b College of Medicine Aldawadmi Shaqra University, Saudi Arabia;
c P. G. Department of Chemistry, Sri Pratap College, Srinagar, J & K, 190001, India;
d School of Biotechnology, Yeungnam University, Gyeongsan 712-749, South Korea
Microorganisms are almost everywhere in nature and infections caused by small pathogenic microorganisms could lead to illnesses even a fatal one [1]. The development of resistance to the currently available antimicrobial agents [2], compounded problems in the therapeutics such as multidrug-resistant gram-positive pathogens, methicillin-resistant Staphylococci aureus (MRSA), penicillin resistant Streptococcus pneumoniae (PRSP) and vancomycin-resistant Enterrococci (VRE) [3, 4]. Therefore, the search for new lead structures and chemical entities (NCEs) for the development of antimicrobial agents is an increasingly important problem in medicinal chemistry. Unfortunately, pharmaceutical companies are more and more leaving this area behind due to economic reasons [5]. Compounds containing the variety of heterocyclic nucleus has been long aimed by the researchers as an antimicrobial agent [6-15]. On the other hand 1, 2, 4-triazine derivatives have been reported to possess biological activities as anti-AIDS [16], anticancer [17], antimicrobial [18-25] antitumor [26, 27], anti-inflammatory and analgesic agents [28], cytotoxicity [29, 30], antileishmanial [31], antiproliferative [32, 33], antinociceptive [34], anticytokine [35], cyclooxygenase-2 inhibitors [36], neuroprotective agents [37], antiviral [38] and antimalarial [39, 40] activities. Besides, indole nucleus is found in a number of biologically active compounds such as anti-inflammatory and analgesic [41-45], antifungal [41, 46], antimicrobial [47, 48], insecticidal activity [41-49], anticancer [41, 50-53], 5-lipoxygenase inhibitors [54], anti-HIV [41, 55], antioxidant [56, 57], antitubercular [41, 58], antiviral [41], plant growth regulator [41], antidepressant, tranquillizing, anticonvulsant [31, 49], cardiovascular [3, 50], antihypertensive [41], antihistaminic [59], opioid antagonist [60], photochemotherapeutic [61], antidiabetic [62], LXR receptor agonist [63], ACAT inhibitor [64] and many other activities. On the other hand hydrazones have been reported to possess antimicrobial [65], antitubercular [66, 67], anticonvulsant [68], analgesic [69], anti-inflammatory [70, 71] antiplatelet [72], anticancer [73-75], antifungal [76], antiviral [77], antitumoral [78, 79], antibacterial [80] and antimalarial [81] activities. Therefore, the biological importance of 1, 2, 4-triazine derivatives, indole nucleus and hydrazone functionality prompted us to design and synthesize some novel moieties which have both the important nucleus and hydrazone functionality and it was believed that their combination will be important to enhance activity.
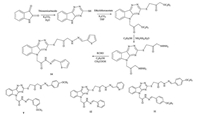
2. Results and discussion 2.1. ChemistryPresent study was undertaken to synthesize some novel 1, 2, 4-triazine derivatives. All the synthesized compounds were then subjected for their antimicrobial evaluation. The target compounds 1-14 were synthesized by four step procedure represented in Scheme 1. First step involves the formation of compound A by the reaction of isatin with thiosemicarbazide in presence of potassium bicarbonate. Subsequently, compound A on reaction with ethylchloroacetate in presence of potassium carbonate yield compound B. Compound B reacted with hydrazine hydrate in ethanol to yield compound C. Finally, compound C reacted with appropriate aldehyde to form hydrazone derivatives (1-14). All the compounds in solid state showed sharp melting points and the elemental analysis was found to be in accordance with ±0.3%. The compounds were stable and soluble in DMSO, methanol and chloroform. All the synthesized compounds were characterized by IR, 1H NMR, 13C NMR, and Mass spectroscopy. Characteristic IR bands provide significant indication for the formation of compounds 1-14. The appearance of characteristic band at/or around 2260 cm-1 and 3250 cm-1 due to SH and NH respectively, provide strong information about the formation of compound A. The formation of compound C was confirmed by the absence of band at/or around 1695 cm-1 and 1685 cm-1 due to the presence of C=O and also by the presence of bands due to C—S and C—N at 662 cm-1 and 1105 cm-1, respectively. The appearance of characteristic band at/or 3280 cm-1 and 3325 cm-1 due to NH and NH2 respectively strongly favor the formation of compound C. The disappearance of bands at around 3325 cm-1 and appearance of bands at 672-679, 1120-1129 and 1603-1618 cm-1 due to C—S, C—N and C=O respectively confirms the formation of compounds 1-14.
|
Download:
|
| Scheme1. Schematic representation of the route adopted for the synthesis of compounds 2-4, 8, 11, 12 and 14 | |
Further confirmation of all the synthesized compounds was done using 1H NMR spectroscopic data. Appearance of a singlet at 3.33 ppm and 11.23 ppm due to SH and NH proton, respectively, provides strong confirmation of the compound A. Structure of compound C was confirmed by the absence of peak at 3.33 ppm and 11.23 ppm due to SH and NH proton and also by the appearance of a triplet and quartet at 1.82 ppm and 4.09 ppm due to CH2 and CH3 protons. Structure of compound C was confirmed by appearance of singlet at 11.09 ppm and 2.50 ppm due to NH and NH2 respectively. Structural confirmation of compounds 1-14 was achieved by the absence of singlet at 11.09 ppm and 2.50 ppm due to NH and NH2 respectively and also by the presence of singlet at 8.11-8.22 ppm due to CH=N proton. The 13C NMR spectra of compounds 1-14 showed some prominent signals, such as signals in the range of 139.00-141.36 ppm, 142.10-143.69 ppm due to presence of CH=N which strongly confirms the formation of hydrazine and some more signals in the range (166.45-167.34) ppm, (169.04-172.77) ppm and (166.45-166.99) ppm due to the presence of C—S and C=O respectively and provide strong recommendation for the formation of these compounds 1-14. Other peaks were also present in the NMR spectra of these compounds at their usual positions described in the experimental section.
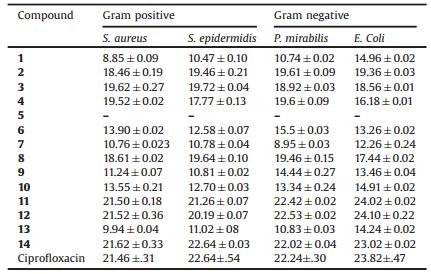
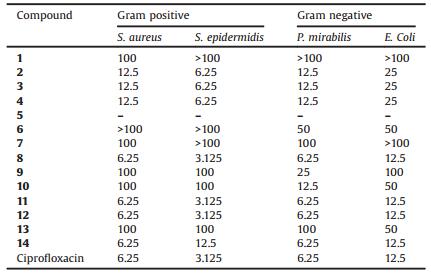
2.2. Antibacterial activityAll the compounds 1-14 were screened for their in vitro antimicrobial activity using the Gram-positive and Gram-negative bacteria. Four different cultures, two each of Gram-negative (Escherichia coli and Proteus mirabilis) and Gram-positive (Staphylococcus aureus and Staphylococcus epidermidis) were treated with the synthesized compounds 1-14 using disk diffusion method. The minimum inhibitory concentration (MIC) is defined as the lowest concentration (highest dilution) required to arrest the growth of bacteria and was determined by using different dilutions of the treatment compounds. The results were compared with positive control, the standard drug ciprofloxacin and negative control, the DMSO. The MIC was evaluated by macro-dilution test using standard inoculums of 10-5 CFL/mL. Serial dilutions of the test compounds, previously dissolved in dimethyl sulfoxide (DMSO), were prepared to final concentrations of 400, 200, 100, 50, 12.5, 6.25, 3.125 μg/mL. The dilutions were added to 24hour old inoculums. The susceptibilityof the bacteria tothe testcompounds was determined by the formation of an inhibitoryzone after18h of incubation at 37 ℃. The inhibition zones (mm) of each compound and the minimum inhibitory concentration are presented in Table 1 and Table 2, respectively. The zone of inhibition was measured at the minimum inhibitory concentration. To compare the antimicrobial effect of the compounds with that of the Ciprofloxacin, the inhibition zone and MIC were considered. We calculated the percent area of inhibition per microgram of compounds and standard which exhibited that compounds 11, 12 and 14 were found to possess better activity against all microorganisms while compound 8 posses similar activity. On the other hand compounds 2, 3 and 4 showed 80% resemblance with standarddrug "Ciprofloxacin"(Fig. 1).Theimportanceofsuch work lies in the possibility that the new compound might be more effective against bacteria for which a thorough investigation regarding the structural activity relationship, toxicity and the biological effects would be helpful in designing more potent antibacterial agents for therapeutic use.
|
|
Table 1 Inhibitory effects of compounds (1-14) on growth of microorganism by halo zone test. Ciprofloxacin used as standard drug and DMSO as negative control |
|
|
Table 2 Representing minimum inhibitory concentration (μg/mL) of 1, 2, 4-triazine derivatives (1-14). Ciprofloxacin was used as standard |

|
Download:
|
| Fig. 1. The graph showing comparative percent area inhibition per μg of the compounds and the Ciprofloxacin against all microorganisms | |
2.3. In vitro cytotoxicity studies
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) is reduced by the succinate dehydrogenase system of mitochondrial living cells to produce water insoluble purple formazan crystals [82, 83] which, after solubilization, can be measured spectrophotometrically. Since the amount of formazan produced is directly proportional to the number of active cells in the culture, MTT has long been used to assess the cell viability in cell proliferation and cytotoxicity [84-86].
In the present study, some newly synthesized 1, 2, 4-triazine derivatives were screened for their antimicrobial activity and then evaluated for their cytotoxicity against human hepatocellular carcinoma cell line (HepG2) to ensure their toxic effect. Ciprofloxacin was used as a reference drug in the experiment. A sub-confluent population of HepG2 cells was treated with increasing concentration of these compounds and the number of viable cells was measured after 48h by MTT cell viability assay. The concentration range of all the compounds was 3.125-100 μmol/L. The cell viability (%) obtained with continuous exposure for 48 h are depicted in Fig. 2. The cytotoxicity of all the compounds was found to be concentration-dependent and results revealed that all the compounds including the reference drug "Ciprofloxacin" showed viability ranging from 95%-100% at the concentration range of 3.125 μmol/L and up to a concentration of 25 μmol/L all the compounds showed a viability of ≥90%. On increasing the concentration range up to 50 and 100 μmol/L the compounds did not show any remarkable toxicity against the HepG2 cell line. All the tested compounds showed a viability of ≥80% at a concentration of 100 μmol/L as depicted in Fig. 2.

|
Download:
|
| Fig. 2. Percent viability of cells after 48h pre-treatment of HepG2 cell line with active compounds of the series and Ciprofloxacin by MTT assay | |
3. Conclusion
New library 1, 2, 4-triazine analogues was synthesized with few steps reactions to make cost effective chemicals that can be used as an antibacterial agent targetting the Gram positive (S. aureus and S. epidermidis) and Gram negative (P. mirabilis and E. coli) bacteria. Considering the individual drug activity of 1, 2, 4-triazine nucleus, indole moiety and hydrazone functionality the scheme was designed in such a way that the target compound will be having all these important structures. All the substituted 1, 2, 4-triazine analogues 1-14 containing indole moiety were screened for their antimicrobial activity against microbes discussed above. Several compounds showed excellent activity better than the standard drug in terms of zone of inhibition and MIC values. Among them, many compounds represented good activity while some compounds found to possess better activity than the standard with less cytotoxicity.
4. ExperimentalSolvents and organic reagents were purchased from Sigma Aldrich, Merck (Germany) and Loba Chemie (India) and were used without further purification. Melting points (mp) were performed using a Mel-temp instrument, and the results were uncorrected. Elemental analyses were performed on HeraeusVario EL Ⅲ analyzer at Central Drug Research Institute, Lucknow, India. The results were within ± 0.4% of the theoretical values. IR spectra were recorded on Perkin-Elmer model 1600 FT-IR RX1 spectrophotometer as KBr discs/ATR mode. 1H NMR and 13C NMR spectra were recorded on Bruker AVANCE 300 MHz spectrometer using DMSO as solvent with TMS as internal standard. Splitting patterns are designated as follows; s, singlet; d, doublet; dd, double doublet; t, triplet; m, multiplet. Chemical shift values are given in ppm. ESIMS was recorded on a MICROMASS QUATTRO Ⅱ triple quadrupole mass spectrometer. Reactions were monitored using thin-layer chromatography (TLC) using commercially available precoated plates (Merck Kieselgel 60 F254 silica). Visualization was achieved with UV light at 254 nm.
4.1. General procedure for the synthesis of compound AA mixture of isatin (10 mmol), thiosemicarbazide (10 mmol) and K2CO3 (15 mmol) in 50 mL of water was refluxed with stirring for 3 h. On cooling the mixture was filtered and precipitated by acidification with acetic acid. The solid was washed with water and dried to obtain as yellow solid. 5H-[1, 2, 4]Triazino[5, 6-b]indole-2-thiol (A): Yellow crystals, yield: 80%; IRvmax 3250, 2560 cm-1; 1H NMR (DMSO-d6, 500 MHz): δ 11.234 (s, 1H, NH), 7.987 (d, 1H, CHAr), 7.429-7.788 (m, Ar-H), 3.332 (s, 1H, SH).
4.2. General procedure for the synthesis of compound BA mixture of compound A (10 mmol), ethyl chloroacetate (10 mmol) in 50 mL of DMF was refluxed at 100 ℃ with stirring for 3 h. Progress of the reaction was monitored by using thin layer chromatographic plates. On cooling the mixture was filtered and precipitated washed with water and dried under vacuum. Ethyl {3-[(ethoxy carbonyl)sulfanyl]-5H-[1, 2, 4]triazino[5, 6-b]indole-5-yl} acetate (B): Yellow crystals, yield: 80%; IRvmax 1695, 1685, 1105, 662 cm-1; 1H NMR (DMSO-d6, 500 MHz): δ 7.488-7.833 (m, Ar-H), 8.351-8.377 (d, 1H, CH-Ar), 5.291 (s, 2H, N-CH2), 4.985 (s, 2H, S-CH2), 4.098 (q, 3H, CH2), 1.826 (t, 3H, CH3).
4.3. General procedure for the synthesis of compound CA mixture of compound B (10 mmol), in ethyl alcohol (10 mL), hydrazine hydrate (10 mmol) was added and resulting mixture was heated to 80 ℃ for 10 h. The mixture was concentrated to obtain solid. The resulting solid was filtered out and washed with water. The recrystalization of the compound C was performed using ethyl alcohol which yielded white solid crystals. S-[5-(2-Hydrazinyl-2-oxoethyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-3-yl] hydrazinecarbothiate (C): White crystals, yield 84%; IRvmax 3325, 3320, 3285, 3280, 1698, 1690, 1118, 690 cm-1; 1H NMR (DMSO-d6, 500 MHz): δ 11.091 (s, 1H, NH), 7.198-7.747 (m, Ar-H), ), 5.324 (s, 2H, N-CH2), 5.008 (s, 2H, S-CH22.507 (s, 2H, NH2).
4.4. General method for preparation of compound 1-14The mixture of compound C and substituted aldehyde in absolute ethanol was taken in a round bottom flask and a few drops of glacial acetic acid added and refluxed for 12-24 h. After cooling, the obtained product was filtered off and recrystallized from ethanol to yield the desired products.
N'-[(E)-Phenylmethylidene]-2-[3-(phenylmethylidene sulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (1): dark yellow crystals, Yield 90%; mp 202-204 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(E)-2-Chlorophenylmethylidene]-2-[3-(2-chlorophenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (2): Yellow crystals, yield 90%; mp 208-210 ℃; IRvmax 3280, 3277, 1688, 1673, 1610, 1603, 1124, 675 cm-1; 1H NMR (DMSO-d6, 500 MHz): δ 11.83 (s, 1H, NH), 11.90 (s, 1H, NH), 8.212 (s, 1H, CH=N), 8.120 (s, 1H, CH=N), 7.898-8.041 (m, Ar-H), 7.738 (s, 1H, Ar-H), 7.724-7.734 (m, Ar-H), 7.319-7.484 (m, Ar-H), 5.616 (s, 2H, N-CH2), 4.092 (s, 2H, S-CH2); 13C NMR (DMSO-d6, 500 MHz): δ 169.16 (C=O), 167.24 (C=O), 166.96 (C-S), 166.25, 164.24, 146.83, 142.18 (CH=N), 141.35 (CH=N), 140.10, 139.23, 138.92, 131.30, 130.72, 129.31, 128.90, 128.73, 128.20, 123.42, 121.82, 117.64, 112.13, 40.69 (CH2), 40.50 (CH2); ESI-MS m/z [M + 1]+, 591.08 (calcd. 591.10); Anal. calc. for C27H22Cl2N8O2S: C, 54.83; H, 3.41; N, 18.94; Found: C, 54.82; H, 3.43; N, 18.90.
N'-[(E)-4-Chlorophenylmethylidene]-2-[3-(4-chlorophenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (3): Brown crystals, yield 90%; mp 206-208 ℃; IRvmax 3281, 3273, 1686, 1674, 1616, 1602, 1121, 677 cm-1; 1H NMR (DMSO-d6, 500 MHz): δ 11.84 (s, 1H, NH), 11.71 (s, 1H, NH), 8.222 (s, 1H, CH=N), 8.124 (s, 1H, CH=N), 7.898-7.947 (m, Ar-H), 7.488-7.494 (m, Ar-H), 7.313-7.481 (m, Ar-H), 5.539 (s, 2H, N-CH2), 4.064 (s, 2H, S-CH2); 13C NMR (DMSO-d6, 500 MHz): δ 169.10 (C=O); 167.28 (C=O), 166.94 (C-S), 166.20, 164.25, 146.89, 142.18 (CH=N), 141.33 (CH=N), 140.10, 139.25, 138.94, 131.30, 130.74, 129.39, 128.90, 128.77, 128.20, 123.45, 121.80, 117.60, 112.16, 40.73 (CH2), 40.52 (CH2); ESI-MS m/z [M + 1] +, 591.08 (calcd. 591.12); Anal. calcd. for C27H22Cl2N8O2S: C, 54.83; H, 3.41; N, 18.94; Found: C, 54.84; H, 3.40; N, 18.91.
N'-[(E)-4-Methylphenylmethylidene]-2-[3-(4-methylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (4): White crystals; yield 80%; mp 198-200 ℃; IRvmax 3289, 3270, 1681, 1672, 1613, 1605, 1124, 676 cm-1; 1H NMR (DMSO-d6, 500MHz): δ 11.82 (s, 1H, NH), 11.83 (s, 1H, NH), 8.344-8.369 (d, 1H, Ar-H), 8.162 (s, 1H, CH=N), 8.105 (s, 1H, CH=N), 7.873 (s, 1H, CH-Ar), 7.733-7.793 (m, Ar-H), 7.305-7.612 (m, Ar-H), 6.754-6.862 (m, Ar-H), 5.541 (s, 2H, N-CH2), 4.152 (2H, s, S-CH2), 2.50 (1H, s, CH3), 2.27 (1H, s, CH3); 13C NMR (DMSO-d6, 500MHz): δ 169.04 (C=O), 167.25 (C=O), 166.97 (C-S), 166.26, 164.26, 146.88, 142.10 (CH=N), 141.36 (CH=N), 140.12, 139.29, 138.99, 131.34, 130.78, 129.35, 128.91, 128.75, 128.26, 123.48, 121.85, 117.67, 112.10, 40.79 (CH2), 24.32 (CH3), 40.49 (CH2), 24.37 (CH3); ESI-MS m/z [M++1], 551.19 (calcd. 551.14); Anal. calcd. for C29H29N8O2S: C, 63.26; H, 4.76; N, 20.35; Found: C, 63.23; H, 4.75; N, 20.37.
N'-[(E)-2-Nitrophenylmethylidene]-2-[3-(2-nitrophenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (5): White crystals; yield 95%; mp 202-204 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(E)-3-Nitrophenylmethylidene]-2-[3-(3-nitrophenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (6): White crystals; yield 96%; mp 202-204 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(E)-4-Nitrophenylmethylidene]-2-[3-(4-nitrophenylmethylidenesulfanyl)-5-H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (7): White crystals; yield 90%; mp 202-204 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(E)-4-Methoxylphenylmethylidene]-2-[3-(4-methoxylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (8): Creamy white crystals, yield 80%; mp 204-206 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(E)-3, 4-Dimethoxylphenylmethylidene]-2-[3-(3, 4-dimethoxylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (9): white crystals White crystals; yield 90%; mp 210-212 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(E)-2-ethoxylphenylmethylidene]-2-[3-(4-methoxylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (10): White crystals; yield 80%; mp 218-220 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(E)-4-Ethoxylphenylmethylidene]-2-[3-(4-methoxylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (11): White crystals; yield 80%; mp 218-220 ℃; IRvmax 3284, 3274, 1686, 1673, 1609, 1608, 1126, 677cm-1; 1H NMR (DMSO-d6, 500MHz): δ 11.72 (s, 1H, NH), 11.80 (s, 1H, NH), 8.167 (s, 1H, CH=N), 8.113 (s, 1H, CH=N), 7.875-7.890 (m, Ar-H), 7.732-7.790 (m, Ar-H), 6.750-6.861 (m, Ar-H), 5.538 (s, 2H, N-CH2), 4.150 (s, 2H, S-CH2), 3.962 (q, 2H, CH2), 3.952 (q, 2H, CH2), 1.320 (t, 3H, CH3), 1.341 (t, 3H, CH3); 13C NMR (DMSO-d6, 500MHz): δ 169.04 (C=O), 167.25 (C=O), 166.97 (C-S), 166.26, 164.26, 146.88, 142.10 (CH=N), 141.36 (CH=N), 140.12, 139.29, 138.99, 131.34, 130.78, 129.35, 128.91, 128.75, 128.26, 123.48, 121.85, 117.67, 112.10, 64.94 (CH2), 64.22 (CH2), 40.79 (CH2), 40.49 (CH2), 14.80 (CH3), 14.71 (CH3); ESIMS m/z [M + 1]+, 611.21 (calcd. 611.22); Anal. calcd. for C31H30N8O4S: C, 60.97; H, 4.95; N, 18.35; Found: C, 60.90; H, 4.93; N, 18.33.
N'-[(Z)-2-Pyridin-2-yl-methylidene]-2-[3-(4-methoxylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (12): Dark yellow crystals; yield 85%; mp 202-204 ℃; IRvmax 3287, 3274, 1680, 1679, 1616, 1606, 1123, 679cm-1; 1H NMR (DMSO-d6, 500MHz): δ 11.73 (s, 1H, NH), 11.69 (s, 1H, NH), 8.631 (s, 1H, Ar-H), 8.530 (d, 1H, Ar-H), 8.154 (s, 1H, CH=N), 8.151 (s, 1H, CH=N), 8.317 (d, 1H, Ar-H), 7.459-7.755 (m, Ar-H), 5.656 (s, 2H, NCH2), 4.148 (s, 2H, S-CH2); 13C NMR (DMSO-d6, 500MHz): δ 169.20 (C=O), 167.24 (C=O), 166.94 (C-S), 166.26, 164.28, 146.86, 142.17 (CH=N), 141.34 (CH=N), 140.13, 139.25, 138.97, 131.33, 130.75, 129.30, 128.92, 128.74, 128.27, 123.49, 121.86, 117.65, 112.12, 40.77 (CH2), 40.45 (CH2); ESI-MS m/z [M++1], 525.15 (calcd. 525.14); Anal. calcd. for C25H20N10O2S: C, 57.24; H, 3.84; N, 26.70; Found: C, 57.26; H, 3.82; N, 26.71.
N'-[(Z)-4-Pyridin-2-yl-methylidene]-2-[3-(4-methoxylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (13): Dark yellow crystals; yield 90%; mp 202-204 ℃; for all the spectroscopic details please see the Supporting information.
N'-[(Z)-Thiophen-2-yl-methylidene]-2-[3-(4-methoxylphenylmethylidenesulfanyl)-5H-[1, 2, 4]triazino[5, 6-b]indol-5-yl]acetohydrazide (14): Dark yellow crystals; yield 92%; mp 202-204 ℃; IRvmax 3286, 3279, 1686, 1674, 1613, 1607, 1124, 679cm-1; 1H NMR (DMSO-d6, 500MHz): δ 8.339-8.365 (d, 1H, Ar-H), 8.111 (s, 1H, CH=N), 8.103 (s, 1H, CH=N), 7.469-7.860 (m, Ar-H), 6.565-6.972 (m, Ar-H), 5.555 (2H, s, N-CH2), 4.153 (2H, s, S-CH2); 13C NMR (DMSO-d6, 500MHz): δ 169.04 (C=O), 167.25 (C=O), 166.97 (C-S), 166.26, 164.26, 146.88, 142.10 (CH=N), 141.36 (CH=N), 140.12, 139.29, 138.99, 131.34, 130.78, 129.35, 40.49 (CH2), 128.91, 128.75, 128.26, 123.48, 121.85, 117.67, 112.10, 40.79 (CH2); ESI-MS m/z [M+1]+, 535.07 (calcd. 535.08); Anal. calcd. for C23H18N8O2S3: C, 51.67; H, 3.39; N, 20.96; Found: C, 51.63; H, 3.41; N 20.98.
4.5. Anti-bacterial activityOrganism cultureand in vitro screening for antibacterial activity was done by the disk diffusion method with minor modifications. S. aureus, S. epidermidis, P. mirabilis, and E. coli was sub cultured in nutrient agar medium and incubated for 18h at 37 ℃. The bacterial cells were suspended for incubation according to the McFarland protocol in saline solution to produce a suspension of about 105 CFU/mL. 10mL of this suspension was mixed with 10mL of sterile antibiotic agar at 40 ℃ and poured on to an agar plate in a laminar flow cabinet. Five paper disks (6.0mm diameter) were fixed onto nutrient agar plate. One milligram of each test compound was dissolved in 100mL DMSO to prepare stock solution. From the stock solution different dilutions of each test compound were prepared and poured over disk plate. Ciprofloxacin was used as a standarddrug (positive control)and DMSO as negative control. The susceptibility of the bacteria to the test compounds was determined by the formation of an inhibitory zone after 18h of incubation at 36 ℃. Table 1 shows the zone of inhibition of the oxadiazoline derivatives (1-14). The results were compared with the positive control and the zone of inhibitions was measured at the minimum inhibitory concentration (MIC). The minimum inhibitory concentration (MIC) was evaluated by the macrodilution test using standard inoculum of 105 CFL/mL. Serial dilutions of the test compounds, previously dissolved in dimethyl sulfoxide (DMSO) were prepared to final concentrations of 400, 200, 100, 50, 12.5, 6.25 and 3.125 μg/mL. To each tube was added 100mL of 24h old inoculums. The MIC, defined as the lowest concentration (highest dilution) required to arrest the growth of bacteria, which inhibits the visible growth after 18h, was determined visually after incubation of 18h at 37 ℃, and the results are presented in
4.6. Cytotoxicity studies (MTT-assay)Cell culture: The human hepatocellular carcinoma cell line (HepG2) was cultured in Dulbecco's modified Eagle's mediumwith 10% fetal bovine serum (heat inactivated), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin B, at 37 ℃ in a saturated humidity atmosphere containing 95% air/5% CO2 [87]. The cell lines were harvested when they reached 80% confluence to maintain exponential growth.
MTT assay: The MTT assay was carried out using HepG2 following the procedure discussed by Mosmann 1983, the assay was performed in triplicate and repeated thrice. Percent viability was defined as the relative absorbance of treated versus untreated control cells [88].
Conflict of interestThe authors have no conflict of interest.
AcknowledgmentThe authors are thankful to UGC (No. F no-43-172/2014 (SR)) and UGC MANF scheme wide letter No. F.40-65(C/M)/2009(SA-Ⅲ/ MANF) for providing financial assistance.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.037.
| [1] | P. Gastmeier, D. Sohr, C. Geffers, et al., Mortality risk factors with nosocomial Staphylococcus aureus infections in intensive care units: results from the German Nosocomial Infection Surveillance System (KISS). Infection 33 (2005) 50–55. |
| [2] | H.C. Neu. The crisis in antibiotic resistance. Science 257 (1992) 1064–1073. DOI:10.1126/science.257.5073.1064 |
| [3] | K.R. Babu, B. Eeshwaraiah, D. Aravind, et al., Synthesis of quinoline analogs: search for antimalarial agents. Monatsh. Chem. 139 (2008) 179–181. DOI:10.1007/s00706-007-0772-5 |
| [4] | A. Dalhoff. Quinolone resistance in Pseudomonas aeruginosa and Staphylococcus aureus. Development during therapy and clinical significance. Infection 22 (1994) S111–S121. DOI:10.1007/BF01793575 |
| [5] | F. von Nussbaum, M. Brands, B. Hinzen, S. Weigand, D. Häbich. Antibacterial natural products in medicinal chemistry-exodus or revival. Angew. Chem. Int. Ed. 45 (2006) 5072–5129. DOI:10.1002/(ISSN)1521-3773 |
| [6] | X. Guo, Y.L. Li, Y.F. Liu, H.Y. Guo, Y.C. Wang. Synthesis and in vitro antibacterial activities of 7-(3-aminopyrrolo [3, 4-c] pyrazol-5(2H, 4H, 6H)-yl) quinolone derivatives. Chin. Chem. Lett. 21 (2010) 1141–1144. DOI:10.1016/j.cclet.2010.06.011 |
| [7] | Y.L. Narasimha Murthy, N. Karthikeyan, G. Boddeti, B.S. Diwakar, E.R. Singh. Design, synthesis and in vitro antibacterial activities of methy-l4((substituted phenyl) [6H-indolo (2, 3-b) quinoxalin-6-yl] methyl-amino)benzoate derivatives. Chin. Chem. Lett. 22 (2011) 567–570. DOI:10.1016/j.cclet.2010.10.055 |
| [8] | T.N. Akhaja, J.P. Raval. Design, synthesis, in vitro evaluation of tetrahydropyrimidine-isatin hybrids as potential antibacterial, antifungal and anti-tubercular agents. Chin. Chem. Lett. 23 (2012) 446–449. DOI:10.1016/j.cclet.2012.01.040 |
| [9] | S. Rostamizadeh, M. Nojavan, R. Aryan, H. Sadeghian, M. Davoodnejad. A novel and efficient synthesis of pyrazolo[3, 4-d]pyrimidine derivatives and the study of their anti-bacterial activity. Chin. Chem. Lett. 24 (2013) 629–632. DOI:10.1016/j.cclet.2013.04.035 |
| [10] | S. Asghari, S. Ramezani, M. Mohseni. Synthesis and antibacterial activity of ethyl-2-amino-6-methyl-5-oxo-4-aryl-56-dihydro-4H-pyrano[3, 2-c]quin oline-3-carboxylate. Chin. Chem. Lett. 25 (2014) 431–434. DOI:10.1016/j.cclet.2013.12.010 |
| [11] | S. Nagarajan, P. Shanmugavelan, M. Sathishkumar, et al., An eco-friendly water mediated synthesis of 1, 2, 3-triazolyl-2-aminopyrimidine hybrids as highly potent anti-bacterial agents. Chin. Chem. Lett. 25 (2014) 419–422. DOI:10.1016/j.cclet.2013.12.017 |
| [12] | S. Asghari, N. Malekian, R. Esmaeilpour, M. Ahmadipour, M. Mohseni. Threecomponent synthesis and antibacterial evaluation of some novel 1, 2-dihydroisoquinoline derivatives. Chin. Chem. Lett. 25 (2014) 1441–1444. DOI:10.1016/j.cclet.2014.05.047 |
| [13] | K.H.M.E. Tehrani, M. Hashemi, M. Hassan, F. Kobarfard, S. Mohebbi. Synthesis and antibacterial activity of Schiff bases of 5-substituted isatins. Chin. Chem. Lett. 27 (2016) 221–225. DOI:10.1016/j.cclet.2015.10.027 |
| [14] | S. Sarva, J.S. Harinath, S.P. Sthanikam, et al., Synthesis, antibacterial and antiinflammatory activity of bis(indolyl)methanes. Chin. Chem. Lett. 27 (2016) 16–20. DOI:10.1016/j.cclet.2015.08.012 |
| [15] | L.Z. Gao, Y.S. Xie, T. Li, et al., Synthesis and antibacterial activity of novel [1, 2, 4] triazolo[3, 4-h][1, 8]naphthyridine-7-carboxylic acid derivatives. Chin. Chem. Lett. 26 (2015) 149–151. DOI:10.1016/j.cclet.2014.09.017 |
| [16] | R.M. Abdel-Rahman. Role of uncondensed 1, 2, 4-triazine compounds and related heterobicyclic systems as therapeutic agents-a review. Pharmazie 56 (2001) 18–22. |
| [17] | Z. El-Gendy, J.M. Morsy, H.A. Allimony, W.R. Abdel-Monem, R.M. AbdelRahman. Synthesis of heterobicyclic nitrogen systems bearing a 1, 2, 4-triazine moiety as anticancer drugs: part Ⅳ. Phosph. Sulfur Silicon Relat. Elem. 178 (2003) 2055–2071. DOI:10.1080/10426500390228738 |
| [18] | T.E.S. Ali. Synthesis and fungicidal activity of some new 4h-chromen-4-ones containing some 1, 3-thiazole, 1, 3-thiazine, 1, 2, 4-triazole and 1, 2, 4-triazine moieties. Phosph. Sulfur Silicon Relat. Elem. 182 (2007) 1717–1726. DOI:10.1080/10426500701313896 |
| [19] | T.E. Ali, W.R. Abdel-Monem. Synthesis of some new 9-heteroarylcarbazole derivatives with expected biological activity. Int. J. Chem. 17 (2007) 303–314. |
| [20] | T.E. Ali, S.A. Abdel-Aziz, H.M. El-Shaaer, F.I. Hanafy, A.Z. El-Fauomy. Synthesis of bioactive 4-oxo-4H-chromenes bearing heterocyclic systems from hydrazonecarbodithioic acid and thiocarbohydrazone. Phosph. Sulfur Silicon Relat. Elem. 183 (2008) 2139–2160. DOI:10.1080/10426500701851291 |
| [21] | F. El-Mariah, M. Hosny, A. Deeb. Pyridazine derivative and related compound, part 18: 1 pyridazino [3', 4': 3, 4]pyrazolo [5, 1-c]-1, 2, 4-triazine-3-carboxylic acid: synthesis, reactions, and antimicrobial activity. Phosph. Sulfur Silicon Relat. Elem. 181 (2006) 2505–2517. DOI:10.1080/10426500600754786 |
| [22] | W.M. Al-Adiwish, M.I.M. Tahir, A. Siti-Noor-Adnalizawati, et al., Synthesis antibacterial activity and cytotoxicity of new fused pyrazolo [5, 1-c][1, 2, 4] triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 64 (2013) 464–476. DOI:10.1016/j.ejmech.2013.04.029 |
| [23] | T.E.S. Ali. Synthesis of some novel pyrazolo[3, 4-d] pyrimidine derivatives bearing 5, 6-diphenyl-1, 2, 4-triazine moiety as potential antimicrobial agents. Eur. J. Med. Chem. 44 (2009) 4385–4392. DOI:10.1016/j.ejmech.2009.05.031 |
| [24] | S.K. Pandey, A. Singh, A. Singh. Nizamuddin, Antimicrobial studies of some novel quinazolinones fused with[1, 2, 4]-triazole, [1, 2, 4]-triazine and [1, 2, 4, 5]-tetrazine rings. Eur. J. Med. Chem. 44 (2009) 1188–1197. DOI:10.1016/j.ejmech.2008.05.033 |
| [25] | W. Lv, B. Banerjee, K.L. Molland, et al., Synthesis of 3-(3-aryl-pyrrolidin-1-yl)-5-aryl-1, 2, 4-triazines that have antibacterial activity and also inhibit inorganic pyrophosphatase. Bioorg. Med. Chem. 22 (2014) 406–418. DOI:10.1016/j.bmc.2013.11.011 |
| [26] | K. Sztanke, W. Markowski, R. Świeboda, B. Polak. Lipophilicity of novel antitumour and analgesic active 8-aryl-2, 6, 7, 8-tetrahydroimidazo[2, 1-c] [1, 2, 4]triazine-3, 4-dione derivatives determined by reversed-phase HPLC and computational methods. Eur. J. Med. Chem. 45 (2010) 2644–2649. DOI:10.1016/j.ejmech.2010.01.068 |
| [27] | K. Sztanke, K. Pasternak, J. Rzymowska, M. Sztanke, M. Kandefer-Szerszeń. Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1, 2, 4-triazine aryl derivatives. Eur. J. Med. Chem. 43 (2008) 1085–1094. DOI:10.1016/j.ejmech.2007.07.009 |
| [28] | H.M. Ashour, O.G. Shaaban, O.H. Rizk, I.M. El-Ashmawy. Synthesis and biological evaluation of thieno[2', 3': 45]pyrimido[1, 2-b][1, 2, 4]triazines and thieno[2, 3-d][1, 2, 4]triazolo[1, 5-a]pyrimidines as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 62 (2013) 341–351. DOI:10.1016/j.ejmech.2012.12.003 |
| [29] | T. Gucký, I. Fryšová, J. Slouka, M. Hajdúch, P. Džubák. Cyclocondensation reaction of heterocyclic carbonyl compounds, Part ⅩⅢ: synthesis and cytotoxic activity of some 3, 7-diaryl-5-(3, 4, 5-trimethoxyphenyl)pyrazolo[4, 3-e][1, 2, 4] triazines. Eur. J. Med. Chem. 44 (2009) 891–900. DOI:10.1016/j.ejmech.2008.05.026 |
| [30] | J. Stýskala, L. Stýskalová, J. Slouka, M. Hajdúch. Synthesis of 2-aryl-4-(benzimidazol-2-yl)-12-dihydro[1, 2, 4]triazino-[4, 5-a]benzimidazol-1-one derivatives with preferential cytotoxicity against carcinoma cell lines. Eur. J. Med. Chem. 43 (2008) 449–455. DOI:10.1016/j.ejmech.2007.01.008 |
| [31] | L. Gupta, N. Sunduru, A. Verma, et al., Synthesis and biological evaluation of new [1, 2, 4]triazino[5, 6-b]indol-3-ylthio-1, 3, 5-triazines and [1, 2, 4]triazino [5, 6-b]indol-3-ylthio-pyrimidines against Leishmania donovani. Eur. J. Med. Chem. 45 (2010) 2359–2365. DOI:10.1016/j.ejmech.2010.02.015 |
| [32] | P. Diana, P. Barraja, A. Lauria, et al., Pyrrolo[2, 1-c][1, 2, 4]triazines from 2-diazopyrroles: synthesis and antiproliferative activity. Eur. J. Med. Chem. 37 (2002) 267–272. DOI:10.1016/S0223-5234(02)01339-9 |
| [33] | F. Krauth, H.M. Dahse, H.H. Rüttinger, P. Frohberg. Synthesis and characterization of novel 1, 2, 4-triazine derivatives with antiproliferative activity. Bioorg. Med. Chem. 18 (2010) 1816–1821. DOI:10.1016/j.bmc.2010.01.053 |
| [34] | K. Sztanke, S. Fidecka, E. Kędzierska, et al., Antinociceptive activity of new imidazolidine carbonyl derivatives.: part 4. Synthesis and pharmacological activity of 8-aryl-34-dioxo-2H. 8H-6, 7-dihydroimidazo[2, 1-c] [1, 2, 4]triazines. Eur. J. Med. Chem. 40 (2005) 127–134. DOI:10.1016/j.ejmech.2004.09.020 |
| [35] | M. Khoshneviszadeh, M.H. Ghahremani, A. Foroumadi, et al., Design, synthesis and biological evaluation of novel anti-cytokine 1, 2, 4-triazine derivatives. Bioorg. Med. Chem. 21 (2013) 6708–6717. DOI:10.1016/j.bmc.2013.08.009 |
| [36] | H. Irannejad, A. Kebriaieeadeh, A. Zarghi, et al., Synthesis, docking simulation, biological evaluations and 3D-QSAR study of 5-Aryl-6-(4-methylsulfonyl)-3-(metylthio)-1, 24-triazine as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. 22 (2014) 865–873. DOI:10.1016/j.bmc.2013.12.002 |
| [37] | H. Irannejad, M. Amini, F. Khodagholi, et al., Synthesis and in vitro evaluation of novel 1, 2, 4-triazine derivatives as neuroprotective agents. Bioorg. Med. Chem. 18 (2010) 4224–4230. DOI:10.1016/j.bmc.2010.04.097 |
| [38] | K. Sztanke, K. Pasternak, B. Rajtar, et al., Identification of antibacterial and antiviral activities of novel fused 1, 2, 4-triazine esters. Bioorg. Med. Chem. 15 (2007) 5480–5486. DOI:10.1016/j.bmc.2007.05.048 |
| [39] | K. Ban, S. Duffy, Y. Khakham, et al., 3-Alkylthio-1, 24-triazine dimers with potent antimalarial activity. Bioorg. Med. Chem. Lett. 20 (2010) 6024–6029. DOI:10.1016/j.bmcl.2010.08.065 |
| [40] | A. Kumar, K. Srivastava, S.R. Kumar, S.K. Puri, P.M.S. Chauhan. Synthesis and bioevaluation of hybrid 4-aminoquinoline triazines as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 18 (2008) 6530–6533. DOI:10.1016/j.bmcl.2008.10.049 |
| [41] | E. Abele, R. Abele, O. Dzenitis, E. Lukevics. Indole and isatin oximes: synthesis, reactions, and biological activity. Chem. Heterocycl. Compd. 39 (2003) 3–35. DOI:10.1023/A:1023008422464 |
| [42] | M.A.A. Radwan, E.A. Ragab, N.M. Sabry, S.M. El-Shenawy. Synthesis and biological evaluation of new 3-substituted indole derivatives as potential antiinflammatory and analgesic agents. Bioorg. Med. Chem. 15 (2007) 3832–3841. DOI:10.1016/j.bmc.2007.03.024 |
| [43] | G.P. Kalaskar, M. Girisha, M.G. Purohit, B.S. Thippeswamy, B.M. Patil. Synthesis and evaluation of in vivo antiinflammatory activity of indole-3-aceetic acids. Indian J. Heterocycl. Chem. 16 (2007) 325–328. |
| [44] | P. Rani, V.K. Srivastava, A. Kumar. Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur. J. Med. Chem. 39 (2004) 449–452. DOI:10.1016/j.ejmech.2003.11.002 |
| [45] | M. Amir, N. Dhar, S.K. Tiwari. Synthesis and anti-inflammatory activity of some new indole and indazole derivatives. Indian J. Chem. 36 (1997) 96–98. |
| [46] | N.M. Przheval'skii, I.V. Magedov, V.N. Drozd. New derivatives of indole. Synthesis of s-(indolyl-3) diethyl dithiocarbamates. Chem. Heterocycl. Compd. 33 (1997) 1475–1476. DOI:10.1007/BF02291655 |
| [47] | H. Panwar, R.S. Verma, V.K. Srivastava, A. Kumar. Synthesis of some substituted azetidinonyl and thiazolidinonyl-1, 3, 4-thiadiazino[6, 5-b]indoles as prospective antimicrobial agents. Indian J. Chem. 45B (2006) 2014–2099. |
| [48] | Y.M. Al-Hiari, A.M. Qaisi, M.M. El-Abadelah, W. Voelter. Synthesis and antibacterial activity of some substituted 3-(aryl)-and 3-(heteroaryl) indoles. Monatsh. Chem. 137 (2006) 243–248. DOI:10.1007/s00706-005-0424-6 |
| [49] | K. Sharma, R. Jain, K.C. Joshi. Synthesis and insecticidal activity of some novel indole derivatives. Indian J. Heterocycl. Chem. 1 (1992) 189. |
| [50] | B.C. Hong, Y.F. Jiang, Y.L. Chang, S.J. Lee. Synthesis and cytotoxicity studies of cyclohepta[b]indoles Benzo[6, 7]cyclohepta[1, 2-b]indoles, indeno[1, 2-b] indoles, and Benzo[a]carbazoles. J. Chin. Chem. Soc. 53 (2006) 647–662. DOI:10.1002/jccs.v53.3 |
| [51] | L. Chacón-Garcı'a, R. Martı'nez. Synthesis and in vitro cytotoxic activity of pyrrolo [2, 3-e] indole derivatives and a dihydro benzoindole analogue. Eur. J. Med. Chem. 37 (2002) 261–266. DOI:10.1016/S0223-5234(01)01328-9 |
| [52] | S. Rossiter, L.K. Folkes, P. Wardman. Halogenated indole-3-acetic acids as oxidatively activated prodrugs with potential for targeted cancer therapy. Bioorg. Med. Chem. Lett. 12 (2002) 2523–2526. DOI:10.1016/S0960-894X(02)00505-X |
| [53] | M.J.R.P. Queiroz, A.S. Abreu, M.S.D. Carvalho, et al., Synthesis of new heteroaryl and heteroannulated indoles from dehydrophenylalanines: antitumor evaluation. Bioorg. Med. Chem. 16 (2008) 5584–5589. DOI:10.1016/j.bmc.2008.04.004 |
| [54] | M.F. Zheng, M.Y. Zheng, D.J. Ye, et al., Indole derivatives as potent inhibitors of 5-lipoxygenase: design, synthesis, biological evaluation, and molecular modeling. Bioorg. Med. Chem. Lett. 17 (2007) 2414–2420. DOI:10.1016/j.bmcl.2007.02.038 |
| [55] | I. Merino, A. Monge, M. Font, et al., Synthesis and anti-HIV-1 activities of new pyrimido[5, 4-b]indoles. Farmaco 54 (1999) 255–264. DOI:10.1016/S0014-827X(99)00035-X |
| [56] | H.Y. Aboul-Enein, I. Kruk, K. Lichszteld, et al., Scavenging of reactive oxygen species by N-substituted indole-2 and 3-carboxamides. Luminescence 19 (2004) 1–7. DOI:10.1002/(ISSN)1522-7243 |
| [57] | O. Talaz, I. Gülçin, S. Göksu, N. Saracoglu. Antioxidant activity of 510-dihydroindeno[1, 2-b]indoles containing substituents on dihydroindeno part. Bioorg. Med. Chem. 17 (2009) 6583–6589. DOI:10.1016/j.bmc.2009.07.077 |
| [58] | N. Karalı, A. Gürsoy, F. Kandemirli, et al., Synthesis and structureantituberculosis activity relationship of 1H-indole-2, 3-dione derivatives. Bioorg. Med. Chem. 15 (2007) 5888–5904. DOI:10.1016/j.bmc.2007.05.063 |
| [59] | S. Battaglia, E. Boldrini, F.D. Settimo, et al., Indole amide derivatives: synthesis, structure-activity relationships and molecular modelling studies of a new series of histamine H1-receptor antagonists. Eur. J. Med. Chem. 34 (1999) 93–105. DOI:10.1016/S0223-5234(99)80044-0 |
| [60] | H. Yu, T. Prisinzano, C.M. Dersch, et al., Synthesis and biological activity of 8bsubstituted hydrocodone indole and hydromorphone indole derivatives. Bioorg. Med. Chem. 12 (2002) 165–168. DOI:10.1016/S0960-894X(01)00689-8 |
| [61] | P. Barraja, L. Sciabica, P. Diana, et al., Synthesis and photochemotherapeutic activity of thiopyrano[2, 3-e]indol-2-ones. Bioorg. Med. Chem. Lett. 15 (2005) 2291–2294. DOI:10.1016/j.bmcl.2005.03.016 |
| [62] | Y.Y. Li, H.S. Wu, L. Tang, et al., The potential insulin sensitizing and glucose lowering effects of a novel indole derivative in vitro and in vivo. Pharmacol. Res. 56 (2007) 335–343. DOI:10.1016/j.phrs.2007.08.002 |
| [63] | S. Kher, K. Lake, I. Sircar, et al., 2-Aryl-N-acyl indole derivatives as liver X receptor (LXR) agonists. Bioorg. Med. Chem. Lett. 17 (2007) 4442–4446. DOI:10.1016/j.bmcl.2007.06.017 |
| [64] | R. Bellemin, A. Decerprit, D. Festal. New indole derivatives as ACAT inhibitors: synthesis and structure-activity relationships. Eur. J. Med. Chem. 31 (1996) 123–132. DOI:10.1016/0223-5234(96)80445-4 |
| [65] | S. Rollas, N. Gulerman, H. Edeniz. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2, 5-disubstituted-1, 34-oxadiazolines. Farmaco 57 (2002) 171–174. DOI:10.1016/S0014-827X(01)01192-2 |
| [66] | A. Imramovsky, S. Polanc, Vinšová, et al., A new modification of anti-tubercular active molecules. Bioorg. Med. Chem. 15 (2007) 2551–2559. DOI:10.1016/j.bmc.2007.01.051 |
| [67] | Y.L. Janin. Antituberculosis drugs: ten years of research. Bioorg. Med. Chem. 15 (2007) 2479–2513. DOI:10.1016/j.bmc.2007.01.030 |
| [68] | J.R. Dimmock, S.C. Vashishtha, J.P. Stables. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 35 (2000) 241–248. DOI:10.1016/S0223-5234(00)00123-9 |
| [69] | P.C. Lima, L.M. Lima, K.C. da Silva, et al., Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 35 (2000) 187–203. DOI:10.1016/S0223-5234(00)00120-3 |
| [70] | U. Salgin-Göksen, N. Gökham-Keleçi, O. Göstaş, et al., 1-Acylthiosemicarbazides, 1, 2, 4-triazole-5(4H)-thiones, 1, 3, 4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 15 (2007) 5738–5751. DOI:10.1016/j.bmc.2007.06.006 |
| [71] | R. Kalsi, M. Shrimali, T.N. Bhalla, J.P. Barthwal. Synthesis and antiinflammatory activity of indolyl azetidinones. Indian J. Pharm. Sci. 52 (1990) 129–134. |
| [72] | G.A. Silva, L.M.M. Costa, F.C.F. Brito, et al., New class of potent antinociceptive and antiplatelet 10H-phenothiazine-1-acylhydrazone derivatives. Bioorg. Med. Chem. 12 (2004) 3149–3158. DOI:10.1016/j.bmc.2004.04.009 |
| [73] | L. Savini, L. Chiasserini, V. Travagli, et al., New α-(N)-heterocyclichydrazones: evaluation of anticancer, anti-HIV and antimicrobial activity. Eur. J. Med. Chem. 39 (2004) 113–122. DOI:10.1016/j.ejmech.2003.09.012 |
| [74] | A. Bijev. New heterocyclic hydrazones in the search for antitubercular agents: synthesis and in vitro evaluations. Lett. Drug Design Discov. 3 (2006) 506–512. DOI:10.2174/157018006778194790 |
| [75] | M. Arshad, A.R. Bhat, S. Pokharel, et al., Synthesis, characterization and anticancer screening of some novel piperonyl-tetrazole derivatives. Eur. J. Med. Chem. 71 (2014) 229–236. DOI:10.1016/j.ejmech.2013.11.008 |
| [76] | C. Loncle, J.M. Brunel, N. Vidal, M. Dherbomez, Y. Letourneux. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 39 (2004) 1067–1071. DOI:10.1016/j.ejmech.2004.07.005 |
| [77] | M.T. Abdel-Aal, W.A. El-sayed, E.H. El-Ashry. Synthesis and antiviral evaluation of some sugar arylglycinoylhydrazones and their oxadiazoline derivatives. Arch. Pharm. Chem. Life Sci. 339 (2006) 656–663. DOI:10.1002/(ISSN)1521-4184 |
| [78] | S.A.M. El-Hawash, A.E. Abdel Wahab, M.A. El-Dewellawy. Cyanoacetic acid hydrazones of 3-(and 4-)acetylpyridine and some derived ring systems as potential antitumor and anti-HCV agents. Arch. Pharm. Chem. Life Sci. 339 (2006) 14–23. DOI:10.1002/(ISSN)1521-4184 |
| [79] | M.T. Cocco, C. Congiu, V. Lilliu, V. Onnis. Synthesis and in vitro antitumoral activity of new hydrazinopyrimidine-5-carbonitrile derivatives. Bioorg. Med. Chem. 14 (2006) 366–372. DOI:10.1016/j.bmc.2005.08.012 |
| [80] | J. Capilla, C. Serena, F. Javier Pastor, M. Ortoneda, J. Guarro. Efficacy of voriconazole in treatment of systemic scedosporiosis in neutropenic mice. Antimicrob. Agents Chemother. 47 (2003) 3976–3978. DOI:10.1128/AAC.47.12.3976-3978.2003 |
| [81] | A. Walcourt, M. Loyevsky, D.B. Lovejoy, V.R. Gordeuk, D.R. Richardson. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 36 (2004) 401–407. DOI:10.1016/S1357-2725(03)00248-6 |
| [82] | H. Garn, H. Krause, V. Enzmann, K. Dröβler. An improved MTT assay using the electron-coupling agent menadione. J. Immunol. Methods 168 (1994) 253–256. DOI:10.1016/0022-1759(94)90062-0 |
| [83] | S.M. Thom, R.W. Horobin, E. Seidler, M.R. Barer. Factors affecting the selection and use of tetrazolium salts as cytochemical indicators of microbial viability and activity. J. Appl. Bacteriol. 74 (1993) 433–443. DOI:10.1111/jam.1993.74.issue-4 |
| [84] | S.R. Kim, M.J. Park, M.K. Lee, et al., Flavonoids of Inula britannica protect cultured cortical cells from necrotic cell death induced by glutamate. Free Radic. Biol. Med. 32 (2002) 596–604. DOI:10.1016/S0891-5849(02)00751-7 |
| [85] | S.H. Kim, J.H. Zo, M.A. Kim, et al., Naringin suppresses the mitogenic effect of lysophosphatidylcholine on vascular smooth muscle cells. Nutr. Res. 23 (2003) 1671–1683. DOI:10.1016/j.nutres.2003.08.001 |
| [86] | H.Y. Lin, S.H. Juan, S.C. Shen, F.L. Hsu, Y.C. Chen. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 66 (2003) 1821–1832. DOI:10.1016/S0006-2952(03)00422-2 |
| [87] | M.K. Gupta, T.V. Neelakantan, M. Sanghamitra, et al., An assessment of the role of reactive oxygen species and redox signaling in norepinephrine-induced apoptosis and hypertrophy of H9c2 cardiac myoblasts. Antioxid. Redox Signal. 8 (2006) 1081–1093. DOI:10.1089/ars.2006.8.1081 |
| [88] | T. Mosmann. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65 (1983) 55–63. DOI:10.1016/0022-1759(83)90303-4 |
 2017, Vol. 28
2017, Vol. 28