b Shanghai Institute of Pharmaceutical Industry, Shanghai 201203, China;
c School of Pharmaceutical Sciences and Chemistry, Dali University, Dali 671000, China
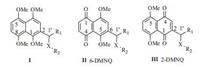
5, 8-Dimethoxy-1, 4-naphthoquinone (DMNQ), methylation of naphthazarin and its derivatives, significantly increased the electrophilicity of the naphthoquinone moiety, assuming more vulnerable to nucleophiles [1-3]. As a result, DMNQ and its derivatives displayed more potent inhibitory actions than corresponding parent compounds [4, 5]. Excellent antitumor activities of reported DMNQ analogues were taken as the convincing examples [6-15]. Likewise, our previous investigation showed that methylation of the naphthazarin nucleus of shikonin and its derivatives was obviously conductive to enhancing the antitumor activity [16-20]. It was found that DMNQ derivatives consisted of two positional isomers, 2-isomers (2-DMNQ) and 6-isomers (6-DMNQ) (Fig. 1). From the standpoint of biological activity, 6-DMNQ exhibited better anticancer activity than the corresponding 2-DMNQ [4, 14-17]. Additionally, extensive researches regarding DMNQ have been conducted around the hydroxyl group, it was decided that a modification of 1'-OH at the side chain of 6-position was responsible for the enhancement of antitumor activity. Therefore, we envisioned that varying substituents (R1, R2 and linker X) attached to the position 6 of naphthoquinone skeleton might confer desirable biological activities.
|
Download:
|
| Fig. 1. The chemical structures of 2-substituted 1, 4, 5, 8-tetramethoxynaphthalene and DMNQs | |
The synthesis of 2-DMNQ through the direct methylation of corresponding naphthazarin analogues was relatively simple, while the preparation of 6-DMNQ was tough. A widely accepted approach to prepare DMNQ derivatives is that oxidative demethylation of 2-substituted 1, 4, 5, 8-tetramethoxynaphthalenes (Fig. 1) with CAN in CH3CN/H2O. Unfortunately, the oxidative demethylation produced the mixture of 2-DMNQ and 6-DMNQ. Although the ratio of the demethylation product was dependent upon the overall electronic effects of the substituted group of 2-substituted tetramethoxynaphthalene [21-23], the detailed influence of the attachments to tetramethoxynaphthalene skeleton (Fig. 1) on the distribution of 6-DMNQ and 2-DMNQ in CAN-mediated oxidation, including the side chain R1, the branched chain R2 and linker X, remained elusive. Thus, this somewhat affected our targetorientated design and synthesis of desired 6-DMNQ derivatives with more potent anticancer activity.
Inspired by the facts mentioned above, we reported herein our interesting findings that CAN-mediated regioselective oxidation of 2-substituted 1, 4, 5, 8-tetramethoxynaphthalenes provided 6-DMNQ in comparatively high yields with EtOAc/H2O (4:1) as the solvent system, which supplemented and enriched the information of substrate-controlled demethylation with CAN, and further shed light on the relationship of oxidative regioselectivity together with antitumor activity among the side chain R1, the branched chain R2 and linker X of 6-DMNQ.
2. Results and discussion 2.1. ChemistryThe synthesis of 2-substituted 1, 4, 5, 8-tetramethoxynaphthalenes was shown in Scheme 1. Aldehyde 1 was synthesized starting from commercially available 1, 5-dihydroxynaphthalene [24]. Alcohols 2a and 2c were obtained by zinc-mediated α-regioselective prenylation of 1 as our previously proposed procedures [25]. Alcohol 2b was afforded using nucleophilic addition reaction. Thiol 3a and 3b were prepared in two steps from corresponding alcohols 2a and 2b according to our previously proposed procedures [16]. 2a or 2c was then coupled with different organic acid to afford 4a-4d in acceptable overall yield in the presence of dicyclohexylcarbodiimide (DCC) and 4-(dimethylamino)pyridine (DMPA), while etherification of 2a or 2c with suitable halohydrocarbon provided 4e-4g in alkali condition. Likewise, compounds 5a-5d were obtained by treatment with corresponding 3a or 3b using the synthetic route as described for 4a-4g. Subsequent oxidation of 5c with m-CPBA quantitatively generated sulfone 9 [26]. Conversion of 2a into 4-methyl-1-(1, 4, 5, 8-tetramethoxynaphthalen-2-yl)pent-3-en-1-amine (7) was achevied by the Mitsunobu and Gabreil reaction [27]. Condensation of 7 with tri-O-methylgallic acid afforded 8 in excellent yield in the presence of DCC and DMAP.

|
Download:
|
| Scheme1. Reagents and conditions: Synthesis of 2-substituted 1, 4, 5, 8-tetramethoxynaphthalenes (a) for 2a: ⅰ. prenyl bromide, Zn, THF, r. t., N2, 2 h; ⅱ. HMPA, 120 ℃, N2, 3 h; (b) for 2b: Mg, THF, C8H17Br, 50 ℃, N2, 3 h; (c) for 2c, prenyl bromide, Zn, THF, r. t., N2, 2 h; (d) ⅰ. 48% HBr, thiourea, EtOH, reflux, N2, 1 h; ⅱ. 40% NaOH, reflux, N2, 2 h; (e) for 4a–4d, 5a, 5b, 8: R2COOH, DCC, DMAP, 0 ℃ to r. t., overnight; (f) for 4e–4g, 5c, 5d: 60% NaH, R2I, DMF, N2, 0 ℃–50 ℃, 14 h; or 40% NaOH, KI, R2Br, EtOH, reflux, 12 h; (g) Ph3P, phthalimide, DEAD, THF, N2, 24 h; (h) 98% N2H4·H2O, EtOH, reflux, N2, 10 h; (i) m-CPBA, NaHCO3, H2O, CH2Cl2, 16 h | |
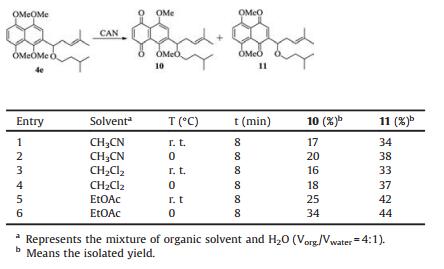
After all the prepared 2-substituted 1, 4, 5, 8-tetramethoxynaphthalenes were prepared, the potential of CAN in preferentially yielding 6-isomers was investigated. Compound 4e, synthesized in multi-gram scales in our lab, was used as a model substrate for probing the reaction conditions. The best ratio of 1 to CAN (1:2.6), optimum reaction temperature (0 ℃) and reaction time (8 min) were established by analysis of a variety of experimental conditions (Table 1). Lowering the reaction temperature from r. t. to 0 ℃ resulted in a slightly higher isolated yield [23]. However, with the temperature continued being reduced, the reaction did not proceed smoothly. As demonstrated in Table 1, treatment of 1, 4, 5, 8-tetramethoxynaphthalene 4e with CAN in homogeneous system CH3CN/H2O produced 2-DMNQ 10 in 17% isolated yield and 6-DMNQ 11 in 34% isolated yield, respectively (Table 1, entry 1). Subsequent oxidation of 4e with CAN using biphasic solvent system CH2Cl2/H2O [18], in contrast to CH3CN/H2O solvent system, the yield of 11 did not change remarkably (Table 1, entry 1-4). However, changing the solvent system from CH3CN/H2O and CH2Cl2/H2O to biphasic condition EtOAc/H2O in the oxidation, we found that the yield of 11 was significantly improved and the ratio of 6-DMNQ and 2-DMNQ was increased from 1:2.1 to 1:1.3 (Table 1, entries 2-6). Therefore, EtOAc/H2O was recommended as the optimal solvent system [28], implying that solvent systems at least partly affected regioselective oxidation.
|
|
Table 1 Different reaction conditions for CAN-mediated oxidation of compound 4e |
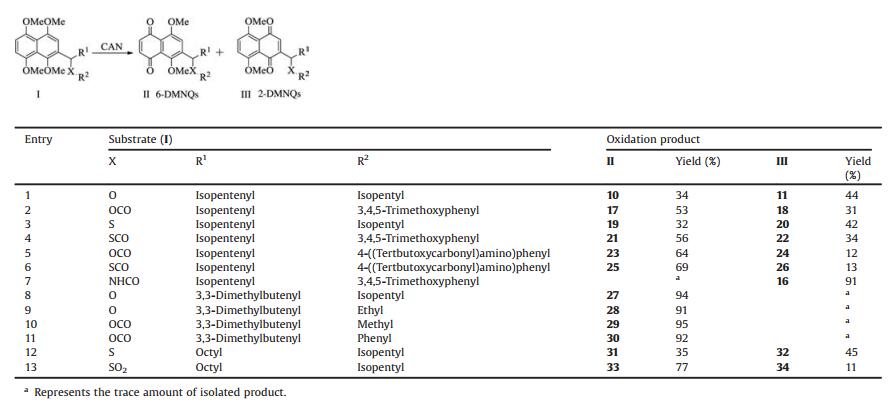
After establishing optimal reaction conditions, we moved on to explore the CAN-mediated demethylation on a range of 2-substituted tetramethoxynaphthalenes, which contained different R1, R2 and the linker X. Initially, the influence of R2 at 1'-OH contained in the side chain was investigated by treatment of compound 4a or 4b with CAN. The replacement of isopentyl group with 3, 4, 5-trimethoxyphenyl moiety in oxidation gave rise to the 6-DMNQ 17 in 53% isolated yield (Table 2, entry 2). Both electrondrawing effects of and steric hindrances of 3, 4, 5-trimethoxyphenyl group were possibly responsible for this increased yield. However, methoxyl group used as electron-donating substituent might reduce the electron-drawing effects of benzoyloxy moiety. This led us to suppose that steric effects of 3, 4, 5-trimethoxyphenyl moiety could play important role in the generation of 6-DMNQ. In view of electron effects and steric hindrances mentioned above, 4-((tertbutoxycarbonyl)amino)phenyl group was thus incorporated into the benzene ring. We found that compared with compound 2, regioselective oxidation of 5 with CAN affored 23 in higher yield (Table 2, entry 5), indicating that steric effects of the branched chain (R2) favored the generation of 6-DMNQ. Similar trend was also observed in sulfur-containing compounds (Table 2, linker X = S, SCO, entries 3, 4 and 6). To be taken together, if R1 was fixed, the introduction of the substituted group with steric hindrances in branched chain (R2) would give the 6-DMNQ as the major product, probably implying that steric hindrances could have influence on CAN-directed regioselective oxidation.
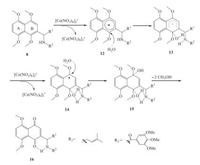
When the oxygen atom of 1'-position in the side chain of 1, 4, 5, 8-tetramethoxynaphthoxynathalene was replaced by nitrogen atom, it was surprising that oxidative demethylation of 8 gave rise to the 2-isomer 16 in an isolated yield of 91% and only a trace amount of 6-isomer was found (Table 2, entry 7). This was possibly ascribed to the overall contribution of the electron-donating effects and the formation of hydrogen bonds. As demonstrated in Fig. 2, the putative reaction mechanism we proposed was carried out via the formation of radical cation 12 and radical 13. The amino group at 1'-position as an electron-donating group could increase the electron density of B ring and facilitate the stability of intermediate 12. On the other hand, intramolecular hydrogen bond interaction could also improve the stability of aryl radical and desire product, and thus helped in the formation of 16.
|
|
Table 2 Oxidation of 2-substituted 1, 4, 5, 8-tetramethoxynaphthalene (4a–g, 5a–d, 8 and 9) with CAN |
|
Download:
|
| Fig. 2. The plausible mechanism for the oxidative demethylation of amide 8 | |
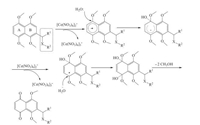
To further examine whether substitution group (R1) exerted an obvious influence on the ratio of the 2-isomer and 6-isomer, the isopentenyl group on position 2 on the naphthalene nucleus was replaced by 3, 3-dimethyl butenyl moiety. As expected, the 6-isomers were the primary demethylated products in the oxidation (Table 2, entries 8-11), and no significant amount of 2-isomer was observed. Unfortunately, a decrease in the yield of 6-isomer was observed when the isopentenyl group was replaced with a much longer carbon chain to eliminate the steric hindrance (Table 2, entry 12). This was completely consistent with previous report that the introduction of electron-donating group at 2-position of 1, 4, 5, 8-tetramethoxynaphthalenes preferentially afforded 2-isomers [21]. However, when sulfur atom in compound 5c was converted into sulfuryl group, demethylation of tetramethoxynaphthalene predominantly gave 6-isomer, which was sevenfold more than the corresponding 2-isomer (Table 2, entry 13). The electron-withdrawing effects of sulfuryl group were responsible for making the electron delocalization of A ring form ary radical easily (Fig. 3). In a word, regardless of the substituent groups (R2), either electron-withdrawing or electron-donating ones, CANmediated oxidation demethylation of tetramethoxynaphthalenes with the bulky substituted group at R1 dominantly yielded the 6-isomers. Further investigation indicated that the steric hindrances of side chain (R1), which facilitated water molecule attacking A ring (Fig. 3), played a pivotal role in the regioselective demethylation. Therefore, we reasoned that steric hindrances (3, 3-dimethyl butenyl etc.) and electron-withdrawing effects were the foremost factors for the generation of the 6-isomers.
|
Download:
|
| Fig. 3. The mechanism of oxidative demethylation of substrate with steric hindrance or electon-withdrawing groups | |
2.2. In vitro cytotoxic activity
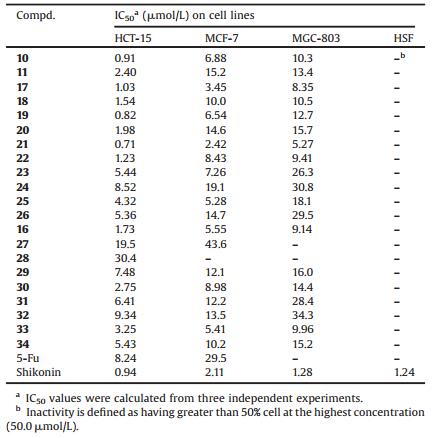
The DMNQ derivatives we prepared above were evaluated for their in vitro cytotoxicity against HCT-15 (colorectal carcinoma), MGC-803 (gastric carcinoma), MCF-7 (breast cancer) and HSF (human skin fibroblast) cells by standard MTT assay [20]. Shikonin and 5-fluorouracil (5-Fu) were used as positive controls. The anticancer potential of these compounds was gauged by IC50 values, which were calculated by linear regression analysis of the concentration-response curves generated from each compound.
From the cytotoxicity data listed in Table 3, the majority of the prepared compounds (10, 11, 17-22) displayed potent inhibitory effects on HCT-15 cell lines. The potencies of some compounds were more superior or comparative to the positive reference compound shikonin (IC50 = 0.94 μmol/L), whereas the positive control 5-FU showed minor inhibitory activity towards MGC-803 cells. Among the dimethylated 1, 4-naphthoquinone derivatives, the IC50 value of the most effective compound 21 against HCT-15 cells was as low as 0.71 μmol/L. Meanwhile, the IC50 values of 21 against MCF-7 and MGC-803 cells were found to be 2.42 and 5.27 μmol/L, respectively. Likewise, the same trend of IC50 values of other compounds was observed among tested tumor cell lines in this category including the best inhibitory effect toward HCT-15, moderate toward MCF-7, but little toward MGC-803, to some degree, which was reflected excellent selectivity towards these cancer cells. Notably, all the prepared compounds exhibited no apparent cytotoxicity towards normal cell line HSF (IC50 > 50 μmol/ L). This observation was of great significance owing to no selectivity between normal and neoplastic cells when administrated by positive control shikonin, although it displayed more potent effects on cancerous cells.
|
|
Table 3 Cytotoxicity of DMNQ derivatives against HCT-15, MCF-7, MGC-803 and HSF cell lines |
Compounds (10, 17, 19, 21, 23, 25, 31 and 33) showed better anticancer activity than their corresponding 2-isomers (11, 18, 20, 22, 24, 26, 32, and 34). This result was in good agreement with our previous report [17]. For example, compound 19 caused significant cytotoxicity at lower concentration (IC50 = 0.82, 6.54 and 12.7 μmol/L), which was superior to the cytotoxicity induced by 20 (IC50 = 1.98, 14.6 and 15.7 μmol/L) in HCT-15 cells, MCF-7 cells and MGC-803 cells, respectively. However, compound 16 bearing benzamido moiety expressed stronger cytotoxic activity with the IC50 value of 1.73 μmol/L against HCT-15 cells than most of 2-isomers, possibly suggesting that the introduction of linker amide to 1'-position favored the enhancement of cytotoxicity. On the other hand, it should be pointed that sulfur-containing 1, 4-naphthoquinone derivatives (19-22, 25 and 26) exhibited potent anticancer activity against the cancer cell lines than the corresponding oxygen-containing ones (10, 11, 17, 18, 23 and 24).
Another interesting observation was that the series 27-30, bearing 3, 3-dimethyl-butenyl moiety instead of isopentenyl one, displayed lower inhibitory effects than shikonin. Additionally, compounds with ester moieties in the side chain showed higher cytotoxicities than those with ether groups, especially for HCT-15 cells. For example, dimethylated derivative 30 containing ester groups (IC50 = 2.75 μmol/L) was approximately 11-fold cytotoxic than corresponding ether compound 28 (IC50 = 30.4 μmol/L). This results indicated that the introduction of bulkier substituents on position 6 of 1, 4-naphthoquinone skeleton might lead to the decline of cytotoxicity.
Among the prepared compounds (19, 20, 31 and 32), replacing the isopentyl moiety with saturated alkyl carbon chain (n = 9) caused a drop in antitumor activity, indicating that different substituents (R1) on position 6 of 1, 4-naphthoquinone skeleton significantly affected inhibitory effects. Meanwhile, it was appealing that compounds (33, 34) with sulfuryl group was found to possess approximately twofold to threefold inhibitory effects against MGC-803 cells than those bearing thioether moiety (31, 32). And our more attention had been attracted to sulfurcontaining 6-substituted 1, 4-naphthoquinone derivatives due to their potential anticancer activities.
3. ConclusionIn summary, CAN-mediated regioselective oxidation of 1, 4, 5, 8-tetramethoxynaphthalenes with various substituents at the 2-position including side chain R1, the branched chain R2 and linker X and its oxidative mechanism were discussed in detail. It was found that 6-DMNQ could be selectively generated by oxidative demethylation with CAN using EtOAc/H2O (4:1) as solvent system in comparatively good yields and meanwhile highly selective generation of the 6-DMNQ had been demonstrated to be largely dependent upon the electron-withdrawing effects and steric hindrances of substituents on position 2 of naphthalene ring. However, electrondonatingeffects or hydrogen bond interaction (X=N) of substituents at the 2-position of 1, 4, 5, 8-tetramethoxynaphthalenes might have extremely negative impactonregioselective synthesis of 6-DMNQ in CAN-mediated oxidation. On the other hand, the results from in vitro antitumor activities showed that the majority of the prepared 6-DMNQ displayed the selective cytotoxic activities toward HCT-15 cells, together with no apparent toxicity toward normal cells. It was fortunate that sulfur-containing 6-DMNQ derivatives were found to show relatively better antitumor activities than oxygencontaining ones. Efforts are ongoing in our research group to exploit the potential sulfur-containing 6-DMNQ derivatives as the promising anticancer agents.
4. Experimental 4.1. ChemistryReagents and solvents were obtained from commercial suppliers. Solvents were dried and purified using standard techniques. All reactions involving air and moisture sensitive were carried out under the atmosphere of nitrogen. Column chromatography analysis was conducted on silica gel (200-300 mesh) from Qingdao Ocean Chemical Factory. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Varian MERCURY plus-400 spectrometer with TMS as an internal standard. HRMS spectra were measured on a Waters Q-TOF Premier Mass Spectrometer.
4.1.1. General procedure for synthesis of target compounds (10 and 11)A solution of CAN (1.42 g, 2.6 mmol) in H2O (2.5 mL) was dropwise added to a solution of 2-substituted 1, 4, 5, 8-tetramethoxynathalene (0.45 g, 1.0 mmol) in EtOAc (10.0 mL) at 0 ℃. After 8 min, the reaction mixture was extracted with EtOAc, washed with brine, dried over anhydrous Mg2SO4, and then concentrated. The residue was purified by flash column chromatography to afford 6-or 2-substituted-5, 8-dimethoxy-1, 4-naphthoquinone.
4.1.2. 6-(1-(Isopentyloxy)-4-methylpent-3-en-1-yl)-5, 8-dimethoxy-1, 4-naphthoquinone (10)This compound was synthesized from 4e. Yield: 34%; yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.42 (s, 1H), 6.72-6.78 (m, 2H), 5.18 (t, 1H, J = 6.6 Hz), 4.71 (t, 1H, J = 6.6 Hz), 3.94 (s, 3H), 3.79 (s, 3H), 3.27-3.37(m, 2H), 2.38-2.45 (m, 1H), 2.26-2.33(m, 1H), 1.68-1.73(m, 1H), 1.65 (s, 3H), 1.48 (s, 3H), 1.41-1.45 (m, 2H), 0.86 (d, 3H, J = 6.6 Hz), 0.83 (d, 3H, J = 6.6 Hz); 13C NMR (100 MHz, CDCl3): δ 184.1, 183.5, 155.3, 150.3, 146.1, 137.9, 136.7, 133.2, 123.8, 118.7, 118.6, 116.2, 74.7, 67.1, 61.2, 55.6, 37.7, 34.8, 24.7, 24.0, 21.6, 21.5, 16.9; HRMS m/z (ESI), calcd. for C23H31O5: 387.2171 [M+H]+, found: 387.2169.
4.1.3. 2-(1-(Isopentyloxy)-4-methylpent-3-en-1-yl)-5, 8-dimethoxy-1, 4-naphthoquinone (11)This compound was synthesized from 4e. Yield: 44%; red oil; 1H NMR (400 MHz, CDCl3): δ 7.23 (s, 2H), 6.73 (s, 1H), 5.11 (t, 1H, J = 7.2 Hz), 4.48 (t, 1H, J = 7.2 Hz), 3.89 (s, 6H), 3.33-3.38 (m, 1H), 3.26-3.31 (m, 1H), 2.36-2.43 (m, 1H), 2.14-2.20 (m, 1H), 1.61-1.68 (m, 1H), 1.58 (s, 3H), 1.46 (s, 3H), 1.35-1.41 (m, 2H), 0.81 (d, 3H, J = 2.0 Hz), 0.79 (d, 3H, J = 2.0 Hz); 13C NMR (100 MHz, CDCl3): δ 184.2, 184.1, 152.7, 152.4, 149.5, 133.2, 132.7, 120.5, 120.1, 119.2, 118.9, 118.5, 74.1, 67.2, 55.9, 55.8, 37.6, 33.3, 24.7, 23.9, 21.6, 21.5, 17.0; HRMS m/z (ESI), calcd. for C23H31O5: 387.2171 [M+H]+, found: 387.2174.
Synthesis of other target compounds 16-34, compounds 2b-9 and their spectroscopic data were given in the Supplementary data.
4.2. Cytotoxic activity assayThe tested compounds were dissolved in suitable amount of dimethyl sulfoxide (DMSO) prior to the experiment to obtain the known concentration of the solution, and then were diluted to various concentrations with culture medium. Cells at 70%-80% confluency were trypsinized and plated into 96-well plates at a density of 1 ×104 cells/mL. After culture for 24 h at 37 ℃, 5% CO2 atmosphere, cells were treated with the tested compound of various concentrations for 72 h and control groups were treated with complete medium alone. The supernatants were removed and replaced by 200 μL RMPI-1640 medium without serum. Then 20 μL 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added to each well. After incubation for another 4 h, the medium was aspirated, and the formazan crystals were dissolved in 100 μL DMSO for each well. In all experiments three replicate wells were used for each compound concentration. The absorbance was measured at a test wave length of 570 nm using a Multiskan MK3 microplate reader (Thermo Scientific, USA). The percentage of survival was calculated as the absorbance ratio of treated cells to that of control cells. Half maximal inhibitory concentration (IC50) values were obtained by linear regression analysis using IBM SPSS statistics software (version 21.0).
AcknowledgmentsThe research was supported by National Natural Science Foundation of China (No. 81373274), Ph.D. Programs Foundation of Ministry of Education China (No. 20120073110068) and Shanghai Biomedical Supporting Funding (No. 15431900600). We are grateful to the Instrumental Analysis Center of Shanghai Jiao Tong University for recording the 1H NMR and 13C NMR spectra.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.034.
| [1] | B.Z. Ahn, D.E. Sok. Michael acceptors as a tool for anticancer drug design. Curr. Pharm. Des. 2 (1996) 247–262. |
| [2] | R.P. Verma, C. Hansch. Elucidation of structure-activity relationships for 2-or 6-substituted-5, 8-dimethoxy-1, 4-naphthoquinones. Bioorg. Med. Chem. 12 (2004) 5997–6009. DOI:10.1016/j.bmc.2004.08.017 |
| [3] | R.P. Verma. Anti-cancer activities of 1, 4-naphthoquinones: a QSAR study. Anticancer Agents Med. Chem. 6 (2006) 489–499. DOI:10.2174/187152006778226512 |
| [4] | Y.J. You, X.G. Zheng, K. Yong, B.Z. Ahn. Naphthazarin derivatives: synthesis, cytotoxic mechanism and evaluation of antitumor activity. Arch. Pharm. Res. 21 (1998) 595–598. DOI:10.1007/BF02975381 |
| [5] | Y.J. You, Y. Kim, G.Y. Song, B.Z. Ahn. (E)-6-(1-alkyloxyiminoalkyl)-5, 8-dimethoxy-1, 4-naphthoquinones: synthesis, cytotoxic activity and antitumor activity. Bioorg. Med. Chem. Lett. 10 (2000) 2301–2303. DOI:10.1016/S0960-894X(00)00447-9 |
| [6] | J.J. Lee, W.Y. Zhang, H. Yi, et al., Anti-proliferative actions of 2-decylamino-5, 8-dimethoxy-1, 4-naphthoquinone in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 411 (2011) 213–218. DOI:10.1016/j.bbrc.2011.06.145 |
| [7] | Y.M. Kanaan, J.R. Das, O. Bakare, et al., Biological evaluation of 2, 3-dichloro-5, 8-dimethoxy-1, 4-naphthoquinone as an anti-breast cancer agent. Anticancer Res. 29 (2009) 191–199. |
| [8] | H.J. Lee, H.J. Lee, G.Y. Song, et al., 6-(1-Oxobutyl)-5, 8-dimethoxy-1, 4-naphthoquinone inhibits lewis lung cancer by antiangiogenesis and apoptosis. Int. J. Cancer 120 (2007) 2481–2490. DOI:10.1002/(ISSN)1097-0215 |
| [9] | S.H. Kim, I.C. Kang, T.J. Yoon, et al., Antitumor activities of a newly synthesized shikonin derivative, 2-hyim-DMNQ-S-33. Cancer Lett. 172 (2001) 171–175. DOI:10.1016/S0304-3835(01)00665-6 |
| [10] | G.Y. Song, Y. Kim, X.G. Zheng, et al., Naphthazarin derivatives (Ⅳ): synthesis, inhibition of DNA topoisomerase I and cytotoxicity of 2-or 6-acyl-5, 8-dimethoxy-1, 4-naphthoquinones. Eur. J. Med. Chem. 35 (2000) 291–298. DOI:10.1016/S0223-5234(00)00129-X |
| [11] | Y. Kim, Y.J. You, B.Z. Ahn. Naphthazarin derivatives (Ⅷ): synthesis, inhibitory effect on DNA topoisomerase-I, and antiproliferative activity of 6-(1-acyloxyalkyl)-5, 8-dimethoxy-1, 4-naphthoquinones. Arch. Pharm. 334 (2001) 318–322. DOI:10.1002/(ISSN)1521-4184 |
| [12] | Y.J. You, B.Z. Ahn. 6-(1-Alkenoyloxyalkyl)-5, 8-dimethoxy-1, 4-naphthoquinone derivatives: synthesis and evaluation of antitumor activity. Arch. Pharm. Res. 21 (1998) 738–743. DOI:10.1007/BF02976768 |
| [13] | G.Y. Song, Y. Kim, Y.J. You, H. Cho, B.Z. Ahn. Naphthazarin derivatives (Ⅶ): antitumor action against ICR mice bearing ascitic S-180 cells. Arch. Pharm. Res. 24 (2001) 190–193. DOI:10.1007/BF02978254 |
| [14] | G.Y. Song, Y. Kim, Y.J. You, et al., Naphthazarin derivatives (Ⅵ): synthesis, inhibitory effect on DNA topoisomerase-I and antiproliferative activity of 2-or 6-(1-oxyiminoalkyl)-5, 8-dimethoxy-1, 4-naphthoquinones. Arch. Pharm. 333 (2000) 87–92. DOI:10.1002/(ISSN)1521-4184 |
| [15] | Y.S. Chung, Y.K. Shin, C.G. Zhan, S. Lee, H. Cho. Synthesis and evaluation of antitumor activity of 2-and 6-[(1, 3-benzothiazol-2-yl)aminomethyl]-5, 8-dimethoxy-1, 4-naphthoquinone derivatives. Arch. Pharm. Res. 27 (2004) 893–900. DOI:10.1007/BF02975839 |
| [16] | L.M. Zhao, T.P. Xie, Y.Q. He, D.F. Xu, S.S. Li. Synthesis and antitumor activity of 6-and 2-(1-substituted-thio-4-methylpent-3-enyl)-5, 8-dimethoxynaphthalene-1, 4-diones. Eur. J. Med. Chem. 44 (2009) 1410–1414. DOI:10.1016/j.ejmech.2008.09.039 |
| [17] | W. Zhou, X. Zhang, L. Xiao, et al., Semi-synthesis and antitumor activity of 6-isomers of 5, 8-O-dimethyl acylshikonin derivatives. Eur. J. Med. Chem. 46 (2011) 3420–3427. DOI:10.1016/j.ejmech.2011.05.006 |
| [18] | R.B. Wang, X. Zhang, H.L. Song, S.S. Zhou, S.S. Li. Synthesis and evaluation of novel alkannin and shikonin oxime derivatives as potent antitumor agents. Bioorg. Med. Chem. Lett. 24 (2014) 4304–4307. DOI:10.1016/j.bmcl.2014.07.012 |
| [19] | R.B. Wang, W. Zhou, Q.Q. Meng, et al., Design, synthesis, and biological evaluation of shikonin and alkannin derivatives as potential anticancer agents via a prodrug approach. ChemMedChem 9 (2014) 2798–2808. DOI:10.1002/cmdc.v9.12 |
| [20] | Y.Y. Yang, H.Q. He, J.H. Cui, et al., Shikonin derivative DMAKO-05 inhibits Akt signal activation and melanoma proliferation. Chem. Biol. Drug Des. 87 (2016) 895–904. DOI:10.1111/cbdd.12722 |
| [21] | Y. Tanoue, A. Terada. The 2-or 6-(α-hydroxyalkyl-and α-oxoalkyl)-5, 8-dimethoxy-1, 4-naphthoquinones from the oxidative demethylation of 2-(α-hydroxyalkyl-and α-oxoalkyl)-1, 4, 5, 8-tetramethoxynaphthalenes with Cerium(Ⅳ) ammonium nitrate, and further demethylations to naphthazarins. Bull. Chem. Soc. Jpn. 61 (1988) 2039–2045. DOI:10.1246/bcsj.61.2039 |
| [22] | V.P. Papageorgiou, A.N. Assimopoulou, E.A. Couladouros, D. Hepworth, K.C. Nicolaou. The chemistry and biology of alkannin shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 38 (1999) 270–301. DOI:10.1002/(ISSN)1521-3773 |
| [23] | M. Kawasaki, F. Matsuda, S. Terashima. Synthetic studies on nogalamycin congeners [2]1, 2 chiral synthesis of the cdef-ring system of nogalamycin. Tetrahedron 44 (1988) 5713–5725. DOI:10.1016/S0040-4020(01)81432-0 |
| [24] | R.B. Wang, X.G. Zheng, W. Zhou, et al., An efficient synthesis of 2-formyl-1, 4, 5, 8-tetramethoxynaphthalene. J. Chem. Res. 34 (2010) 520–521. DOI:10.3184/030823410X12843836823191 |
| [25] | L.M. Zhao, D.F. Xu, W. Zhou, S.S. Li. Concise formal synthesis of (±)-shikonin via a highly a-regioselective prenylation of 1, 4, 5, 8-tetramethoxynaphthalene-2-carbaldehyde. Lett. Org. Chem. 5 (2008) 234–236. DOI:10.2174/157017808783955899 |
| [26] | J.S. Foot, G.M.P. Giblin, A.C. Whitwood, R.J.K. Taylor. Unexpected Z-stereoselectivity in the Ramberg-Bäcklund reaction of diarylsulfones leading to cisstilbenes: the effect of aryl substituents and application in the synthesis of the integrastatin nucleus. Org. Biomol. Chem. 3 (2005) 756–763. DOI:10.1039/B418426B |
| [27] | J. Yang, K.L. Hong, P.V. Bonnesen. Synthesis of N1-tritylethane-1, 1, 2, 2-d4-1, 2-diamine: a novel mono-protected C-deuterated ethylenediamine synthon. J. Label. Compd. Radiopharm. 55 (2012) 463–466. DOI:10.1002/jlcr.v55.13 |
| [28] | N.K. Sharma, K.N. Ganesh. Regioselective oxidation of N-alkylpyrrolidines to pyrrolidin-5-ones by RuCl3/NaIO4. Tetrahedron Lett. 45 (2004) 1403–1406. DOI:10.1016/j.tetlet.2003.12.072 |
 2017, Vol. 28
2017, Vol. 28