b College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450052, China
Cancer chemotherapy with metal-based drugs has gained momentum since the fortuitous discovery of cisplatin [1, 2]. Although they have been widely used in clinic practice for several decades, the efficacy of platinum-based drugs is limited by side effects and intrinsic or acquired drug resistance [3, 4].
Recently, new metal-based anticancer agents have been developed that are synthesized based on complexes with transition metals such as copper (Cu), zinc (Zn), cobalt (Co), and nickel (Ni) [5, 6]. A common approach for inhibiting tumor proliferation is to exploit the metal complex-forming property of artificial nucleases by interfering with DNA binding [7, 8]. For example, the antitumor DNA-binding activities of N-phthaloylglycine in Ni(Ⅱ), Cu(Ⅱ), and Zn(Ⅱ) complexes have been reported [9]. Cu(Ⅱ) is an essential trace element in organisms that functions as a catalytic cofactor of enzymes associated with angiogenesis, iron acquisition, and cell growth [10, 11]. The position of the ligand is important in the design of metal-based agents. Benzimidazole derivatives can bind to the minor groove of DNA and thus have antitumor, antiparasitic, antibacterial, and antiviral activities that are useful for drug development [12-16]. Nitrogen heterocyclic benzimidazole (NHB; Fig. 1) complexed with Cu(Ⅱ) or Co(Ⅱ) has demonstrated protein kinase-inhibitory and anti-inflammatory activities [17-19].

|
Download:
|
| Fig. 1. Molecular structure of ligand | |
Based on these observations, in the present study we synthesized and characterized two NHB-metal complexes, Cu(p-2-bmb)(OH)(ClO4) and Co2(p-2-bmb)Cl4 (complexes 1 and 2, respectively). Both complexes showed antiproliferative activity against SMMC7721 cells, and complex 1 permeated the cell membrane and entered the nucleus and mitochondrion. Moreover, complex 1 was found to interact with DNA and induce apoptosis. These results demonstrate that NHB-based Cu(Ⅱ) complexes are promising as candidate antitumor agents.
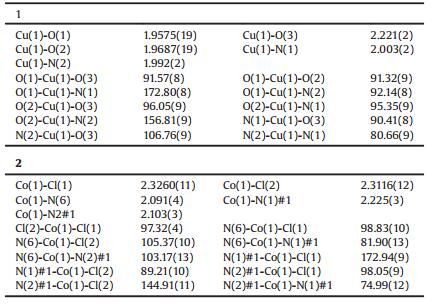
2. Results and discussion 2.1. Crystal structure of Cu(p-2-bmb)(OH)(ClO4) (1) and Co2(p-2-bmb)2Cl4 (2)The molecular structure of the mononuclear Cu(Ⅱ) complex belonging to the monoclinic system with the P-1 space group is shown in Fig. 2a. The penta-coordinated Cu(Ⅱ) complex consisted of Cu(Ⅱ) ion bound to two nitrogen and three oxygen atoms, with O3 at the top of tetragonal pyramid formed by these coordinated atoms; O1, O2, N1, and N2 constituting an approximately planar tetragonal pyramid underside; and Cu(Ⅱ) occupying the center of this plane. The angle of N1-Cu(1)-O1 was 173.05(9)°, while that of N2-Cu(1)-O2 was 156.67(10)°. Cu-O distances ranged from 1.956 (2) to 2.219(2) Å, and Cu-N bond lengths were 1.992(2) and 2.002 (2) Å for Cu1-N2 and Cu1-N1, respectively, as previously reported [20, 21].

|
Download:
|
| Fig. 2. The schematic view of coordination environment of complex 1 (a) and complex 2 (b) (ball-and-stick model) | |
X-ray single crystal diffraction analysis revealed that complex 2 had a binuclear structure based on benzimidazolyl ligand and crystallized in monoclinic space group P-1. The Co(Ⅱ) was pentacoordinated in a tortuous tetragonal pyramidal geometry (Fig. 2b) completed by two Cl atoms (Cl1, Cl2) provided by the decomposition of chloroform during the synthesis as well as three N atoms (N1 and N2 from one p-2-bmb molecule and N6 from the other). N6a occupied apical positions, and the coplanar N(1)-Co(1)-Cl(1) and N(2)-Co(1)-Cl(2) angles were 172.94(9)° and 144.91(11)°, respectively. Co-N distances were 2.091(4)-2.225(3) Å and Co-Cl bond lengths were 2.3116(12) and 2.3260(11) Å for Co1-Cl2 and Co1-Cl1, respectively, which is comparable to those of a similar complex that was previously reported [22, 23].
Crystallographic data for complexes 1 and 2 have been deposited with the Cambridge Crystallographic Data Centre < 1477869-1477870>. These data can be freely accessed at www.ccdc.cam.ac.uk/conts/retrieving.html, or obtained from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223-336-033).
2.2. Cytotoxicity analysisThe cytotoxicity of complexes 1 and 2 was evaluated in SMMC7721, BGC823, and HCT116 carcinoma cell lines after 24, 48, and 72 h with the MTT assay, with cisplatin dissolved in PBS solution and ligand used as reference samples (Table S1 in supporting information). The two compounds showed time-and dose-dependent effects on cell viability (Fig. 3), with complex 1 showing especially strong cytotoxicity in SMMC7721 cells (IC50 = 39.2 ± 1.47 μmol/L, 72 h) and less potency in BGC823 (IC50 > 80 μmol/L, 72 h) and HCT 116 (IC50 = 43.5 ± 4.83 μmol/L, 72 h) cells. The result of better cytotoxicity of complex 1 took us forward to perform DNA binding behaviors and further studies against SMMC7721 cells.

|
Download:
|
| Fig. 3. Inhibition curve showing inhibition rate verses drug concentration (μmol/L) against HCT116, BGC823, SMMC7721 cell lines for 24 h, 48 h, 72 h. (a, b, c) Inhibition curve for complex 1; (d, e, f) Inhibition curve for complex 2 | |
2.3. Cellular uptake studies
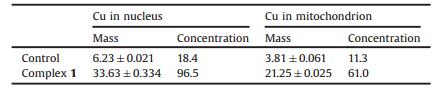
Cu content in SMMC7721 cells was measured by ICP-MS (Table 1). Complex 1 penetrated the cell membrane and entered the nucleus and mitochondrion; Cu2+ concentrations in these organelles were 96.5 ng/mg and 61.0 ng/mg, respectively, which were approximately 5-fold higher than the levels in untreated control cells. These results indicate that complex 1 can accumulate within cells, which is consistent with previously reported in vitro better cytotoxicity and DNA damage-inducing effects [24].
|
|
Table 1 Cu content in the nucleus and mitochondrion of SMMC7721 cells. Values were calculated based on data from at least three independent replicates and are expressed as mass (ppb) and concentration (ng/mg) |
2.4. DNA binding and cleavage activity
DNA is the target of various antitumor metal complexes. As such, the interaction between DNA and complex 1 was investigated by electronic absorption titration and fluorescence and CD spectroscopy, as detailed below.
2.4.1. Stability analysisThe stability of the complex 1 was determined by dissolving the compound in Tris-HCl/NaCl buffer for different times (10 and 30 min and 24, 48, and 72 h) followed by UV-vis spectroscopy analysis. The characteristic absorption intensity was stable over time, although a slight shift in absorption peak and minor hypochromicity were observed in the spectra after 72 h (Fig. S1 in Supporting information), indicating that complex 1 is stable in Tris-HCl/NaCl buffer solution.
Thermogravimetric analysis of air-stable complex 2 (Fig. S2 Supporting information) indicated that the weight loss of 12.57% as temperature was increased from 30 ℃ to 220 ℃ is attributable to the loss of disordered solvent molecules, which is in agreement with the endothermic peak near 215.4 ℃ in the differential scanning calorimetry spectrum. The further decrease in weight indicated that the framework of complex 2 began to collapse at 261.1 ℃, with two endothermic peaks observed at 288.9 ℃ and 345.8 ℃.
2.4.2. DNA interaction studiesThe binding nature of the complex 1 to DNA was assessed by UV-vis spectroscopy. The electronic absorption spectrum of complex 1 in Tris-HCl/NaCl buffer (Fig. 4) at increasing concentrations of CT-DNA revealed strong, increasing absorptivity (hyperchromicity = 64.5%) at around 264 nm without a bathochromic shift, which was contributed by hydrogen bonding or van der Waals interactions from the planarity and extended aromaticity between NHB and the DNA base pairs [24]. A weak hyperchromicity occurred at about 289 nm corresponding to charge transfer of the absorption bands of complex affected by the increasing amounts of CT-DNA [25, 26]. These results suggest that complex 1 moderately binds to DNA.

|
Download:
|
| Fig. 4. Absorption spectra of complex 1 (20 μmol/L) in 5 mmol/L Tris-HCl/50 mmol/ L NaCl buffer upon the addition of calf thymus DNA ([CT-DNA]/[complex]:0.125, 0.25, 0.5, 1, 2, 3, 4, 5, 6); Arrow shows change in intensity with increasing concentration of CT-DNA | |
Ethidium bromide (EB) is a fluorescent probe that is useful for studying the mode of agent-DNA interaction [27]. Due to the quenching effect of solvent molecules, free EB alone does not emit appreciable fluorescence; however, it can produce intense emission by intercalating between adjacent DNA base pairs. We used EB to assess whether complex 1 can bind to CT-DNA via intercalation. The emission spectrum revealed that in the absence and upon continuous addition of complex 1 to EB-CT-DNA, emission intensity significantly decreased by 35.9% (Fig. 5), which may suggest that a portion of EB molecules bound to DNA were displaced from their binding sites by the complex [28, 29]. The above data were analyzed according to the Stern-Volmer equation [29]:

|
Download:
|
| Fig. 5. Emission spectra of EB bound to DNA in the absence and the presence of complex 1, [EB] = 24.5μmol/L, [DNA] = 120.9 μmol/L, [Complex]/[DNA] = r (r = 0– 0.6). Arrow shows the intensity changes upon increasing the concentration of the complex. Inset: Stern–Volmer quenching curves | |
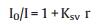
|
where I0 and I represent the fluorescence intensities in the absence and presence of the complex, respectively; and r is the concentration ratio of complex to DNA. Ksv was obtained as the slope of I0/I vs. r, which was used to assess quenching efficiency. The plot (insets in Fig. 5) revealed that the quenching reaction conformed to the Stern-Volmer equation and showed the binding capacity of the complex to DNA. The plot of I0/I vs. [complex]/[DNA] showed that the Ksv value is 0.79 for complex 1. The apparent binding constant (Kapp) is given by using the equation [30]: KEB[EB] = Kapp[complex], where the complex concentration was the value at a 50% reduction of fluorescence intensity of EB. The Kapp value is estimated as 2.13 ×106 (mol/L)-1, which is less than the binding constant of EB as a a classical DNA intercalator (KEB = 1.0 × 107 (mol/L)-1, [EB] = 24.5μmol/L), but is higher than some reported intercalating agents [30].
CD allows the direct monitoring of conformational changes in DNA during interactions with other molecules [31]. There were two main absorption peaks in the CD spectrum of CT-DNA, including positive and negative bands at 275 and 245 nm, respectively, that were due to π ¡ π base pair stacking and helicity, respectively [31, 32]. The CD spectrum of DNA revealed hypochromicity at 275 nm with a 1-4 nm bathochromic shift and a negligible change at 245 nm (Fig. 6), indicating that π…π base pair stacking—which stabilizes the DNA double helix structure— was reduced [33]. The CD spectrum of DNA hypochromicity at 275 nm with bathochromic shift, along with a weak change occurred at about 289 nm and the significantly decreased emission intensity of the bound EB molecules suggested that complex 1 interacts with DNA due to partial intercalation.

|
Download:
|
| Fig. 6. CD spectra of CT-DNA and 1 in 5 mmol/L Tris–HCl/50 mmol/L NaCl buffer solution according to the increasing concentration of [Complex]/[CT-DNA] ratios (r = 0.0, 0.2, 0.4, 0.6, 0.8, 1.0) at room temperature | |
2.4.3. DNA cleavage activity
The DNA cleavage ability of complex 1 was examined by agarose gel electrophoresis using supercoiled pBR322 plasmid DNA. The supercoiled form of DNA (Form Ⅰ) migrates most rapidly on the gel, followed by the linear and nicked forms (Forms Ⅲ and Ⅱ, respectively) [34]. We found that the amount of supercoiled pBR322 (Form Ⅰ) gradually decreased whereas those of the nicked circular and linear plasmids (Forms Ⅱ and Ⅲ, respectively) increased at higher concentrations of complex 1 in the presence of Vc, especially at 125 μmol/L, suggesting that double-stranded DNA was fully cleaved (Fig. 7).

|
Download:
|
| Fig. 7. Cleavage of pBR322 supercoiled plasmid DNA without and with added Vc by the complex 1 in a Tris-HCl buffer containing 50 mmol/L Tris-HCl/50 mmol/L NaCl at pH 7.4 and 37 ℃ with an incubation time of 2 h. Lane 1, DNA; lane 2, DNA + Vc; lane 3, DNA + 1 (125 μmol/L); lane 4-9, DNA + Vc + 1 (10, 25, 50, 75, 100 and 125μmol/L, respectively) | |
2.5. Analysis of DNA damage
DNA cleavage was confirmed with the comet assay. Following treatment with different concentrations of complex 1, damaged DNA was visible as migrating fragments in the comet tails, in contrast to control samples with no DNA damage (Fig. 8C). This phenomenon demonstrated that complex 1 did a great damage to nucleic acid. To further prove the death mechanism of complex 1 against the cancer cells, treated cells were stained with Hoechst 33258 and AO/EB to visualize nuclear morphology. According to the previous studies, chemotherapeutic agents, like cisplatin and the other an array of metal complexes, induced tumor efficient apoptosis accompanied with extensive DNA damages, which can be readily respond to changes in cell nuclear chromatin [16]. Hoechst 33258 staining is a common approach to assess apoptotic features, like nuclear condensation and nuclear fragmentation [35]. Acridine orange (AO) is a vital dye used to stain both live and dead cells, and EB can be easily stain only cells that have lost membrane integrity. When the treated cells are stained with AO/EB, the living cells always show uniformly green, and green accompanied with different degrees of yellow, or orange can be detected in the apoptotic cells, whereas the dead cells are always red [36]. As shown in Fig. 8, SMMC7721 cells treated with complex 1 for 48 h exhibit features of apoptosis such as nuclear shrinkage, chromatin condensation, membrane blebbing, and orange fluorescence, which is accordance with benzimidazole derivatives based complexes induced DNA fragmentation and apoptosis in cancer cells in our previous report [37]. These findings suggest that complex 1 induces apoptosis in tumor cells.

|
Download:
|
| Fig. 8. The morphological changes of SMMC7721 cells after treatment with complex 1 at 40 μmol/L as observed under a fluorescent microscope for 24 h. (A) Hoechst 33258 staining; (B) AO/EB staining; (C) DNA damage types/classes (comets) | |
2.6. Analysis of cell cycle progression and effect of complex 1 on Δψm
To clarify the inhibitory effect of complex 1 on SMMC7721 cell proliferation, cell cycle progression was assessed by flow cytometry. After the treatment by different concentrations of complex 1 solution, we observed that complex 1 arrested cells in G2/M phase in a concentration-dependent manner (Fig. S3 in Supporting information), which was consistent with the results obtained by Hoechst 33258 and AO/EB staining [35].
Mitochondrial membrane permeability, as reflected by Δψm, was detected in SMMC7721 cells treated with various concentrations of complex 1 for 24 h by rhodamine 123. Compared to control cells, the fractions of cells with mitochondrial depolarization increased as a function of complex 1 concentration, with 80 μmol/L inducing collapse of Δψm (Fig. S4 in Supporting information). The result indicated that the apoptosis of SMMC7721 cells induced by complex 1 might be mediated by the mitochondrial pathway.
3. ConclusionIn this study, we generated two novel metal complexes based on NHB. Complex 1 showed good cytotoxicity against SMMC7721 cells by accumulating in the nucleus and mitochondria and causing structural changes, DNA damage and cleavage, and a decrease in Δψm, leading to apoptosis after 24 h. The decrease in Δψm indicated that apoptosis was mediated by the mitochondrial pathway, which merits further examination in future studies. Our results highlight the potential of NHB-based Cu(Ⅱ) complexes as anticancer agents.
4. Experimental 4.1. Materials and general methodsAll reagents were obtained commercially and used as received without further purification. Ethidium bromide (EB), 3-(4, 5-dimathylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), dimethyl sulphoxide (DMSO), bisbenzimide (Hoechst 33258), acridine orange (AO), propidium iodide, and calf thymus (CT)-DNA were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Cell Mitochondria Isolation kit was obtained from Beyotime Institute of Biotechnology (Shanghai, China). Other reagents such as potassium iodide (KI), sodium azide (NaN3) were of standard analytical purity and obtained from Sinopharm (Shanghai, China).
Elemental analysis was carried out on a Flash EA 1112 elemental analyzer (Thermo Fisher Scientific, Waltham, MA, USA). Electrospray ionization mass spectra (ESI-MS) were measured on a Thermo Scientific LCQ fleet ESI-MS spectrometer. Infrared spectra with a KBr pellet were obtained using a Fourier transform infrared spectrometer (PerkinElmer, Waltham, MA, USA) in the region of 400-4000 cm-1. Circular dichroism (CD) spectra were obtained on an MOS-500 instrument (Bio-Logic Science Instruments, Seyssinet-Pariset, France). Thermogravimetric experiments were performed on a Mettler Toledo TGA/SDTA instrument at a heating rate of 10 ℃ min-1.
SMMC7721 human hepatocellular carcinoma, BGC823 human gastric cancer, and HCT116 human colon cancer cell lines were obtained from Xiamen University (Xiamen, China). Cells were cultured in Roswell Park Memorial Institute (RPMI)1640 medium (SMMC7721) or high-glucose Dulbecco's Modified Eagle's Medium containing GlutaMax I (BGC823 and HCT116 cells) supplemented with 10% fetal bovine serum (FBS; Gemini Bio, West Sacramento, CA, USA) and penicillin/streptomycin (100 ×) as antibiotics (Genview, USA) at 37 ℃ under a humidified atmosphere of 5% CO2 (Heraeus, Hanau, Germany). Absorbance readings in the MTT assay were obtained using an Infinite M1000 Pro microplate reader (Tecan, Morrisville, NC, USA). Cycletest Plus DNA reagent was from BD Biosciences (Franklin Lakes, NJ, USA). Cisplatin was purchased from Shanghai Energy Chemical Co. (Shanghai, China).
4.2. SynthesisSynthesis of Cu(p-2-bmb)(OH)(ClO4) (1): Cu(ClO4)2·6H2O (0.03 mmol, 0.0111 g), p-2-bmb (0.02 mmol, 0.0065 g), methanol (MeOH; 0.5 mL), and chloroform (1.5 mL) were placed in a tightly closed glass reactor (10 mL) that was heated at 85 ℃ for 60 h, then gradually cooled to room temperature at a rate of 5 ℃ h-1. Green rectangular crystals formed at the bottom of the glass reactor, with a yield of 51% (based on Cu). Elemental analysis (%) calcd. for C19H17ClCuN6O6: C, 43.52; H, 3.27; N, 16.03; Found: C, 43.50; H, 3.38; N, 16.11. ESI-MS (MeOH) 525.29 [M+H]+. IR (KBr/pellet, cm-1): 3104 (w), 2918 (w), 1602 (m), 1495 (m), 1483 (m), 1441 (m), 1327 (m), 1291 (m), 1109 (s), 1060 (s), 790 (w), 756 (s), 623 (s).
Synthesis of Co2(p-2-bmb)2Cl4 (2): The procedure for the synthesis of complex 2 was similar to that of complex 1, except that CoCl2 (0.03 mmol, 0.0039 g), p-2-bmp (0.02 mmol, 0.0065 g), acetonitrile (1.5 mL), and chloroform (0.5 mL) were used as the materials. Maple-colored polygon crystals were obtained; the yield (based on Co) was 47%. ESI-MS (MeOH) 912.83 [M+H]+. IR (KBr/ pellet, cm-1): 3089 (w), 3064 (w), 3029 (w), 2980 (m), 2254 (w), 1603 (m), 1590 (m), 1478 (s), 1454 (s), 1438 (s), 1400 (s), 1299 (s), 763 (s), 748 (s).
4.3. Crystal structure determinationComplexes 1 and 2 were crystallized as described above. Highquality crystals obtained from the parent liquid were mounted on a glass fiber. X-ray single crystal diffraction data were collected on a SuperNova system with graphite monochromated Cu-Kα radiation (λ = 1.54184 Å) at 293(2) K. The OLEX2 crystallographic program was used to resolve and refine the data [38]. All non-hydrogen atoms were refined with anisotropic thermal parameters. The final cycle of full-matrix least-squares refinement was based on observed reflections and variable parameters; a conventional refinement of complex 1 converged reasonably well. The contribution of disordered solvent in complex 2 was subtracted from the corresponding diffraction patterns by the squeeze procedure using PLATON software [39]. Crystallographic data such as crystal parameters and refinements for the two complexes are listed in Table 2, and the relevant bond lengths and angles are shown in Table 3.
|
|
Table 2 Crystallographic data and structural refinement for complexes 1 and 2 |
|
|
Table 3 Selected bond lengths (Å) and angles (°) for complexes 1 and 2 |
4.4. Evaluation of cytotoxicity
The cytotoxicity of complexes 1 and 2 in SMMC7721, BGC823, and HCT116 human tumor cells was evaluated with the MTT assay. Complexes were dissolved in DMSO to obtain stock solutions, with a final DMSO concentration of < 0.5% in each well [31]. Cell suspensions were seeded in a 96-well plate and cultured for 24 h at 37 ℃ in a 5% CO2 incubator before treatment with different concentrations (5-80μmol/L) of complex solutions. Control wells were prepared by adding the same volume of culture medium containing DMSO. MTT reagent (20μL of a 5 mg/ml solution) was then added to each well, followed by incubation at 37 ℃ for 4 h. The formazan crystals that were formed were dissolved with 150mL DMSO, and absorbance was measured at 492 nm using a microplate reader (Tecan, Männedorf, Switzerland). The half-maximal inhibitory concentration (IC50) was calculated by plotting percent viability vs. complex concentration; the final values represent averages of triplicate samples and were measured as the percentage ratio of absorbance of treated relative to untreated control cells.
4.5. Analysis of cellular uptakeCells were cultured with different concentrations of complex 1 for 12 h at 37 ℃ and 5% CO2. Cell nuclei were extracted using the Cell Mitochondria Isolation kit according to the manufacturer's instructions. Briefly, cells were collected and washed three times with phosphate-buffered saline (PBS), then resuspended with mitochondria separation reagent (to which 1 mmol/L phenylmethylsulfonyl fluoride was added just before use) on ice for 10 min. The mixture was transferred to a glass homogenizer (10 ml) and disrupted. Nuclei and mitochondria were obtained by centrifugation at 600 and 11000 × g, respectively, for 10 min at 4 ℃. Nuclei and mitochondria were mineralized by successive treatment with 65% HNO3 (100μL, 1 h), 30% H2O2 (50μL, 1 h), and 36%-38% HCl (100μL, 2 h) at 95 ℃. The total volume of each sample was made up to 1 mL with ultrapure water. Cellular metal levels are expressed as ng Cu per mg protein. An aliquot of sample was used to determine protein concentration from a calibration curve generated by the Bradford method, with bovine serum albumin used as a reference standard. Cu2+ concentration in cells was determined by inductively coupled plasma-mass spectrometry (ICP-MS) using a VG Elemental Plasma-Quad Ⅱ instrument (Thermo Optek, Franklin, MA, USA) and is expressed as nanograms of metal per milligram of protein.
4.6. Morphological analysisThe morphological assay was carried out as described in our previous report [39]. Briefly, cells were seeded on cover slips for 24 h and treated with complex 1 solution (40μmol/L) for another 24 h; untreated cells served as the control group. The cover slips were washed twice with PBS before and after fixation with a mixture of glacial acetic acid and MeOH (1:3, v/v) for 5 min. A 50-μL volume of Hoechst 33258 (5μg/mL) was added to the cover slips followed by incubation at room temperature for 10 min, and cells were then visualized under an Axiovert 200 M fluorescence microscope (Zeiss, Jena, Germany). After washing with PBS, 20μL AO/EB solution (100μg/mL each in PBS) was added for 3 min at room temperature, followed by immediate observation under a fluorescence microscope.
4.7. Single-cell gel electrophoresisThe comet assay was performed as previously described [40], with slight modification. SMMC7721 cells (2 ×105) were seeded overnight in 6-well plates and then incubated with complex 1 solution (0, 40, and 80μmol/L) for 24 h. The medium was removed and cells were washed three times with PBS, then resuspended in 1 mL PBS. About 1000 cells mixed with 1% low-melting point agarose were transferred to comet slides with solidified 0.5% normal melting point agarose, which were immersed in lysis buffer composed of 2.5 mol/L NaCl, 0.1 mol/L ethylenediamine tetraacetic acid (EDTA), 0.01 mol/L Tris base, 1% Triton-X100, and 10% DMSO (pH 13) at 4 ℃ for 120 min. The slides were submerged horizontally in alkaline electrophoresis buffer (0.3 mol/L NaOH and 0.5 mol/L EDTA) in an electrophoresis tank for 20 min at 4 ℃, and subjected to 25 V for 30 min. The slides were then neutralized with PBS, stained with EB (50 μg/mL) at room temperature for 15 min, and visualized under a fluorescence microscope.
4.8. DNA binding experiments and DNA cleavage assayDNA binding experiments were performed in buffer composed of 5 mmol/L Tris-HCl and 50 mmol/L NaCl (pH 7.20) at 25 ℃. CTDNA in Tris-HCl/NaCl buffer was stored at 4 ℃ and used within 4 days of preparation [41]. CT-DNA concentration in the buffer solution was determined with a Genesys 10S ultraviolet-visible light (UV-vis) spectrophotometer (Daly City, CA, USA) at 260 nm, using 6600 mol/L/cm as the molar absorption coefficient; this yielded a ratio of UV absorbance at 260 nm and 280 nm of about 1.80-1.90, indicating that the CT-DNA was completely free of protein interference [42].
In vitro stability is essential for complexes used in biological applications. Complexes 1 were soluble at 4 ×10-5 mol/L in buffer composed of 5 mmol/L Tris-HCl and 50 mmol/L NaCl (pH 7.4) containing 0.5% DMSO. Complex stability was assessed at 10 and 30 min and 24, 48, and 72 h by UV-vis spectrophotometry.
4.8.1. DNA binding experimentsAbsorption titration of complex 1 in buffer was carried out by adding increasing amounts of DNA stock solution to a fixed concentration of complex 1 (20 μmol/L). After 10 min of incubation, complex-DNA solutions were analyzed by UV-vis spectrophotometry.
Comparative binding of complexes to CT-DNA was monitored in an EB-bound CT-DNA solution in Tris-HCl/NaCl buffer. Samples were mixed with 100 μL CT-DNA (120.9 μmol/L) and 100 μL EB (24.5μmol/L) and then incubated at 37 ℃ for 30 min. Complex 1 (0, 8, 24, 30, 60, 100, 120, and 160 μmol/L) was added to the samples with stirring, followed by incubation at 37 ℃ for 2 h and measurement of absorbance on a microplate reader.
Samples were prepared at increasing [complex]/[CT-DNA] ratios (r = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0). After incubation at 25 ℃ for 1 h, all CD spectra in the range of 220-320 nm were obtained with an MOS-50 spectropolarimeter (Bio-Logic Science Instruments) at room temperature, with subtraction of the background (buffer solution).
4.8.2. DNA cleavage assayDNA cleavage by complex 1 was evaluated with pBR322 plasmid DNA in Tris-HCl/NaCl buffer by agarose gel electrophoresis with and without ascorbic acid (Vc) as a reductant. A 5-μL volume of varying concentrations of complex 1 (10-125 μmol/L), 1 μL pBR322 DNA, and 1 mmol/L Vc were made up to a final volume of 10 μL by adding 50 mmol/L Tris-HCl/50 mmol/L NaCl solution. After mixing, samples were incubated at 37 ℃ for 2 h followed by electrophoresis on a 1% agarose gel in Tris/acetic acid/EDTA buffer for 1 h at 85 V. After staining for 5 min with EB, the gel was imaged (Thmorgan, Beijing, China).
4.9. Flow cytometry analysisSMMC7721 cells were cultured in 10% FBS-supplemented RPMI1640 culture medium with different concentrations of complex 1 solution for 48 h at 37 ℃. Cells were collected and washed once with PBS, then treated with the Cycletest Plus DNA Reagent kit (BD Biosciences, Franklin Lakes, NJ, USA). Cell cycle distribution was evaluated with a Guava EasyCyte 6-2I flow cytometer (Millipore, Billerica, MA, USA).
The effect of complex 1 on Δψm in SMMC7721 cells was evaluated using rhodamine 123 fluorescent probe. Cells were seeded in 6-well plates and treated with different concentrations of complex 1 solution (0, 10, 20, and 40 μmol/L). After 24 h, the medium was removed and cells were washed twice with PBS, and 2 μL rhodamine 123 (2 mmol/L) were added to the cell suspension (2 mL). After incubation at 37 ℃ in the dark for 30 min, samples were analyzed by flow cytometry.
AcknowledgmentThis study was supported by the National Natural Science Foundation of China (Nos. 21371046, 21401041).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.025.
| [1] | H.H. Chen, M.T. Kuo. Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy. Met. Base. Drugs 2010 (2010) 1–7. |
| [2] | A. Dash, M.D. Galsky, A.J. Vickers, et al., Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107 (2006) 506–513. DOI:10.1002/(ISSN)1097-0142 |
| [3] | W. Dong, S.J. Lippard. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug. Discov. 4 (2005) 307–320. DOI:10.1038/nrd1691 |
| [4] | M.M. Gottesman. Mechanism of Cancer Drug Resistance. Annu. Rev. Med. 53 (2002) 615–627. DOI:10.1146/annurev.med.53.082901.103929 |
| [5] | K. Kulprachakarn, Y.L. Chen, X.L. Kong, et al., Copper(Ⅱ) binding properties of hepcidin. J. Biol. Inorg. Chem. 21 (2016) 329–338. DOI:10.1007/s00775-016-1342-2 |
| [6] | W. Huang, S. Kong, Z. Wang, C. Pan, H. Zhu. Ni(Ⅱ) ternary complex based on antimicrobial drug enoxacin: synthesis and biological properties. Chin. J. Chem. 32 (2015) 1169–1175. |
| [7] | I. Neundorf, J. Hoyer, K. Splith, et al., Cymantrene conjugation modulates the intracellular distribution and induces high cytotoxicity of a cell-penetrating peptide. Chem. Commun. 43 (2008) 5604–5606. |
| [8] | S. Thota, S. Vallala, R. Yerra, E.J. Barreiro. Design, synthesis, characterization, cytotoxic and structure activity relationships of novel Ru(Ⅱ) complexes, Chinese. Chem. Lett. 26 (2015) 721–726. |
| [9] | N. Arshada, N. Abbasa, M.H. Bhattia, et al., Synthesis, crystal structure DNA binding and in vitro biological studies of Ni(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) complexes of N-phthaloylglycine. J. Photochem. Photobiol. B. 117 (2012) 228–239. DOI:10.1016/j.jphotobiol.2012.10.003 |
| [10] | K. Zarschler, M. Kubeil, H. Stephan. Establishment of two complementary in vitro assays for radiocopper complexes achieving reliable and comparable evaluation of in vivo stability. Rsc. Adv. 4 (2014) 10157–10164. DOI:10.1039/c3ra47302c |
| [11] | R. Turnaturi, V. Oliveri, M. Viale, M. Monticone, G. Vecchio. Antiproliferative and antioxidant activity of glycoconjugates of dithiocarbamates and their Copper(Ⅱ) and Zinc(Ⅱ) complexes. ChemPlusChem. 80 (2016) 1786–1792. |
| [12] | X. Zhang, J. Huang, F. Hu, P. Yu, E. Hua. Synthesis and bioactivity evaluation of 2-arylbenzimidazole analogues. Asian J. Chem. 7 (2014) 1891–1894. |
| [13] | R.V. Patel, P.K. Patel, P. Kumari, D.P. Rajani, K.H. Chikhalia. Synthesis of potential antitubercular and antimicrobial s triazine-based scaffolds via Suzuki crosscoupling reaction. Eur. J. Med. Chem. 53 (2012) 209–225. |
| [14] | M. Hegde, K.S.S. Kumar, E. Thomas, K.S. Rangappa. A novel benzimidazole derivative binds to the DNA minor groove and induces apoptosis in leukemic cells. RSC Adv. 5 (2015) 93194–93208. DOI:10.1039/C5RA16605E |
| [15] | X.J. Wang, M.L. Yang, L.P. Zhang, et al., Design of novel bis-benzimidazole derivatives as DNA minor groove binding agents. Chin. Chem. Lett. 25 (2014) 589–592. DOI:10.1016/j.cclet.2014.01.019 |
| [16] | S. Liu, W. Cao, L. Yu, et al., Chen, Zinc(Ⅱ) complexes containing bisbenzimidazole derivatives as a new class of apoptosis inducers that trigger DNA damage-mediated p53 phosphorylation in cancer cells. Dalton Trans. 42 (2013) 5932–5940. DOI:10.1039/c3dt33077j |
| [17] | F. Arjmand, B. Mohani, S. Ahmad. Synthesis antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(Ⅱ) complex. Eur. J. Med. Chem. 40 (2005) 1103–1110. DOI:10.1016/j.ejmech.2005.05.005 |
| [18] | M.Z. Wisniewski, T. Glowiak, A. Opolski, J. Wietrzyk. Synthesis, characterization and antiproliferative activity of the Co(Ⅱ), Ni(Ⅱ), Cu(Ⅱ) Pd (Ⅱ) and Pt(Ⅱ) complexes of 2-(4-Thiazolyl)benzimidazole (Thiabendazole). Met. Base. Drugs 8 (2000) 189–194. |
| [19] | J. Zhao, K. Peng, Y. Guo, et al., Photoluminescent and cytotoxic properties of multinuclear complexes and multinuclear-based polymers with group 12 metals and a tripodal ligand. New J. Chem. 39 (2015) 6016–6024. DOI:10.1039/C5NJ00222B |
| [20] | E. Balogh-Hergovich, J. Kaizer, G. Speier, V. Fu1lo1p, L. Pa'rka'nyi. Quercetin 2, 3-dioxygenase mimicking ring cleavage of the flavonolate ligand assisted by copper. Synthesis and characterization of Copper(Ⅰ) Complexes [Cu(PPh3)2(fla)] (fla=Flavonolate) and [Cu(PPh3)2(O-bs)] (O-bs=O-Benzoylsalicylate). Inorg. Chem. 38 (1999) 3787–3795. DOI:10.1021/ic990175d |
| [21] | J. Hernandezgil, 'S. Ferrer, A. Castiñeiras, F. Lloret. Antisymmetric exchange in triangular tricopper(Ⅱ) complexes: correlation among structural, magnetic, and electron paramagnetic resonance parameters. Inorg. Chem. 51 (2012) 985–1001. DOI:10.1021/ic2020034 |
| [22] | H. Ackermann, R. Leo, W. Massa, B. Neumuller, K. Dehnicke. Phosphoraneiminato-acetato complexes of cobalt and cadmium with M4N4 heterocubane structure. Anorg. Allg. Chem. 626 (2000) 284–289. DOI:10.1002/(ISSN)1521-3749 |
| [23] | G. Lyubartseva, S. Parkin. Tetra-ethyl-ammonium (2, 2'-bipyridine)tetracyanidocobaltate(Ⅲ) sesquihydrate acetonitrile solvate, Acta. Crystallogr. 66 (2010) 475–476. |
| [24] | K.M. Vyas, R.N. Jadeja, D. Patel, R.V. Devkar, V.K. Gupta. A new pyrazolone based ternary Cu(Ⅱ) complex: synthesis, characterization, crystal structure, DNA binding, protein binding and anti-cancer activity towards A549 human lung carcinoma cells with a minimum cytotoxicity to non-cancerous cells. Polyhedron. 65 (2013) 262–274. DOI:10.1016/j.poly.2013.08.051 |
| [25] | W. Zhou, X. Wang, M. Hu, C. Zhu, Z. Guo. A mitochondrion-targeting copper complex exhibits potent cytotoxicity against cisplatin-resistant tumor cells through multiple mechanisms of action. Chem. Sci. 5 (2014) 2761–2770. DOI:10.1039/C4SC00384E |
| [26] | C.H. Wang, W.C. Shi, H.C. Chang, et al., Preparation and characterization of amino-linked heterocyclic carbene Palladium Gold, and Silver complexes and their use as anticancer agents that act by triggering apoptotic cell death. J. Med. Chem. 54 (2011) 5245–5249. DOI:10.1021/jm101096x |
| [27] | J. Lu, Q. Sun, J. Li, et al., Two water-soluble copper(Ⅱ) complexes: synthesis, characterization, DNA cleavage, protein binding activities and in vitro anticancer activity studies. J. Inorg. Biochem. 137 (2014) 46–56. DOI:10.1016/j.jinorgbio.2014.03.015 |
| [28] | S. Tabassum, M. Zaki, M. Ahmad, et al., Synthesis and crystal structure determination of copper(Ⅱ)-complex: in vitro DNA and HSA binding, pBR322 plasmid cleavage, cell imaging and cytotoxic studies. Eur. J. Med. Chem. 83 (2014) 141–154. DOI:10.1016/j.ejmech.2014.06.018 |
| [29] | M.Y. Qin, S.P. Liu, Z.F. Liu, X.L. Hu. Fluorescence quenching reaction of tetracaine hydrochloride with erythrosine and their analytical applications. Spectrosc. Spect. Anal. 28 (2008) 389–393. |
| [30] | H. Gopinathan, N. Komathi, M.N. Arumugham. Synthesis, structure DNA binding, cleavage and biological activity of cobalt (Ⅲ) complexes derived from triethylenetetramine and 1, 10 phenanthroline ligands. Inorg. Chim. Acta. 416 (2014) 93–101. DOI:10.1016/j.ica.2014.03.015 |
| [31] | I. Yousuf, F. Arjmand, S. Tabassum, et al., Mechanistic insights into a novel chromone-appended Cu(Ⅱ) anticancer drug entity: in vitro binding profile with DNA/RNA substrates and cytotoxic activity against MCF-7 and HepG2 cancer cells. Dalton Trans. 44 (2015) 10330–10342. DOI:10.1039/C5DT00770D |
| [32] | S. Tabassum, G.C. Sharma, F. Arjmand. New modulated design and synthesis of chiral CuⅡ/SnIV bimetallic potential anticancer drug entity: in vitro DNA binding and pBR322 DNA cleavage activity. Spectrochim. Acta A 90 (2012) 208–217. DOI:10.1016/j.saa.2012.01.020 |
| [33] | F. Arjmand, S. Parveen, Mohd. Afzal, Mohd. Shahid. Synthesis, characterization, biological studies (DNA binding, cleavage, antibacterial and topoisomerase I) and molecular docking of copper(Ⅱ) benzimidazole complexes. J. Photochem. Photobiol. B 114 (2012) 15–26. DOI:10.1016/j.jphotobiol.2012.05.003 |
| [34] | X.Q. Chen, J.Y. Wang, T.Y. Zhang, L.Z. Zhang, X.J. Peng. Synthesis and DNA cleavage activity of diiron(Ⅲ) complex bearing pyrene group. Chin. Chem. Lett. 19 (2008) 342–344. DOI:10.1016/j.cclet.2007.12.028 |
| [35] | X. Wang, C. Wang, Y. Sun, C. Sun, Y. Zhang, X. Wang, K. Zhao. Taxol Produced from Endophytic Fungi Induces Apoptosis in Human Breast, Cervical and Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. 16 (2015) 125–131. DOI:10.7314/APJCP.2015.16.1.125 |
| [36] | A. Byczkowska, A. Kunikowska, A. Kaźmierczak. Determination of ACCinduced cell-programmed death in roots of Vicia faba ssp. minor seedlings by acridine orange and ethidium bromide staining. Protoplasma 250 (2013) 121–128. DOI:10.1007/s00709-012-0383-9 |
| [37] | J. Zhao, Y. Guo, J. Hu, et al., Potential anticancer activity of benzimidazolebased mono/dinuclear Zn(Ⅱ) complexes towards human carcinoma cells. Polyhedron 102 (2015) 163–172. DOI:10.1016/j.poly.2015.09.057 |
| [38] | F.N. Gonsior. Synthesis of mesomeric betaine compounds with imidazoliumenolate structure. Beilstein. J. Org. Chem. 8 (2012) 390–397. DOI:10.3762/bjoc.8.42 |
| [39] | G.N. Li, C.W. Gao, H. Xie, et al., New luminescent cyclometalated iridium(Ⅲ) complexes containing fluorinated phenylisoquinoline-based ligands: synthesis, structures, photophysical properties and DFT calculations. Chin. Chem. Lett. 27 (2016) 428–432. DOI:10.1016/j.cclet.2015.12.007 |
| [40] | X. Pu, Z. Wang, J.E. Klaunig. Cryopreservation of human blood for alkaline and Fpg-modified comet assay. Toxicol. Mech. Methods 16 (2016) 1–6. |
| [41] | D. Mahendiran, R.S. Kumar, V. Viswanathan, D. Velmurugan, A.K. Rahiman. Targeting of DNA molecules, BSA/c-Met tyrosine kinase receptors and antiproliferative activity of bis(terpyridine)copper(ii) complexes. Dalton Trans. 45 (2016) 7794–7814. DOI:10.1039/C5DT03831F |
| [42] | M. Milkiewicz, O. Hudlicka, R. Shiner, S. Egginton, M.D. Brown. Vascular endothelial growth factor mRNA and protein do not change in parallel during non-inflammatory skeletal muscle ischaemia in rat. J. Physiol. 577 (2006) 671–678. DOI:10.1113/jphysiol.2006.113357 |
 2017, Vol. 28
2017, Vol. 28