a, the results of these catalysts for the synthesis of 6a were tabulated in Table 2.
|
|
Table 2 Comparing the catalytic activity of sulfamic acid for the synthesis of 6a with reported catalysts. |
It is clear from Table 2, our methodology is more efficient than previous reports because sulfamic acid is cheap when compared with other catalysts like Ionic liquids, B2O3-ZrO2, easily handling eco-friendly nature.
The optimised reaction conditions were applied for the synthesis of other derivatives such as 6(b-h). Results of this study were presented in Table 3 and it concluded that the substitution on pyrazole ring does not exert any effect on the efficiency of the reaction.
|
|
Table 3 One-pot synthesis of 4-pyrazolylmethylene pyrazol-5(4H)-ones 6(a-n).a |
Further, we have also examined recycling of the catalyst for model reaction (Scheme 2) under optimised conditions and the results are tabulated in Table 4. Sulfamic acid was insoluble in many organic solvents hence it can be easily separated from the reaction mixture. After completion of reaction, ethyl acetate was added to extract the product and the insoluble catalyst was separated by simple separating technique. The separated catalyst was reused for five times with fresh starting materials without losing its catalytic activity and slight reduction of products yields. This might be due to loss of small amount of catalyst during handling.
|
|
Table 4 Recyclability of the catalyst.a |
To find the structure of recovered catalyst, the IR spectra of fresh sulfamic acid were compared with 1st and 5th time recovered sulfamic acid (Fig. 1). It is observed that there are no differences in the IR spectra. Hence, it can be concluded that the structure of recovered sulfamic acid is stable under the applied conditions even after 5th time recovery and reuse.

|
Download:
|
| Fig. 1. IR spectra of sulfamic acid. | |
The plausible mechanism of this reaction is explained by the following sequence of reactions (Scheme 6). It consist two steps, in the first step ethylacetoacetate 5a gets protonated in the presence of sulfamic acid. The protonated ethyl acetoacetate reacts with phenyl hydrazine 2 with subsequent cyclisation to form compound 9a. Knoevenagel condensation involves in the second step of mechanism. In this step, 4a undergoes protonation with sulfamic acid, which reacts with 9a to form alcohol intermediate. The latter undergoes protonation followed by elimination of water molecule to yield desire product 6a.
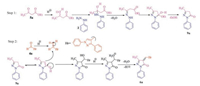
|
Download:
|
| Scheme 6. Plausible mechanism for the synthesis of 6a. | |
3. Conclusions
In summary, we have reported a bi-functional, efficient, and green methodology for the multi component reaction using sulfamic acid as solid acid catalyst. This method offers many advantages, like eco-friendly, economically cheaper, avoiding of hazardous organic solvents, short reaction time, without isolation of intermediates and high yields.
4. Experimental 4.1. Material and methodsAll the chemicals sulfamic acid, derivatives of acetophenones, phenyl hydrazine and β-ketoesters were obtained from commercial sources & are used without purification. Melting points were determined in open capillary tubes on Cintex melting point apparatus and are uncorrected. Pre-coated TLC silica gel plates (Kieselgel 60 F254, Merck) were used for monitoring reactions and the spots are visualized under UV lamp (254 nm). IR spectra were recorded using Perkin-Elmer spectrum version 10.03.02 instrument in KBr Pellets. 1H NMR and 13C NMR spectra were recorded in CDCl3/DMSO-d6 on a Bruker (400 MHz) or Varian Mercury 400 MHz spectrometer. Proton chemical shifts are presented in δ ppm with reference to TMS. Mass spectra were recorded on an Agilent LC-MS instrument giving only M+ values.
4.2. Synthesis of 4-((1, 3-diphenyl-1H-pyrazol-4-yl) methylene)-1-phenyl pyrazolin-5(4H)-one 6(a-n)One-pot synthesis of 6(a-n): A mixture of appropriate 1, 3-diphenylpyrazolin-5-(4H)-4-carbaldehyde 4(a-h) (5 mmol), β-ketoesters 5(a, b) (5 mmol), phenyl hydrazine 2 (5 mmol) and sulfamic acid (20 mol%) was thoroughlygrind with pestle in an open mortar at room temperature. Reaction progress was monitored by TLC. Upon completion of the reaction ethyl acetate (5mL× 3) was added to the mixture, shaked well and insoluble catalyst was separated by simple separating methods. The combined organic layers were concentrated under reduced pressure to get the clean products 6(a-n). Purification of the crude products was done by recrystallization from suitable solvent, which afforded respective 4-(1H-pyrazol-4-yl) methylene-1H-pyrazol-5(4H)-ones 6(a-n). All the synthesized products are stable, coloured solids and authenticity of these compounds was established based on their melting points and spectral analysis (IR, 1H NMR and LC-MS).
Stepwise synthesis: A mixture of appropriate 1, 3-diphenylpyrazolin-5-(4H)-4-carbaldehyde 4(a-h) (5 mmol), pyrazolones 9(a, b) (5 mmol) and sulfamic acid (20 mol%) was thoroughly grind with pestle in an open mortar at room temperature. Reaction progress was monitored by TLC. Upon completion of the reaction, isolation and purification of the crude products 6(a-n) was carried out by using above methodology. All the synthesized products are stable, coloured solids and authenticity of these compounds was established based on their melting points and spectral analysis (IR, 1H NMR and LC-MS).
Spectral data for compound 6(a-n):
(Z)-3-Methyl-1-phenyl-4-(1-phenyl-3-p-tolyl-1H-pyrazol-4-yl)methylene-1H-pyrazol-5(4H)-one (6a): IR (KBr, cm-1): 3125.7, 2982, 1675; 1H NMR (400 MHz, DMSO-d6/TMS): δ 2.42 (s, 3H, -CH3), 7.38-7.99 (m, 15H, aromatic protons and 1H olefinic proton), 10.88 (s, 1H pyrazole ring proton); LC-MS: m/z 405 (Q + 1).
(Z)-3-Methyl-4-((3-(3-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl) methylene)-1-phenyl-1H-pyrazol-5(4H)-one (6b): IR (KBr, cm-1): 3539, 312, 3152, 1625, 1590, 778; 1H NMR (400 MHz, DMSO-d6/ TMS): δ 2.30 (s, 3H, -CH3), 7.18-7.96 (m, 14H, aromatic protons and 1H olefinic proton), 10.60 (s, 1H, pyrazole ring proton); LC-MS: m/z 450 (Q + 1).
(Z)-4-((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (6c): IR (KBr, cm-1): 3108, 3072, 2902, 1624, 1573, 743; 1H NMR (400 MHz, DMSO-d6/TMS): δ 2.39 (s, 3H, CH3), 7.20-8.48 (m, 14H, aromatic protons and 1H olefinic proton), 10.66 (s, 1H, pyrazole ring proton); LC-MS: m/z 439 (Q + 1).
(Z)-3-Methyl-1-phenyl-4-((1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)methylene)-1H-pyrazol-5(4H)-one (6d): IR (KBr cm-1): 3128.7, 2985, 1670; 1H NMR (400 MHz, DMSO-d6/TMS): δ 2.30 (s, 3H, -CH3), 3.41(s, 3H, -CH3), 7.26-7.71 (m, 15H, aromatic protons and 1H olefinic proton), 9.22 (s, 1H, pyrazole ring proton); LC-MS: m/z 419 (Q + 1).
(Z)-4-((3-(4-Bromophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (6e): IR (KBr cm-1): 3125.7, 2982, 1675; 1H NMR (400 MHz, DMSO-d6/TMS): δ 2.34 (s, 3H, -CH3), 7.18-7.14 (m, 14H, aromatic protons and 1H olefinic proton), 9.25 (s, 1H, pyrazole ring proton); LC-MS: m/z 494 (Q + 1).
(Z)-4-((3-(4-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (6f): IR (KBr, cm-1): 3325, 3158, 1620, 1574, 1435; 1H NMR (400 MHz, DMSOd6/TMS): δ 2.33 (s, 3H, CH3), 3.62 (s, 3H, OCH3), 7.26-7.72 (m, 15H, aromatic protons and 1H olefinic proton), 9.63 (s, 1H pyrazole ring proton); LC-MS: m/z 435 (Q + 1).
(Z)-4-((3-(3-Nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1, 3-diphenyl-1H-pyrazol-5(4H)-one (6h): IR (KBr, cm-1): 1625, 1590, 778; 1H NMR (400 MHz, DMSO-d6/TMS): δ 7.28-7.71 (m, 20H, aromatic protons and 1H olefinic proton), 9.44 (s, 1H, pyrazole ring proton); LC-MS: m/z 512 (Q + 1).
(Z)-4-((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1, 3-diphenyl-1H-pyrazol-5(4H)-one (6i): IR (KBr, cm-1): 1625, 1590, 778; 1H NMR (400 MHz, DMSO-d6/TMS): δ 7.25-7.91 (m, 20H, aromatic protons and 1H olefinic proton), 9.42 (s, 1H, pyrazole ring proton); LC-MS: m/z 499 (Q-1).
(Z)-1, 3-diphenyl-4-((1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl) methylene)-1H-pyrazol-5(4H)-one (6j): IR (KBr, cm-1): 3325, 3158, 1620, 1574, 1435; 1H NMR (400 MHz, DMSO-d6/TMS): δ 2.33 (s, 3H, -CH3), 7.25-7.91 (m, 20H, aromatic protons and 1H olefinic proton), 7.27 (s, 1H, pyrazole ring proton); LC-MS: m/z 481 (Q + 1).
(Z)-Ethyl-4-((3-methyl-5-oxo-1-phenyl-1H-pyrazol-4(5H)-ylidene)methyl)-1-phenyl-1H-pyrazole-3-carboxylate (6m): IR (KBr, cm-1): 1720, 1625, 1354, 792; 1H NMR (400 MHz, DMSO-d6/TMS): δ 1.4 (s, 3H, -CH3), 2.4 (s, 3H, -CH3), 4.4(s, 2H, -CH2), 7.2-7.2 (m, 10H, aromatic protons and 1H olefinic proton), 10.1 (s, 1H, pyrazole ring proton); LC-MS: m/z 399 (Q -1).
(Z)-4-((3-Methyl-5-oxo-1-phenyl-1H-pyrazol-4(5H)-ylidene) methyl)-1-phenyl-1H-pyrazole-3-carboxylic acid (6n): IR (KBr, cm-1): 3353, 1756, 1625, 1342, 778; 1H NMR (400 MHz, DMSO-d6/ TMS): δ 2.18(s, 3H, -CH3), 7.23-7.84 (m, 11H, aromatic protons and 1H olefinic proton), 10.31 (s, 1H, pyrazole ring proton), 13.10 (s, br, 1H, -COOH D2O exangeble); LC-MS: m/z 371 (Q -1).
AcknowledgementThe authors are very thankful to authorities of Jawaharlal Nehru Technological University for providing laboratory facilities and they are indebted to University Grants Commission, Govt. of India for providing financial support to one of them (K.V.S) in form of UGC-SRF scheme.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.02.005
| [1] | B.F. Wahaba, E. Abdel, H.A. Mohameda, et al., Design and synthesis of new 4-pyrazolin-yl-1, 2, 3-triazoles and 1, 2, 3-triazol-4-yl-pyrazolin-1-yl-thiazoles as potential antimicrobial agents. Eur. J. Med. Chem. 52 (2012) 263–268. DOI:10.1016/j.ejmech.2012.03.023 |
| [2] | I.V. Magedov, M. Manpadi, S.S. Van, et al., Discovery and investigation of antiproliferative and apoptosis-inducing properties of new heterocyclic podophyllotoxin analogues accessible by a one-step multicomponent synthesis. J. Med. Chem. 50 (2007) 5183–5192. DOI:10.1021/jm070528f |
| [3] | G.C. Rovnyak, R.C. Millonig, J. Schwartz, V.J. Shu. Synthesis and antiinflammatory activity of hexahydrothiopyrano[4, 3-c] pyrazoles and related analogs. J. Med. Chem. 25 (1982) 1482–1488. DOI:10.1021/jm00354a018 |
| [4] | E. Palaska, M. Aytemir, I.T. Uzbay, D. Erol. Synthesis and antidepressant activities of some 3, 5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 36 (2001) 539–543. DOI:10.1016/S0223-5234(01)01243-0 |
| [5] | A. Sener, M.K. Sener, I. Bildmci, R. Kasimogullari, Y. Akcamur. Studies on the reactions of cyclic oxalyl compounds with hydrazines or hydrazones: synthesis and reactions of 4-benzoyl-1-(3-nitrophenyl)-5-phenyl-1Hpyrazole-3-carboxylic acid. J. Heterocycl. Chem. 39 (2002) 869–875. DOI:10.1002/jhet.v39:5 |
| [6] | X.H. Liu, P. Cui, B.A. Song, et al., Synthesis structure and antibacterial activity of novel 1-(5-substituted-3-substituted-4, 5-dihydropyrazol-1-yl)ethanone oxime ester derivatives. Bioorg. Med. Chem. 16 (2008) 4075–4082. DOI:10.1016/j.bmc.2008.01.035 |
| [7] | E. Akbas, I. Berber. Antibacterial and antifungal activities of new pyrazolo[3, 4-d]pyridazin derivatives. Eur. J. Med. Chem. 40 (2005) 401–405. DOI:10.1016/j.ejmech.2004.12.001 |
| [8] | G.A. Wachter, R.W. Hartmann, T. Sergejew, G.L. Grun, D. Ledergerber. Tetrahydronaphthalenes: influence of heterocyclic substituents on inhibition of steroidogenic enzymes P450 arom and P45017. J. Med. Chem. 39 (1996) 834–841. DOI:10.1021/jm950377t |
| [9] | S.A. Rostoma, M.A. Shalaby, M.A. Demellawy. Polysubstituted pyrazoles, part 5. Synthesis of new 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur. J. Med. Chem 38 (2003) 959–974. DOI:10.1016/j.ejmech.2003.08.003 |
| [10] | L.R. Dias, R.R.S. Salvador. Pyrazole carbohydrazide derivatives of pharmaceutical interest. Pharmaceuticals 5 (2012) 317–324. DOI:10.3390/ph5030317 |
| [11] | E. Conchona, B. Aboaba, M. Roy, et al., Synthesis in vitro antiproliferative activities, and Chk1 inhibitory properties of indolylpyrazolones and indolylpyridazinedione. Eur. J. Med. Chem. 41 (2006) 1470–1477. DOI:10.1016/j.ejmech.2006.06.012 |
| [12] | P. Manojkumar, T.K. Ravi, S. Gopalakrishnan. Antioxidant and antibacterial studies of arylazopyrazoles and arylhydrazonopyrazolones containing coumarin moiety. Eur. J. Med. Chem. 44 (2009) 4690–4694. DOI:10.1016/j.ejmech.2009.07.004 |
| [13] | R.V. Ragavana, V. Vijayakumara, N.S. Kumari. Synthesis of some novel bioactive 4-oxy/thio substituted-1H-pyrazol-5(4H)-ones via efficient cross-Claisen condensation. Eur. J. Med. Chem. 44 (2009) 3852–3857. DOI:10.1016/j.ejmech.2009.04.010 |
| [14] | D. Zimmermann, Y.L. Janin, L. Brehm, et al., 3-Pyrazolone analogues of the 3-isoxazolol metabotropic excitatory amino acid receptor agonist homo-AMPA. Synthesis and pharmacological testing. Eur. J. Med. Chem 34 (1999) 967–976. DOI:10.1016/S0223-5234(99)00122-1 |
| [15] | P.N. Dube, S.S. Bule, V. Yogesh, R.U. Manoj, K.R. Pravin. Synthesis of novel 5-methyl pyrazol-3-one derivatives and their in vitro cytotoxic evaluation. Med. Chem. Res. 24 (2015) 1070–1076. DOI:10.1007/s00044-014-1201-z |
| [16] | A. Shamsuzzaman, A. Mashrai, M.A. Anis, H. Dar, M. Khanam, Danishuddin, A. U. Khan. Synthesis, evaluation and docking studies on steroidal pyrazolones as anticancer and antimicrobial agents. Med. Chem. Res. 23 (2014) 348–362. DOI:10.1007/s00044-013-0636-y |
| [17] | S.K. Lanke, N. Sekar. Pyrazole based solid state emissive NLOphores with TICT characteristics: Synthesis, DFT and TDDFT studies. Dyes Pigm. 126 (2016) 62–75. DOI:10.1016/j.dyepig.2015.11.014 |
| [18] | R.V. Hangarge, M.S. Shingare. Environmentally benign synthesis of 3-methyl-4-[(1, 3-diphenyl-1H-pyrazol-4-yl)-methylene]-1-phenylpyrazolin-5-(4H) ones in an ionic liquid. Mendeleev Commun. 13 (2003) 79–80. DOI:10.1070/MC2003v013n02ABEH001721 |
| [19] | S.S. Chobe, S. Bhaskar, M. Dawane, Khaled, et al., An ecofriendly synthesis and DNA binding interaction study of some pyrazolo [1, 5-a] pyrimidines derivatives. Bioorg. Med. Chem. Lett. 22 (2012) 7566–7572. DOI:10.1016/j.bmcl.2012.10.027 |
| [20] | S.S. Santosh, M.R. Balaji, R.V. Hangarge, P.T. Patil, M.K. Dongare, M.S. Shingare. Borate Zirconia Mediated Knoevenagel Condensation Reaction in Water. J. Kor. Chem. Soc 49 (2005) 377–380. DOI:10.5012/jkcs.2005.49.4.377 |
| [21] | B.B. Toure, D.G. Hall. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 109 (2009) 4439–4486. DOI:10.1021/cr800296p |
| [22] | S. Bondock, W. Fadaly, M.A. Metwally. Recent trends in the chemistry of 2-aminobenzothiazoles. J. Sulfur Chem. 30 (2009) 74–107. DOI:10.1080/17415990802588033 |
| [23] | B. Ganem. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 42 (2009) 463–472. DOI:10.1021/ar800214s |
| [24] | A. Domling. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106 (2006) 17–89. DOI:10.1021/cr0505728 |
| [25] | K. Tanaka, F. Toda. Solvent-free organic synthesis. Chem. Rev. 100 (2016) 1025–1074. |
| [26] | F. Toda. Solid state organic reactions. Synlett 5 (1993) 303–312. |
| [27] | G. Centi, P. Ciambelli, S. Perathoner, P. Russo. Environmental catalysis: trends and outlook. Catal. Today 75 (2002) 3–15. DOI:10.1016/S0920-5861(02)00037-8 |
| [28] | R. Nagarajan, C.J. Magesh, P.T. Perumal. Inter-and intramolecular imino DielsAlder reactions catalyzed by sulfamic acid: a mild and efficient catalyst for a one-pot synthesis of tetrahydroquinolines. Synthesis 1 (2004) 69–74. |
| [29] | M. Xia, Y.D. Lu. A novel direct and one-pot Mannich synthesis of fluorinated β-aminobutanones with sulfamic acid as a green catalyst. J. Fluorine Chem. 127 (2006) 1119–1124. DOI:10.1016/j.jfluchem.2006.05.020 |
| [30] | S.D. Mitragotri, D.M. Pore, U.V. Desai, P.P. Wadgaonkar. Sulfamic acid: An efficient and cost-effective solid acid catalyst for the synthesis of β-aminophosphonates at ambient temperature. Catal. Commun. 9 (2008) 1822–1826. DOI:10.1016/j.catcom.2008.02.011 |
| [31] | L. Wu, S. Ma, F. Yan, C. Yang. Sulfamic-acid-catalyzed simple and efficient synthesis of 4-aryl-3-methyl-1-phenyl-H-benzo[g]pyrazolo[3, 4-b] quinoline-5, 10-diones under solvent-free conditions. Monatsh. Chem. 141 (2010) 565–568. DOI:10.1007/s00706-010-0282-8 |
| [32] | B. Zakerinasab, M.A. Nasseri, H. Hassani, M.M. Samieadel. Application of Fe3O4@SiO2@sulfamic acid magneticnanoparticles as recyclable heterogeneous catalystfor the synthesis of imine and pyrazole derivativesin aqueous medium. Res. Chem. Intermed. 42 (2016) 3169–3181. DOI:10.1007/s11164-015-2204-1 |
| [33] | V. Sandeep, N. Wamanrao, M.K. Jeevan, V.G. Sumit, N.K. Nandkishor. Sulfamic acid catalysed one-pot three-component condensation for the synthesis of 1, 4-dihydropyrano[2, 3-c]pyrazoles. J. Chem. Res. 2008 (2008) 278–279. DOI:10.3184/030823408X321051 |
| [34] | J.P. Li, J.K. Qiu, H.J. Li, G.S. Zhang. An efficient three-component one-pot preparation of 1, 4-dihydropyridines containing novel substituted pyrazole under su lfamic acid catalysis. Chin. J. Chem. 29 (2011) 511–514. DOI:10.1002/cjoc.201190114 |
| [35] | M.R. Shetty, S.D. Shriniwas. Sulfamic acid (H2NSO3H): a low-cost, mild, and efficient catalyst for the synthesis of substituted n-phenylpyrazoles under solvent-free conditions. Synth. comm. 42 (2012) 1411–1418. DOI:10.1080/00397911.2010.540365 |
 2017, Vol. 28
2017, Vol. 28