b Key Laboratory of National Health and Family Planning Commission on Parasitic Disease Control and Prevention, Jiangsu Provincial Key Laboratory on Parasite and Vector Control Technology, Jiangsu Institute of Parasitic Diseases, Wuxi 214064, China;
c Medical School, Jiangnan University, Wuxi 214122, China;
d Suzhou Kemu Veterinary Pharmaceutical Co., Ltd., Meiyan High-technology Development Zone, Suzhou 215225, China
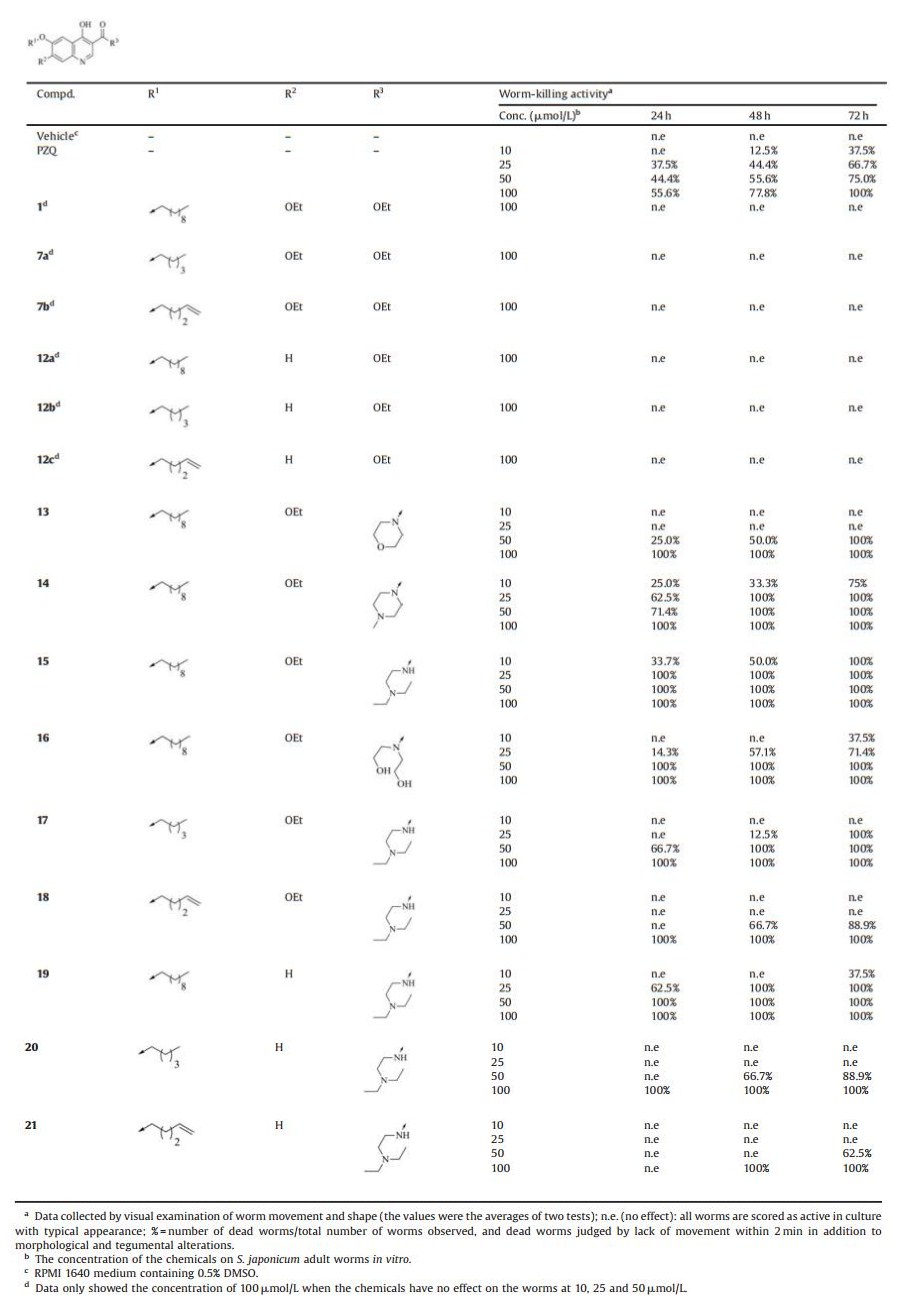
Schistosomiasis is a neglected tropical disease (NTD) caused by blood-dwelling trematodes belonging to the genus Schistosoma. Schistosoma haematobium, Schistosoma japonicum, and Schistosoma mansoni are the main species parasitizing humans [1]. It has been estimated that 779 million people are at risk for schistosomiasis transmission, with 207 million infected in 76 countries and territories [1, 2]. In China, the disease caused by Schistosoma japonicum remains a major public health concern with more than 280 thousand people infected [3]. In the absence of a vaccine, praziquantel (PZQ) is the only drug recommended by the World Health Organization for the treatment and control of schistosomiasis through mass drug administration (MDA) program for more than three decades [4]. The massive and exclusive use of PZQ has triggered concerns about the emergence of drug resistant parasite, and evidence of drug resistance has been reported [4-6]. Therefore, it is imperative to develop new antischistosomal agents for the treatment of schistosomiasis. Drug repositioning is attractive option in the case of tropical diseases for which the finance and resources to support de-novo drug development are limited [7]. Artemisinin and its derivatives, which are a vital conerstone in treatment and control of malaria, were now considered as alternative chemotherapies against S. japonicum [7]. Decoquinate (1) is an old and inexpensive coccidiostat. Like Artemisinin, Decoquinate exhibited strong antimalarial activity. [8-10]. We hypothesized that Decoquinate, acting similarly as Artemisinin, may also possess antischistosomal activity. Using decoquinate as a template, a series of compounds were designed and synthesized, with the aim to improve aqueous solubility and other drug like properties, and their antischistosomal activities were evaluated against S. japonicum adult worms in vitro. (Fig. 1). Among them, compound 15 killed 100% of adult S. japonicum in 72 h at the concentration of 10 μmol/L in vitro, while praziquantel (PZQ) (10 μmol/L) caused 37.5% of adult S. japonicum death in 72 h. The result indicated that compound 15 had stronger worm-killing activity than PZQ in vitro and could serve as a promising lead compound to develop novel antischistosomal agents (Fig. 1).
|
Download:
|
| Fig. 1. Structures of novel decoquinate derivatives | |
2. Results and discussion 2.1. Chemistry
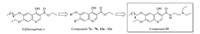
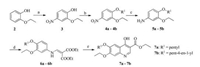
Based on the structure of decoquinate 1, compounds 7a-7b, 12a-12c and 13-21 were designed and synthesized (Table 1). Phenolic compound 2 was nitrated with 65% nitric acid in the presence of catalytic amounts of iron(Ⅲ) nitrate nonahydrate to give compound 3 in 52%. After alkylation of hydroxyl group on compound 3, compound 4a and 4b were obtained. Nitro compounds (4a and 4b) were reduced by Fe/NH4Cl to produce amino compounds (5a and 5b), which reacted with ethoxymethylenemalonic diethyl ester (EMME) to give the intermediates 6a and 6b using similar methods reported by wang group (11). The last cycle-closing reaction was carried out by heating the intermediates 6a and 6b in diphenyl ether at 260 ℃ to obtain the key intermediates 7a and 7b (Scheme 1) [11]. By removal of ethoxy group from decoquinate, intermediates 12a-12c were synthesized using similar procedures to compounds 7a-7b (Scheme 2). Ester compounds 1, 7a-7b, 12a-12c were treated with various amine to afford the target compounds 13-21 (Scheme 3).
|
|
Table 1 Worm-killing activities against S. japonicum adult worms in vitro by compounds DQ 1, 7a–7b, 12a–12c, 13–21 |
|
Download:
|
| Scheme1. Synthesis of compounds 7a and 7b. Reagents and conditions: (a) con HNO3 (65%), Fe(NO3)3·9H2O, THF, 0 ℃ to r. t., 52%; (b) R1Br, K2CO3, CH3CN, reflux, 74%–82%; (c) Fe, NH4Cl, EtOH, reflux, 65%–70%; (d) ethoxymethylenemalonic diethyl ester (EMME), EtOH, reflux, 68%–70%; (e) diphenyl ether, 260 ℃, 55%–67% | |

|
Download:
|
| Scheme2. Synthesis of compounds 12a–12c. Reagents and conditions: (a) R1Br, K2CO3, CH3CN, reflux, 78%–82%; (b) Fe, NH4Cl, EtOH, reflux, 65%–70%; (c) ethoxymethylenemalonic diethyl ester (EMME), EtOH, reflux, 68%–70%; (d) diphenyl ether, 260 ℃, 55%–66% | |
|
Download:
|
| Scheme3. Synthesis of compounds 13–21. Reagents and conditions: (a) amine, 100 ℃, 40%–60%. | |
2.2. Worm-killing activities and structure-activity relationships
All the synthesized compounds were evaluated their wormkilling activities against adult S. japonicum in vitro, using vehicle as negative control and PZQ as positive control [12-16], and the results were detailed in Table 1. We initially prepared intermediates 7a-7b and 12a-12c by the route outlined in Schemes 1 and 2, in which we replaced decyl group on 1 with pentyl group (compound 7a) or pent-4-en-1-yl group (compound 7b), or removed ethoxy group (compound 12a) from 1, or substituted decyl group on compound 12a with pentyl group (compound 12b) or pent-4-en-1-yl group (compound 12c). We noticed that none of them showed worm-killing activity against adult S. japonicum in vitro at the concentration of 100 μmol/L. To explore novel antischistosomal agents and investigate the SARs, amide compounds 13-21 were synthesized (Scheme 3). As for compounds 13-16 converted from decoquinate 1, all of them obviously exhibited worm-killing activities against adult S. japonicum in vitro. Among amide compounds with six-membered ring at R3 position, compound 13 with morpholino group showed lower antischistosomal activities than compound 14 with 4-methylpiperazin-1-yl group, which killed 75% of adult S. japonicum at the concentration of 10 μmol/L in 72 h. Opening the piperazine ring on compound 14 led to compound 15 with (2-(diethylamino)ethyl)amino group, which completely killed worms at the concentration of 10 μmol/L in 72 h and exhibited stronger worm-killing activity than PZQ in vitro. In addition, replacement of morpholino group (compound 13) with bis(2-hydroxyethyl)amino group (compound 16) increased worm-killing activity. The results demonstrated that ringopening strategy at R3 position significantly improved wormkilling activity. Converting decyl group into pentyl group, the antischistosomal activities of compound 17 was obviously decreased compared to compound 15. Introduction of C=C bond into R1 position, the activity of compound 18 dropped significantly compared to compound 15 and 17. These results demonstrated that long saturated alky group at R1 position could have a beneficial effect on worm-killing activity. Among the compounds without ethoxy group (19-21), compounds 19-21 showed lower wormkilling activities than corresponding compounds with ethoxy group (15, 17-18). These results demonstrated that ethoxy group on R2 position took positive effect on worm-killing activity.
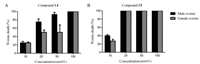
Furthermore, the activities against female or male adult worms were also observed. The mortality rate was 75.0% for male worms or 50.0% for female worms in 24 h at 25 μmol/L of compound 14. When the concentrationwas increased to 50 μmol/L, compound 14 killed 92.8% of male worms and 50.0% of female worms in 24 h. (Fig. 2A). The mortality rate was 40.0% for male worms or 27.4% for female worms in 24 h at 10 μmol/L of compound 15 (Fig. 2B). However, the result of statistical analysis showed that there were no differences between male and female worms killing activity of compounds 14 or 15.
|
Download:
|
| Fig. 2. Killing activity of compounds 14 (A) and 15 (B) against male and female adult S. japonicum after 24 h | |
The morphological alternations of Schistosoma japonicum worms incubated with compound 15 in vitro after 48 h were observed using an inverted microscopy (Leica, Wetzlar, Germany). The surface of worms in negative control was smooth. The head sucker and the ovarian structure in the body were clearly visible. However, the body of worms incubated with compound 15 became dimmed. The head and the structure in the body was blurred. The worms curled up and there were many vacuoles on the surface of worms (Fig. 3).

|
Download:
|
| Fig. 3. Morphological investigation of Schistosoma japonicum worms after in vitro incubation with compound 15. After 48 h or in the case of death, schistosomes were monitored using an inverted microscopy (Leica, Wetzlar, Germany). (A) Negative control. (B) Vehicle control. (C) 10 μmol/L of compound 15. (D) 25 μmol/L of compound 15 | |
2.3. Cytotoxicity assay of compounds 14 and 15 on Hela cells [14]
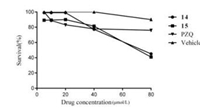
The cytotoxic assessment of the compounds 14 and 15 on the Hela cells was observed at different concentrations (5, 10, 20, 40, 80 μmol/L) for 48 h using the MTT method [14] and the viability of the Hela cells treated with compound 14, 15 and PZQ at the concentration of 20 μmol/L was 99%, 90% and 83% respectively (Fig. 4). The results indicated that compounds 14 showed lower cytotoxicity than PZQ, and the difference was statistically significant (P < 0.05). Meanwhile, compound 15 has the similar cytotoxicity to PZQ, and the difference was not statistically significant (P > 0.05).
|
Download:
|
| Fig. 4. Cytotoxic assessment of compounds 14, 15 and PZQ on Hela cells | |
3. Conclusion
Given the emergence of PZQ resistant parasites in the near future, it is undoubtedly urgent to develop new chemotherapy for schistosomiasis. In this paper, we synthesized a series of decoquinate derivatives as novel antischistosomal agents against adult S. japonicum in vitro, and identified compound 15 as potent antischistosomal agent. The study of these structurally diverse decoquinate derivatives would likely provide meaningful information for developing new potent antischistosomal agents.
4. Experimental 4.1. General procedure for the synthesis of compounds 7a-7b (exemplified by 7a)Nitric acid (10 mL, 65%) was added dropwisely into a mixture of 2-ethoxyphenol (10.00 g, 72.50 mmol) and iron (Ⅲ) nitrate nonahydrate (900 mg, 2.23 mmol) in THF (250 mL) through a constant pressure dropping funnel at 0 ℃ for 30 min. Then the mixture was stirred at room temperature for 30 min and quenched by water (100 mL) and dichloromethane (150 mL). The aqueous phase was extracted with dichloromethane (100 mL × 3), then the combined organic phases were then processed in the usual way and chromatographed to afford compound 3 (6.90 g, 52%) as solid. The mixture of compound 3 (5.00 g, 27.30 mmol), potassium carbonate (15.10 g, 109.20 mmol) and 1-bromopentane (3.57 mL, 28.70 mmol) in MeCN (50 mL) was refluxed overnight, then the mixture was diluted with EtOAc (200 mL), the organic phases were then processed in the usual way and chromatographed to afford compound 4a (5.10 g, 74%) as solid. A solution of compound 4a (5.00 g, 19.70 mmol) in ethanol/water (120 mL/120 mL) was treated with iron powder (3.32 g, 59.30 mmol) and ammonium chloride (3.17 g, 59.30 mmol) and the mixture was heated to reflux overnight. The mixture was then filtered and concentrated under reduced pressure. The resulting oil was partitioned between water and ethyl acetate, and the organic phase was then washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to provide the desired compound 5a (2.87 g, 65%) as black oil without purification to use for next step. The mixture of Ethoxymethylenemalonic diethyl ester (EMME, 2.42 g, 11.21 mmol) and compound 5a (2.50 g, 11.21 mmol) in EtOH (100 mL) was refluxed overnight. After removal of EtOH, the mixture was chromatographed to afford compound 6a (3.01 g, 68%) as yellow oil. Diphenyl ether (10 mL) was heated to 260 ℃ and compound 6a (1.00 g, 2.54 mmol) was added. The reaction mixture was stirred at 260 ℃ for15 min, and then cooled to room temperature. EtOAc (25 mL) was added to the mixture and the obtained solid was filtrated, washed with EtOAc and dried to obtain 7a (590 mg, 67%) as solid.
4.2. General procedure for the synthesis of compounds 12a-12c (exemplified by 12a)The mixture of compound 8 (2.78 g, 20.0 mmol), potassium carbonate (11.03 g, 80 mmol) and 1-bromodecane (4.55 mL, 22.0 mmol) in MeCN (50 mL) was refluxed overnight, then the mixture was diluted with EtOAc (200 mL), the organic phases were then processed in the usual way and chromatographed to afford compound 9a (4.47 g, 80%) as solid. A solution of compound 9a (4.47 g, 16.0 mmol) in ethanol (100 mL) and water (100 mL) was treated with iron powder (2.69 g, 48.0 mmol) and ammonium chloride (2.54 g, 48.0 mmol) and the mixture was heated to reflux overnight. The mixture was then filtered and concentrated under reduced pressure. The resulting oil was partitioned between water and ethyl acetate, and the organic phase was then washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to provide the desired compound 10a(2.59 g, 65%)as blackoil without purification to use for next step. The mixture of Ethoxymethylenemalonic diethyl ester (EMME, 2.08 mL, 10.4 mmol) and compound 10a (2.59 g, 10.4 mmol) in EtOH (100 mL) was refluxed overnight. After removal of EtOH, the mixture was chromatographed to afford compound 11a (2.97 g, 68%) as yellow oil. Diphenyl ether (10 mL) was heated to 260 ℃ and compound 11a (2.97 g, 7.08 mmol) was added. The reaction mixture was stirred at 260 ℃ for15 min, and then cooled to room temperature. EtOAc (25 mL) was added to the mixture and the obtained solid was filtrated, washed with EtOAc, and dried to obtain 12a (1.45 g, 55%) as solid.
4.3. General procedure for the synthesis of compounds 13-21 (exemplified by 13)The mixture of decoquinate 1 (100 mg, 0.24 mmol) and morpholine (209 μL, 2.4 mmol) was stirred at 100 ℃ for 12 h, cooled to room temperature and diluted with dichloromethane (0.5 mL). Then petroleum ester was added to the mixture. The solid was precipitated, filtrated and dried to obtain 13 (60 mg, 55%).
4.4. Worm-killing activity of compounds DQ 1, 7a-7b, 12a-12c, 13-21 on S. japonicum adult worms in vitroStock solutions of compounds DQ 1, 7a-7b, 12a-12c, 13-21 and praziquantel were prepared by dissolving the drugs (1 mg) in dimethyl sulfoxide (DMSO, 0.4 mL) and adding RPMI 1640 medium (0.6 mL). S. japonicum worms obtained from mice (C57BL/6, female, 22-24 g, each infected with 50 cercariae) were washed in RPMI 1640 medium, kept at pH 7.5 with HEPES (20 mmol/L) and supplemented with penicillin (100 UI/mL), streptomycin (100 mg/mL) and 10% fetal bovine serum (FBS, Gibco). After washing, 8-15 adult worms were transferred to each well of a 24-well culture plate containing 2 mL of the same medium. The worms were cultured for 30 to 60 min at 37 ℃ in a humid atmosphere containing 5% CO2, and then different concentrations of compounds DQ 1, 7a-7b, 12a-12c, 13-21 (10, 25, 50, 100 μmol/L) diluted with RPMI 1640 medium were added. Control worms were treated with equal volumes of RPMI 1640 or DMSO, and worms treated with praziquantel (10, 25, 50, 100 μmol/L) were also observed. The worm mobility, tegumental alterations and parasite survival were monitored under an inverted microscope (Leica, Wetzlar, Germany) at 24, 48 and 72 h. Parasite death was defined as having no motor activity during 2 min of continuous observation as well as morphological and tegumental alterations. The tests were repeated two times when compounds showed worm killing activity below the concentration of 100 μmol/L.
4.5. Cytotoxic assay of compounds 14 and 15 on Hela cellsCytotoxicity assays were assessed using Hela cells. Cells in the exponential growth phase were collected by centrifugation and a concentration of 4-5 ×104/mL was created. 100 μL of the cell suspension was added to each well of a 96-well plate and cultured at 37 ℃ in a humid atmosphere with 5% CO2 overnight. Compounds (at 5, 10, 20, 40 and 80 μmol/L) were added. Each concentration was assayed in triplicate, and control wells containing no drug were assayed at the same time. The cells were cultured for 48 h at 37 ℃ in a humid atmosphere with 5% CO2. Then 20 μL of MTT (5 mg/mL) was added to each well and the culture conditions were maintained for 4 h. The medium was removed and DMSO (150 μL) was added into each well. The value of absorbance of each well was measured (at 570 nm) with a microplate reader to assess the toxic effect of the compounds on the vertebrate cells.
4.6. Statistical analysisCell survival rates after exposure to compounds 14, 15 and PZQ were determined using chi-square tests. SPSS 13.0 was used for the statistical analyses. Differences between mean values were considered to be significant at the level of 5%.
AcknowledgementsThis work was supported by Laboratory Research Fund funded by Key Laboratory of National Health and Family Planning Commission on Parasitic Disease Control and Prevention, Jiangsu Provincial Key Laboratory on Parasite and Vector Control Technology, Parasitic Disease Prevention and Control Platform (No. WK014-002), fund from the Department of Science and Technology of Jiangsu Province (No. BY2016022-37), fund from the National Natural Science Foundation of China (Nos. 21472069, 30972581, 8120316), fund from the Natural Science Foundation of Jiangsu Province (Nos. BK2012544, BK20151120), fund from the scientific research projects of Jiangsu Provincial Commission of Health and Family Planning (No. H201635), fund from the scientific research projects of Wuxi City Commission of Health and Family Planning (No. Q201656), fund from the Ministry of Education of China (No. JUSRP51516) and Jiangsu Science and Technology Department (No. BM2015024).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.036.
| [1] | A. Danso-Appiah, P.L. Olliaro, S. Donegan, D. Sinclair, J. Utzinger. Drugs for treating Schistosoma mansoni infection. Cochrane Database Syst. Rev. 2 (2013) CD000528. |
| [2] | B. Gryseels, K. Polman, J. Clerinx, L. Kestens. Human schistosomiasis. Lancet 368 (2006) 1106–1118. DOI:10.1016/S0140-6736(06)69440-3 |
| [3] | H. Zheng, L.J. Zhang, R. Zhu, et al., Schistosomiasis situation in People's Republic of China in 2011. Chin. J. Schistosomiasis Control 24 (2012) 621–626. |
| [4] | C.R. Caffrey, W.E. Secor. Schistosomiasis: from drug deployment to drug development. Curr. Opin. Infect. Dis. 24 (2011) 410–417. DOI:10.1097/QCO.0b013e328349156f |
| [5] | F.F. Stelma, I. Talla, S. Sow, et al., Efficacy and side effects of praziquantel in an epidemicfocusofSchistosomamansoni. Am.J.Trop.Med.Hyg 53 (1995) 167–170. DOI:10.4269/ajtmh.1995.53.167 |
| [6] | W. Wang, L. Wang, Y.S. Liang. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res. 111 (2012) 1871–1877. DOI:10.1007/s00436-012-3151-z |
| [7] | Y.X. Liu, W. Wu, Y.J. Liang, et al., New uses for old drugs: the tale of artemisinin derivatives in the elimination of Schistosomiasis japonica in China. Molecules 19 (2014) 15058–15074. DOI:10.3390/molecules190915058 |
| [8] | T.G. Nam, C.W. McNamara, S. Bopp, et al., A chemical genomic analysis of decoquinate, a Plasmodium falciparum cytochrome b inhibitor. ACS Chem. Biol. 6 (2011) 1214–1222. DOI:10.1021/cb200105d |
| [9] | H. Wang, Q. Li, S. Reyes, et al., Nanoparticle formulations of decoquinate increase antimalarial efficacy against liver stage Plasmodium infections in mice. Nanomedicine 10 (2014) 57–65. DOI:10.1016/j.nano.2013.07.010 |
| [10] | R.M. Beteck, D. Coertzen, F.J. Smit, et al., Straightforward conversion of decoquinate into inexpensive tractable new derivatives with significant antimalarial activities. Bioorg. Med. Chem. Lett. 26 (2016) 3006–3009. DOI:10.1016/j.bmcl.2016.05.024 |
| [11] | C.-R. Yan, J. Xu, Y.-B. Weng, et al., A series of ethyl 6-arylmethoxy-7-alkoxy-4-hydroxy-3-quinolinecarboxylates: synthesis and anticoccidial activity against Eimeria tenella. Chem. Biol. Drug Des. 72 (2008) 314–319. DOI:10.1111/jpp.2008.72.issue-4 |
| [12] | W.-L. Wang, L.J. Song, X. Chen, et al., Synthesis and SAR studies of praziquantel derivatives with activity against Schistosoma japonicum. Molecules 18 (2013) 9163–9178. DOI:10.3390/molecules18089163 |
| [13] | W.-L. Wang, L.J. Song, G.P. Wang, et al., 2, 3, 4-tetrahydroisoquinoline derivatives. Chin. J. Org. Chem. 33 (2013) 2588–2595. DOI:10.6023/cjoc201308017 |
| [14] | L.J. Song, H. Luo, W.H. Fan, et al., Oxadiazole-2-oxides may have other functional targets, in addition to SjTGR, through which they cause mortality in Schistosoma japonicum. Parasite Vectors 9 (2016) 26. DOI:10.1186/s13071-016-1301-3 |
| [15] | F.F. Yang, L.J. Song, X.R. Yin, et al., Activity of gossypol against Schistosoma japonicum. Latin Am. J. Pharm. 35 (2016) 1660–1663. |
| [16] | W.-L. Wang, L.J. Song, X. Chen, et al., Synthesis of triclabendazole derivatives and biological evaluation against Schistosoma japonicum. Latin Am. J. Pharm. 35 (2016) 2274–2278. |
 2017, Vol. 28
2017, Vol. 28